Abstract
Carbonic anhydrases (CAs, EC 4.2.1.1) are ubiquitous metalloenzymes involved in biosynthetic processes, transport, supply, and balance of CO2/HCO3- into the cell. In Bacteria, CAs avoid the depletion of the dissolved CO2/HCO3- from the cell, providing them to the central metabolism that is compromised without the CA activity. The involvement of CAs in the survival, pathogenicity, and virulence of several bacterial pathogenic species is recent. Here, we report the kinetic properties of the recombinant γ-CA (EcoCAγ) encoded in the genome of Escherichia coli. EcoCAγ is an excellent catalyst for the physiological CO2 hydration reaction to bicarbonate and protons, with a kcat of 5.7 × 105 s−1 and kcat/KM of 6.9 × 106 M−1 s−1. The EcoCAγ inhibition profile with a broad series of known CA inhibitors, the substituted benzene-sulphonamides, and clinically licenced drugs was explored. Benzolamide showed a KI lower than 100 nM. Our study reinforces the hypothesis that the synthesis of new drugs capable of interfering selectively with the bacterial CA activity, avoiding the inhibition of the human α -CAs, is achievable and may lead to novel antibacterials.
1. Introduction
Nature developed a fascinating system for recycling CO2Citation1,Citation2. Plant, algae, and photosynthetic prokaryotes through the photosynthetic process can convert light energy into chemical energyCitation1. The last is stored in the carbohydrates, and the carbon dioxide (CO2) is fixed into biomass. In the aerobic environment, carbon from the biomass returns to the atmosphere by the action of O2-requiring decomposers, such as bacteria and fungiCitation3, while, in an environment characterised by the absence of oxygen, the anaerobic microbes decompose the biomass releasing methane and CO2Citation4. The carbon cycle is essential for the life on the Earth since carbon is a critical component in controlling the planet temperature, a crucial food ingredient for sustaining the entire living organisms, and, finally, an energy source for driving the global economyCitation5. A necessary enzyme involved in the carbon cycle, which has the function to enhance the photosynthesis via a mechanism that concentrates and supplies CO2 up to 1000-fold close to the ribulose-1,5-bisphosphate carboxylase (RuBisCO), is the carbonic anhydrase (CA, EC 4.2.1.1) Citation6. Several CA-classes have been identified, such as α, β, γ, δ, ζ, η, θ, and ιCitation7–9. The eight CA-classes, showing a low sequence similarity, different folds and structures, are considered phylogenetically distinct. This exceptional sequence and structural divergence evolved, reflecting a convergent evolution of the CA-classes since they target a common substrate, the CO2, and catalyse the same reaction, the simple but physiologically crucial reaction of carbon dioxide hydration/dehydration to bicarbonate and protons (CO2 + H2O ⇄ HCO3- + H+)Citation10–17. The CO2 hydratase reaction is catalysed at very high rates, with a pseudo-first-order kinetic constant (kcat) ranging from 104 to 106 s−1 (18, 19), which is about 67,000–7,000,000 times higher than the uncatalyzed reaction with a kcat=0.15 s−1Citation18,Citation19. In addition to the involvement in photosynthetic processes, CAs accomplish the transport, supply, and balance of CO2 and bicarbonate into the cell, but also pH homeostasis, secretion of electrolytes, and participate in several biosynthetic processesCitation20,Citation21. The homeostasis of H+ and CO2/HCO3- is involved in many physiological and pathological conditionsCitation18,Citation19,Citation22–26. In Bacteria, for example, the primary CA function is to avoid the depletion of the dissolved inorganic carbon (CO2/HCO3-) from the cell, providing them quickly to the central metabolism, which might be compromised without the CA activityCitation7,Citation8,Citation16,Citation27–29. A charming example is represented by the β-CA (CynT) encoded by the genome of the bacterium Escherichia coli, a Gram-negative bacterium typically colonising the lower intestine of warm-blooded organismsCitation30–32. The E. coli cyn operon contains three genes, CynT, CynS, and CynX encoding for a β-CA, a cyanase, and an unknown protein, respectivelyCitation33. CynT catalysing the CO2 hydration produces HCO3-, whose depletion from the bacterial cells is avoided since the cyanase uses it as a substrate to produce ammonia and CO2Citation33–35.
Numerous examples support the importance of CA activity in the growth of bacteria. For example, the deletion of a gene encoding for the β-CA from Ralstonia eutropha allowed the heterotrophic growth of the mutant only at an elevated concentration of CO2 compared to wild-typeCitation36. In E. coli, a second β-CA, CynT2, is essential for the growth of the microorganism at atmospheric pCO2Citation37,Citation38. The lost of CA genes in some Proteobacteria, such as those belonging to the genera Buchnera and Rickettsia, determined their adaptation only in high-CO2 nichesCitation39. More interesting, are the discovery of the involvement of CAs in the survival, pathogenicity, and virulence of several species of human pathogens, such as Helicobacter pyloriCitation40–42, Vibrio choleraeCitation43, Brucella suisCitation44–47, Salmonella entericaCitation48, and Pseudomonas aeruginosaCitation49 among others.
In this context, we focalised our interest in the inhibition profile of the CAs encoded by the genome of E. coli, a bacteria generally coexisting in a mutually beneficial state with the hostCitation50. In some cases, it may become a severe pathogenCitation51–53 or can cause diseases if the host defences are weakenedCitation54. The E. coli genome encodes for β-CAs (Cyn T and CynT2), γ- and ι-CAs. Here, we cloned, overexpressed, and purified the γ-CA (EcoCAγ) enzyme. The recombinant EcoCAγ was subjected to the investigation of its kinetic properties since only the three-dimensional structure was determined in 2012Citation55. Besides, we explored the EcoCAγ inhibition profile with a broad series of substituted benzene-sulphonamides and clinically licenced drugs, which generally inhibit the CAs in the nanomolar rangeCitation56–58. The EcoCAγ inhibition profile was compared with those obtained for the two human isoforms (hCA I and hCA II) with the prospect of gaining new scientific knowledge in the design of potentially new inhibitors capable of blocking efficiently and selectively the CA activity encoded by the pathogenic microbes.
2. Materials and methods
2.1. Chemicals and instruments
All the chemicals used in this study were of reagent grade and purchased from Sigma. The Affinity column (His-Trap FF) and the AKTA-Prime purification system were bought from GE Healthcare. The SX20 Stopped-Flow and SDS–PAGE and Western-Blot apparatus were obtained by AppliedPhotophysics and BioRAD, respectively.
2.2. Cloning, expression and purification of the recombinant E.coli γ-CA
The synthetic E. coli gene encoding for the EcoCAγ (Accession number: WP_009008373.1) was synthesised by the Invitrogen GeneArt (ThermoFisher Scientific), a company specialised in gene synthesis, and cloned into the expression vector pET100D-Topo/γ-CA. Briefly, the gene was designed to produce the recombinant EcoCAγ as fusion proteins with a tag containing nucleotides encoding for six histidines (His-Tag) at the amino terminus of neosynthetized recombinant protein. Competent E. coli BL21 (DE3) codon plus cells (Agilent) were transformed as described by Del Prete et al. Citation59. Isopropyl β-D-1-thiogalactopyranoside (IPTG) at the concentration of 1 mM was added to the cellular culture to overexpress the recombinant EcoCAγ. After growth, the cells were harvested and disrupted by sonication. Cellular extract was purified using a nickel affinity column (His-Trap FF), which allows the interaction between the matrix functionalised with Ni2+ ions and the His-Tag at the N-terminus of the protein. The HisTrap column (1 ml) was equilibrated with 20 ml equilibration buffer (50 mM Tris, 20 mM imidazole and 150 mM sodium chloride, pH 7.5) at 1 ml/min. The supernatant from the cellular lysate was loaded onto the column at 1 ml/min, and eluted from the column by fluxing imidazole (300 mM) at a flow of 0.5 ml/min in a buffer composed of 50 mM Tris and 300 mM sodium chloride, pH 7.5. The recovered EcoCAγ was 90% pure. The protein quantification was carried out by Bradford method (BioRAD) Citation60.
2.3. Enzyme activity for monitoring the EcoCAγ purification
The CA activity assay was performed as described by Capasso et al. Citation61. Briefly, the assay was based on the monitoring of pH variation due to the catalysed conversion of CO2 to bicarbonate. Bromothymol blue was used as the indicator of pH variation. The assay was performed at 0 °C and a CO2-satured solution was used as substrate. The enzyme activity was calculated by measuring the time required for Bromothymol blue to change from blue to yellow. This time is inversely related to the quantity of enzyme present in the sample and allows the calculation of the Wilbur-Anderson units as described previouslyCitation61.
2.4. Sds-PAGE, protonography and Western-Blot
A 12% Sodium Dodecyl Sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) prepared as described by LaemmliCitation62 was used, loading on the gel the recovered EcoCAγ from the affinity column. The gel was stained with Coomassie Brilliant Blue-R. To perform the protonography, wells of 12% SDS-PAGE gel were loaded with samples mixed with loading buffer not containing 2-mercaptoethanol and not subjected to boiling, in order to avoid protein denaturation. The gel was run at 150 V until the dye front ran off the gel. Following the electrophoresis, the 12% SDS-PAGE gel was subject to protonography to detect the yellows bands due to the hydratase activity on the gel as described previouslyCitation63–66. In addition, EcoCAγ was transferred to a PVDF (polyvinylidene fluoride) membrane with transfer buffer (25 mM Tris, 192 mM glycine, 20% methanol) using Trans-Plot SD Cell (Bio-Rad, Hercules, CA, USA). His-Tag Western blot was carried out using the Pierce Fast Western Blot Kit (Thermo Scientific, Waltham, MA, USA). Blotted membrane was placed in the wash blot solution Fast Western 1 Wash Buffer to remove transfer buffer. Primary Antibody Working Dilution was added to the blot and incubated for 30 min at room temperature (RT) with shaking. Invitrogen anti-His antibody (1:10000) was used. Afterwards, the blot was removed from the primary antibody solution and incubated for 10 min with the FastWestern Optimised HRP ReagentWorking Dilution. Subsequently, the membrane was washed two times in about 20 ml of FastWestern 1Wash Buffer. Finally, the membrane was incubated with the detection reagent working solution and incubated for 1 min, at room temperature, and then developed with X-ray film.
2.5. Kinetic parameters and inhibition constants
The CO2 hydration activity performed by the EcoCAγ was monitored using an Applied Photophysics stopped-flow instrumentCitation67. Phenol red (at a concentration of 0.2 mM) was used as indicator, working at the absorbance maximum of 557 nm, with 20 mM TRIS (pH 8.3) as buffer, and 20 mM NaClO4 (for maintaining constant the ionic strength), following the initial rates of the CA-catalysed CO2 hydration reaction for a period of 10–100 s. To determine the kinetic parameters by Lineweaver-Burk plots and the inhibition constants, a concentration of CO2 between 1.7 to 17 mM was used. At least six measurements of the original 5–10% reaction were used to assess the initial velocity for each inhibitor. The uncatalyzed rates were identically determined and detracted from the total observed rates. Stock inhibitor solutions (10–100 mM) were prepared in distilled-deionized water and dilutions up to 0.01 mM were done with the buffer test. Inhibitor and enzyme solutions were preincubated together for 15 min at room temperature prior to assay, in order to allow for the formation of the E-I complex or for the eventual active site mediated hydrolysis of the inhibitor. The inhibition constants were obtained by non-linear least-squares methods using PRISM 6 and the Cheng-Prusoff equation, as reported earlierCitation12,Citation14,Citation68, and represent the mean from at least three different determinations. h CAI and hCA II were recombinant enzymes obtained in-house.
3. Results and discussion
3.1. Primary structure analysis
Because the genome of Escherichia coli was full sequenced, it was designed and polymerised the synthetic gene encoding for the EcoCAγ polypeptide chain. The sequence of the synthetic gene and its corresponding protein are shown in . The EcoCAγ nucleotide sequence consists of an open reading frame of 771 nucleotides, encoding for a polypeptide chain of 256 amino acid residues (). The EcoCAγ was aligned with the YrdA amino acid sequence, which corresponded to the γ-CA crystallised in 2012 (55). As shown in , EcoCAγ has the same polypeptide chain of the YrdA protein, except for the presence of 72 additional amino acids at the N-terminus. In bacteria genes are often found in a cluster on the chromosome, which are under control of a single promoterCitation69. This gene organisation is known as an operon. Thus, it is possible that the additional 72 residues belong to a different protein encoded by the E. coli operon, which includes the gene encoding for the E.coli γ-CA, too. As described in the literature, γ-CA is a homotrimer with three zinc-containing active sites located at the interfaces between two monomersCitation55. Each monomer is structurally characterised by a tandemly-repeated hexapeptide, which mostly shows an aliphatic residue, usually Ile, Val, or Leu, at the first position, a well-conserved residue is glycine at position two, and a residue Ala, Ser, Cys, Val, Thr, or Asn at position five () Citation70. This repeated hexapeptide is essential for the left-hand fold of the trimeric β-helix structures, which contradistinguish the canonical γ-CAs and putative acetyltransferases/acyltransferasesCitation55. YrdA was crystallised without the extra 72 residues at the N-terminus because the authors considered only the polypeptide chain with the conserved hexapeptide-repeat motifs (182 amino acid residues), which generally typify all the γ-CA sequences ().
Figure 1. Nucleotide and translated amino acid sequences of the EcoCAγ. The amino acid residues and the open reading frame are indicated by capital letters, in bold and not in bold, respectively, *, indicate the stop codon.
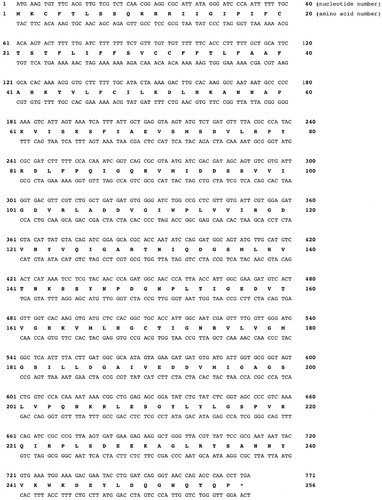
Figure 2. Pairwise comparison of EcoCAγ amino acid sequence with the YrdA polypeptide chain. The pairwise alignment was performed with the programme Blast Global Align. The accession numbers of the aligned sequences are WP_009008373.1 (EcoCAγ) and P0A9W9 (YrdA). Legend: The extra 72 amino acid residues are in green bold; the identical amino acid residues are between the two aligned sequences; the amino acid residues of a typically repeated hexapeptide are reported in bold blue; the three histidines coordinating the metal ion are in red bold; the catalytically relevant residues, which participate in a network of hydrogen bonds with the catalytic water molecule, are represented in black bold; a hyphen shows gaps.

Next paragraphs report 1) the heterologous expression and purification of the EcoCAγ full amino acid sequence (containing the extra 72 residues at the N-terminus of the polypeptide chain); 2) the determination of the kinetic parameters of EcoCAγ using the stopped-flow technique; and 3) the inhibition profile of EcoCAγ with a broad range of small molecules, which generally inactivate this class of enzyme.
3.2. Heterologous expression and purification
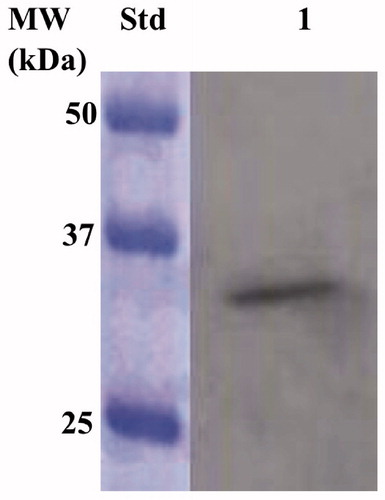
IPTG ((Isopropyl β-D-1-thiogalactopyranoside) induction of E. coli BL21 (DE3) cells transformed with the expression plasmid containing the complete EcoCAγ gene resulted in the production of the recombinant γ-CA as a chimeric polypeptide chain obtained by the fusion of the N-terminus of the EcoCAγ protein to the C-terminus of a soluble peptide (about 4.0 kDa) containing six histidines (His-Tag). This strategy has been adopted to improve the solubility, purification, and detection of the recombinant EcoCAγ expressed in its complete form (plus the extra 72 residues at the N-terminus). After the sonication and centrifugation, most of the CA activity was recovered in the soluble fraction of the E. coli cell extract. The expression of the chimeric EcoCAγ was confirmed by Western Blot (WB) analysis using an anti-His-Tag antibody (). As expected, analysing the E. coli cellular extract, the specific antibody for the His-Tag tail recognised the fusion protein as a band with a molecular weight of about 33.0 kDa. A subunit molecular mass of 32.4 kDa was calculated on the amino acid sequence translated from the chimeric gene encoding for the chimeric EcoCAγ.
Figure 3. Western blot analysis performed on the supernatant coming from the E. coli cellular extract obtained after the sonication and centrifugation. Lane STD, molecular markers, M.W. starting from the top: 50 kDa, 37 kDa, and 25 kDa; lane 1, overexpressed chimeric EcoCAγ.
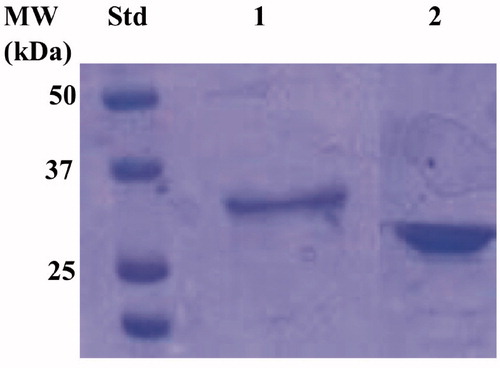
The recombinant enzyme was purified to homogeneity using the affinity chromatography, as demonstrated by the SDS-Page analysis (). As a result, the electropherogram profile of EcoCAγ showed a band at the same position identified in the western-blot analysis (33.0 kDa) under reducing conditions ().
Figure 4. Electropherogram of the SDS-PSAGE carried out on the recombinant chimeric EcoCAγ. Legend: lane STD, molecular markers, M.W. starting from the top: 50 kDa, 37 kDa, and 25 kDa; lane 1, chimeric EcoCAγ; Lanes 2 commercial bovine CA (bCA) used as control. The band at a molecular weight of about 33.0 kDa represented the chimeric EcoCAγ purified by affinity chromatography.
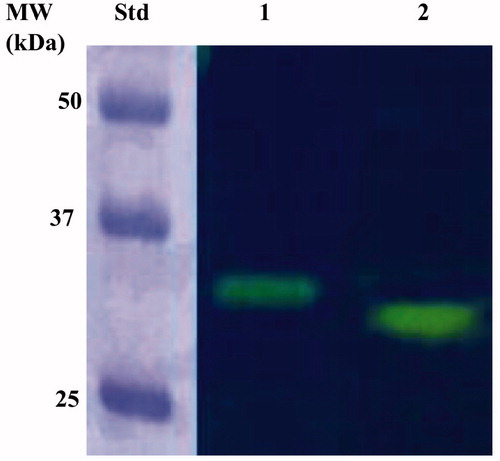
The full sequence of the recombinant EcoCAγ was also subjected to protonography, a powerful and elegant technique able to reveal, as a yellow band on the polyacrylamide gel, the production of ions (H+) developed during the CO2 hydration reactionCitation63,Citation65. The protonography analysis demonstrated that the complete EcoCAγ polypeptide chain was catalytically active. The protonogram showed a single hydratase activity band on the gel with a molecular weight of about 33.0 kDa, corresponding to the monomeric form of the chimeric EcoCAγ (). This was not a surprise since the protonography analysis requires the elimination of SDS at the end of the electrophoretic run. Even if all the γ-CAs are active as trimers, the yellow band appeared in the position of the inactive monomeric form because the SDS purging leads to the rearrangement of the γ-CA monomers in the gel. As a result, EcoCAγ correctly refolded and generated the active trimeric forms of the γ-CA at the position of the monomer. This is described for other eukaryotic and prokaryotic CA classes, tooCitation63
Figure 5. Protonogram of the EcoCAγ eluted from the affinity resin. The yellow band on the protonogram corresponds to the enzyme activity responsible for the drop of pH from 8.2 to the transition point of the dye in the control buffer (pH 6.8), due to the hydratase activity. Lane STD, molecular markers starting from the top: 50 kDa, 37 kDa, and 25 kDa); Lane 2, purified EcoCAγ; Lane 3, commercial bovine CA (bCA) used as a positive control.
3.3. Determination of the kinetic parameters using the stopped-flow technique
The CO2 hydratase activity of the soluble enzyme and its kinetic constants were determined using the stopped-flow technique (). These results were compared with the kinetic parameters of the two mammalian α-CA isoforms (h CAI and h CAII), as well as with the β-CA from the same microorganism and the α-, β-, and γ- CAs from Vibrio cholerae ().
Table 1. Kinetic parameters for the CO2 hydration reaction catalysed by the α-, β-, and γ-class CA enzymes: hCA I and II (α-class CAs), and VchCAα at 20 °C and pH 7.5 in 10 mM HEPES buffer; VchCAβ, VchCAγ; CynT2 and EcoCAγ determined at 20 °C, pH 8.3 in 20 mM TRIS buffer and 20 mM NaClO4. Inhibition data with the clinically used sulphonamide AZA (5-acetamido-1,3,4-thiadiazole-2-sulphonamide) are also provided.
As shown in , EcoCAγ is an excellent catalyst for the physiological CO2 hydratase reaction to bicarbonate and protons, with a kcat of 5.7 × 105 s−1 and catalytic efficiency (kcat/KM) of 6.9 × 106 M−1 s−1. EcoCAγ kcat was similar to those obtained for other bacterial CAs belonging to the β-or γ- classes, as well as for the human isoform hCA I. Interestingly, the catalytic efficiency of EcoCAγ resulted to be two orders of magnitude lower with respect to that of hCA II and one order with respect to VchCAγ (same class of EcoCAγ) and the other enzymes reported in . The investigation of the kinetic properties of the CAs is important because even if these enzymes belong to the same or different CA-classes the steric hindrance of the amino acid residues surrounding the catalytic pocket is responsible for the increase/decrease of the parameters related to the affinity of the enzyme for the substrate (KM). EcoCAγ was also inhibited by the sulphonamide acetazolamide (KI = 248 nM), a well-known pharmacological CA inhibitor (). The acetazolamide resulted to be a very sensitive inhibitor of the human isoform h CAII (KI=12 nM) and the α-CA from Vibrio cholerae (KI=6.8 nM), wheras for the other CAs reported in it was less effective, with KIs in the range of 227–473 nM. Thus, the KI variation can be attributed to the interaction and steric hindrance of the amino acid residues of the catalytic pocket interacting with the inhibitor. The structural differences, which affect the CA-classes or CAs belonging to the same class, highlight the possibility of designing specific and selective inhibitors for this superfamily of enzymes.
3.4. Sulphonamide inhibition profile
Among the CAIs, a library of 42 compounds, 1–24 and AAZ-EPA, represent an important group of simple aromatic/heterocyclic sulphonamides (including one sulfamate), which are able to inhibit the CA ().
Figure 6. The 42 compounds used to study the EcoCAγ inhibition profile. Forty-one sulphonamides and one sulfamate (TPM) were exploited. On the left, the series 1–24; on the right and grey, the clinically used drugs.
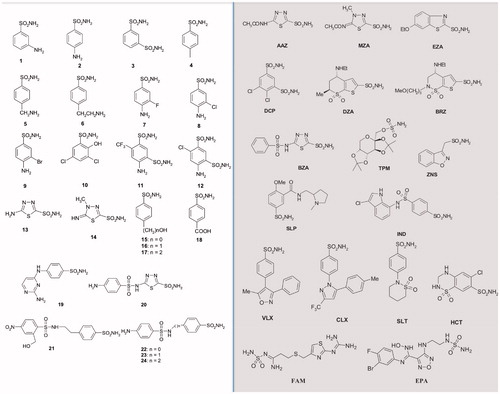
The series AAZ-EPA includes licenced drugs used for the following clinical treatments: glaucoma, epilepsy, idiopathic intracranial hypertension, diuretic, duodenal ulcers, migraine, Parkinson’s disease, obesity, cancer, osteoarthritis, rheumatoid arthritis, diet, etc.Citation56–58. Most of the sulphonamides bind in a tetrahedral geometry to the Zn(II) ion in the deprotonated state, establishing with the amino acid residues of the catalytic site an extended network of hydrogen bondsCitation18,Citation19,Citation22, as well as the aromatic/heterocyclic part of the inhibitor interacts with the hydrophilic and hydrophobic residues of the catalytic cavityCitation18,Citation19.
Since CAs are considered a valuable target for impairing the microbe vitality or their virulence, the in vitro exploration of the EcoCAγ inhibition profile with such inhibitors is crucial for obtaining potent and selective families of new pharmacological agents. New drugs are needed considering that the emergence arisen from the resistance to the existing antimicrobial medicines is one of the most severe problems afflicting the human community. reports the inhibition profile of EcoCAγ, which was compared with the inhibitory behaviour of hCA I, hCA II, and VchCAγ reported earlier by our groupCitation17,Citation56,Citation71. From the data of , the following can be observed:
Table 2. Inhibition of hCA I, hCA II, EcoCAγ and VchCAγ with sulphonamides 1–24 and the clinically used drugs AAZ-EPA.
Among the sulphonamides and sulfamate used to determine the EcoCAγ inhibition profile, only one inhibitor resulted in a KI lower than 100 nM. This is the case of the benzolamide (BZA) whit a KI = 94 nM. Generally, BZA is clinically used in the treatment of glaucoma. The two human isoforms, hCA I and hCA II, resulted very sensitive to such inhibitor with a KI of 15 and 9 nM, respectively. The Vibrio enzyme was inhibited with a KI = 78 nM. V. cholerae enzyme showed a large number of nanomolar inhibitors with a KI below 100 nM, such as compounds 2, 3, 4, 5, 6, 7, 8, 9, 11, 13, 14, 15, 24, EZA, DZA, BRZ, BZA, the sulfamate TMP, SLP, and IND. Except for BZA, these inhibitors inhibited EcoCAγ with KIs in the range 193–1013 nM for the series 1–24, and KIs of 387-5538 nM for the proposed licenced drugs. The analysis of the three-dimensional structures of EcoCAγ and VchCAγ (not available at this moment) will allow the identification of the structural factors responsible for the KI variations, and thus the possibility to design efficient and selective inhibitors of the bacterial enzymes.
Most of the inhibitors considered in the present study were moderate inhibitors of EcoCAγ with KIs in the range 160–944 nM, such as compounds from 1 to 9, 13, 14, 20, 21, AZA, MZA, DCP, TMP, ZNS, SLP, IND, VLX, CLX, SLT, FAM, and EPA. It is important to note that some of these inhibitors were very sensitive versus the human isoform h CAII and harmful inhibitors for the human isoform h CA I (KI = 6.6 – 78.5 µM). The zonisamide (ZNS) an aliphatic primary sulphonamide, was also a very weak inhibitor for the bacterial enzymes (KIs= 725 nM) but effective towards the human isoenzymes (KIs= 35–56 nM). The KI differences reported in the evidenced that the inhibition pattern is almost different also between the human isoenzymes corroborating the idea that the comparison of the inhibition profile represents a potent tool for developing new specific inhibitors. For example, it is possible to tune and/or design specific inhibitors for the isoforms based on their structural differences, even if the isoenzymes show a high percentage of amino acid sequence identity.
Several substituted benzene-sulphonamides, such as 10, 11, 12, 15, 16, 17, 18, 19, 22, 23, 24, EZA, DZA, and BRZ, were ineffective week inhibitors of the EcoCAγ, showing KIs in the range of 1.0–5.5 µM. Moreover, most of these inhibitors, such as the compounds 11, 24, EZA, DZA, and BRZ, inhibited the Vibrio enzyme (VchCAγ) with KIs =73–93 nM.
The behaviour of EcoCAγ is somewhat challenging to explain observing the different inhibition profiles and comparing them to the orthologous VchCAγ and the two human isoforms (hCA I and hCA II). EcoCAγ seems less or not inhibited by most of the substituted benzene-sulphonamides and clinically licenced drugs. However, considering that also the two human isoforms showed a sulphonamide inhibition pattern different from each other, as well as from the bacterial enzymes, it is reasonable to support the thesis concerning the synthesis of new drugs capable of interfering selectively with EcoCAγ or VchCAγ activity, avoiding the inhibition of the human CAs (α-class enzymes).
4. Conclusions
Escherichia coli is an opportunistic pathogen typically colonising the lower intestine of warm-blooded organismsCitation51–54. In the present manuscript, we focussed our interest on the EcoCAγ (γ-CA) encoded by the E. coli genome since bacterial CAs are considered a valuable target for impairing the microbe vitality or the bacterial virulence. EcoCAγ was cloned, expressed, purified, and investigated for its kinetic properties, the last not explored previously even if the three-dimensional structure was solved in 2012 (55). EcoCAγ resulted in an excellent catalyst for the physiological CO2 hydratase reaction to bicarbonate and protons, with a kcat of 5.7 × 105 s−1 and catalytic efficiency (kcat/KM) of 6.9 × 106 M−1 s−1. A broad range of substituted benzene-sulphonamides and clinically licenced drugs were used to determine the inhibition profile of EcoCAγ, the ortholog enzyme VchCAγ, and the possible off-targets hCA I and hCA II. Among the sulphonamides and the one sulfamate used as inhibitors, only one of them resulted in a KI lower than 100 nM. This is the case of the benzolamide (BZA) whit a KI= 94 nM. Generally, BZA is clinically used in the treatment of glaucoma. All the other inhibitors had KIs > 100 nM. Surprisingly, for most of the inhibitors used, EcoCAγ showed 0.5 <Kis< 0.5 µM, evidencing that enzyme form E. coli was less or not inhibited by most of the substituted benzene-sulphonamides and clinically licenced drugs. As a consequence, the difference in sulphonamide inhibition pattern of the two human isoforms, as well as the two orthologs bacterial enzyme fortifies the thesis that the synthesis of new drugs is capable of interfering selectively with EcoCAγ or VchCAγ activity, avoiding the inhibition of the human CAs (α-class enzymes).
Acknowledgements
We are grateful to Giovanni Del Monaco for technical assistance.
Disclosure statement
The authors state no conflict of interests.
References
- Wang QK. [Responses of forest soil carbon pool and carbon cycle to the changes of carbon input]. Ying Yong Sheng Tai Xue Bao 2011;22:1075–81.
- Sellers PJ, Schimel DS, Moore B, 3rd, et al. Observing carbon cycle-climate feedbacks from space. Proc Natl Acad Sci USA 2018;115:7860–8.
- Lettinga G. Digestion and degradation, air for life. Water Sci Technol 2001;44:157–76.
- Offre P, Spang A, Schleper C. Archaea in biogeochemical cycles. Annu Rev Microbiol 2013;67:437–57.
- Piao S, Wang X, Wang K, et al. Interannual variation of terrestrial carbon cycle: issues and perspectives. Glob Chang Biol 2020;26:300–18.
- Atkinson N, Feike D, Mackinder LC, et al. Introducing an algal carbon-concentrating mechanism into higher plants: location and incorporation of key components. Plant Biotechnol J 2016;14:1302–15.
- Supuran CT, Capasso C. An overview of the bacterial carbonic anhydrases. Metabolites 2017;7:56.
- Capasso C, Supuran CT. An overview of the alpha-, beta- and gamma-carbonic anhydrases from bacteria: can bacterial carbonic anhydrases shed new light on evolution of bacteria? J Enzyme Inhib Med Chem 2015;30:325–32.
- Del Prete S, Nocentini A, Supuran CT, Capasso C. Bacterial ι-carbonic anhydrase: a new active class of carbonic anhydrase identified in the genome of the Gram-negative bacterium Burkholderia territorii. J Enzyme Inhib Med Chem 2020;35:1060–8.
- Del Prete S, De Luca V, De Simone G, et al. Cloning, expression and purification of the complete domain of the η-carbonic anhydrase from Plasmodium falciparum. J Enzyme Inhib Med Chem 2016;31:54–9.
- Del Prete S, Vullo D, De Luca V, et al. Cloning, expression, purification and sulfonamide inhibition profile of the complete domain of the η-carbonic anhydrase from Plasmodium falciparum. Bioorg Med Chem Lett 2016;26:4184–90.
- Del Prete S, Vullo D, De Luca V, et al. Anion inhibition profiles of the complete domain of the η-carbonic anhydrase from Plasmodium falciparum. Bioorg Med Chem 2016;24:4410–4.
- Annunziato G, Angeli A, D'Alba F, et al. Discovery of new potential anti-infective compounds based on carbonic anhydrase inhibitors by rational target-focused repurposing approaches. ChemMedChem 2016;11:1904–14.
- Del Prete S, Vullo D, De Luca V, et al. Anion inhibition profiles of α-, β- and γ-carbonic anhydrases from the pathogenic bacterium Vibrio cholerae. Bioorg Med Chem 2016;24:3413–7.
- Abdel Gawad NM, Amin NH, Elsaadi MT, et al. Synthesis of 4-(thiazol-2-ylamino)-benzenesulfonamides with carbonic anhydrase I, II and IX inhibitory activity and cytotoxic effects against breast cancer cell lines. Bioorg Med Chem 2016;24:3043–51.
- Capasso C, Supuran CT. An overview of the carbonic anhydrases from two pathogens of the oral cavity: Streptococcus mutans and Porphyromonas gingivalis. Curr Top Med Chem 2016;16:2359–68.
- Del Prete S, Vullo D, De Luca V, et al. Comparison of the sulfonamide inhibition profiles of the α-, β- and γ-carbonic anhydrases from the pathogenic bacterium Vibrio cholerae. Bioorg Med Chem Lett 2016;26:1941–6.
- Supuran CT. Advances in structure-based drug discovery of carbonic anhydrase inhibitors. Expert Opin Drug Discov 2017;12:61–88.
- Supuran CT. Structure and function of carbonic anhydrases. Biochem J 2016;473:2023–32.
- Nishimori I, Onishi S, Takeuchi H, Supuran CT. The alpha and beta classes carbonic anhydrases from Helicobacter pylori as novel drug targets. Curr Pharm Des 2008;14:622–30.
- Morishita S, Nishimori I, Minakuchi T, et al. Cloning, polymorphism, and inhibition of beta-carbonic anhydrase of Helicobacter pylori. J Gastroenterol 2008;43:849–57.
- Supuran CT. How many carbonic anhydrase inhibition mechanisms exist? J Enzyme Inhib Med Chem 2016;31:345–60.
- De Simone G, Monti SM, Alterio V, et al. Crystal structure of the most catalytically effective carbonic anhydrase enzyme known, SazCA from the thermophilic bacterium Sulfurihydrogenibium azorense. Bioorg Med Chem Lett 2015;25:2002–6.
- Supuran CT. Carbonic anhydrase inhibition and the management of neuropathic pain. Expert Rev Neurother 2016;16:961–8.
- Ozensoy Guler O, Supuran CT, Capasso C. Carbonic anhydrase IX as a novel candidate in liquid biopsy. J Enzyme Inhib Med Chem 2020;35:255–60.
- Blandina P, Provensi G, Passsani MB, et al. Carbonic anhydrase modulation of emotional memory. Implications for the treatment of cognitive disorders. J Enzyme Inhib Med Chem 2020;35:1206–14.
- Supuran CT, Capasso C. Biomedical applications of prokaryotic carbonic anhydrases. Expert Opin Ther Pat 2018;28:745–54.
- Supuran CT, Capasso C. Carbonic anhydrase from porphyromonas gingivalis as a drug target. Pathogens 2017;6:30.
- Capasso C, Supuran CT. Bacterial, fungal and protozoan carbonic anhydrases as drug targets. Expert Opin Ther Targets 2015;19:1689–704.
- Allocati N, Masulli M, Alexeyev MF, Di Ilio C. Escherichia coli in Europe: an overview. Int J Environ Res Public Health 2013;10:6235–54.
- Conway T, Cohen PS. Commensal and pathogenic Escherichia coli metabolism in the gut. Microbiol Spectr 2015;3: 23.
- Robins-Browne RM, Holt KE, Ingle DJ, et al. Are Escherichia coli pathotypes still relevant in the era of whole-genome sequencing? Front Cell Infect Microbiol 2016;6:141
- Guilloton MB, Lamblin AF, Kozliak EI, et al. A physiological role for cyanate-induced carbonic anhydrase in Escherichia coli. J Bacteriol 1993;175:1443–51.
- Kozliak EI, Guilloton MB, Gerami-Nejad M, et al. Expression of proteins encoded by the Escherichia coli cyn operon: carbon dioxide-enhanced degradation of carbonic anhydrase. J Bacteriol 1994;176:5711–7.
- Guilloton MB, Korte JJ, Lamblin AF, et al. Carbonic anhydrase in Escherichia coli. A product of the cyn operon. J Biol Chem 1992;267:3731–4.
- Kusian B, Sultemeyer D, Bowien B. Carbonic anhydrase is essential for growth of Ralstonia eutropha at ambient CO(2) concentrations. J Bacteriol 2002;184:5018–26.
- Cronk JD, Endrizzi JA, Cronk MR, et al. Crystal structure of E. coli beta-carbonic anhydrase, an enzyme with an unusual pH-dependent activity. Protein Sci 2001;10:911–22.
- Merlin C, Masters M, McAteer S, Coulson A. Why is carbonic anhydrase essential to Escherichia coli? J Bacteriol 2003;185:6415–24.
- Ueda K, Nishida H, Beppu T. Dispensabilities of carbonic anhydrase in proteobacteria. Int J Evol Biol 2012;2012:324549
- Modak JK, Tikhomirova A, Gorrell RJ, et al. Anti-Helicobacter pylori activity of ethoxzolamide. J Enzyme Inhib Med Chem 2019;34:1660–7.
- Ronci M, Del Prete S, Puca V, et al. Identification and characterization of the α-CA in the outer membrane vesicles produced by Helicobacter pylori. J Enzyme Inhib Med Chem 2019;34:189–95.
- Buzas GM. [Helicobacter pylori – 2010]. Orv Hetil 2010;151:2003–10.
- Abuaita BH, Withey JH. Bicarbonate induces Vibrio cholerae virulence gene expression by enhancing ToxT activity. Infect Immun 2009;77:4111–20.
- Kohler S, Ouahrani-Bettache S, Winum JY. Brucella suis carbonic anhydrases and their inhibitors: towards alternative antibiotics? J Enzyme Inhib Med Chem 2017;32:683–7.
- Singh S, Supuran CT. 3D-QSAR CoMFA studies on sulfonamide inhibitors of the Rv3588c β-carbonic anhydrase from Mycobacterium tuberculosis and design of not yet synthesized new molecules. J Enzyme Inhib Med Chem 2014;29:449–55.
- Ceruso M, Vullo D, Scozzafava A, Supuran CT. Sulfonamides incorporating fluorine and 1,3,5-triazine moieties are effective inhibitors of three β-class carbonic anhydrases from Mycobacterium tuberculosis. J Enzyme Inhib Med Chem 2014;29:686–9.
- Carta F, Maresca A, Covarrubias AS, et al. Carbonic anhydrase inhibitors. Characterization and inhibition studies of the most active beta-carbonic anhydrase from Mycobacterium tuberculosis, Rv3588c. Bioorg Med Chem Lett 2009;19:6649–54.
- Rollenhagen C, Bumann D. Salmonella enterica highly expressed genes are disease specific. Infect Immun 2006;74:1649–60.
- Lotlikar SR, Kayastha BB, Vullo D, Khanam , et al. Pseudomonas aeruginosa β-carbonic anhydrase, psCA1, is required for calcium deposition and contributes to virulence. Cell Calcium 2019;84:102080.
- Del Prete S, De Luca V, Nocentini A, et al. Anion inhibition studies of the beta-carbonic anhydrase from Escherichia coli. Molecules 2020;25:2564–77.
- Pitout JD. Extraintestinal pathogenic Escherichia coli: a combination of virulence with antibiotic resistance. Front Microbiol 2012;3:9
- Nataro JP, Kaper JB. Diarrheagenic Escherichia coli. Clin Microbiol Rev 1998;11:142–201.
- Moriel DG, Bertoldi I, Spagnuolo A, et al. Identification of protective and broadly conserved vaccine antigens from the genome of extraintestinal pathogenic Escherichia coli. Proc. Natl. Acad. Sci. U.S.A 2010;107:9072–7.
- Agus A, Massier S, Darfeuille-Michaud A, et al. Understanding host-adherent-invasive Escherichia coli interaction in Crohn's disease: opening up new therapeutic strategies. Biomed Res Int 2014;2014:567929.
- Park HM, Park JH, Choi JW, et al. Structures of the γ-class carbonic anhydrase homologue YrdA suggest a possible allosteric switch. Acta Crystallogr D Biol Crystallogr 2012;68:920–6.
- Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–81.
- Nguyen K, Ahlawat R, Famotidine. Treasure Island (FL): StatPearls; 2020.
- Komiya T, Huang CH. Updates in the clinical development of epacadostat and other Indoleamine 2,3-Dioxygenase 1 Inhibitors (IDO1) for human cancers. Front Oncol 2018;8:423
- Del Prete S, Vullo D, Ghobril C, et al. Cloning, purification, and characterization of a beta-carbonic anhydrase from Malassezia restricta, an opportunistic pathogen involved in dandruff and seborrheic dermatitis. Int J Mol Sci 2019;20:2447–58.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–54.
- Capasso C, De Luca V, Carginale V, et al. Biochemical properties of a novel and highly thermostable bacterial α-carbonic anhydrase from Sulfurihydrogenibium yellowstonense YO3AOP1. J Enzyme Inhib Med Chem 2012;27:892–7.
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970;227:680–5.
- De Luca V, Del Prete S, Supuran CT, Capasso C. Protonography, a new technique for the analysis of carbonic anhydrase activity. J Enzyme Inhib Med Chem 2015;30:277–82.
- Del Prete S, De Luca V, Iandolo E, et al. Protonography, a powerful tool for analyzing the activity and the oligomeric state of the γ-carbonic anhydrase identified in the genome of Porphyromonas gingivalis. Bioorg Med Chem 2015;23:3747–50.
- Del Prete S, De Luca V, Supuran CT, Capasso C. Protonography, a technique applicable for the analysis of η-carbonic anhydrase activity. J Enzyme Inhib Med Chem 2015;30:920–4.
- Del Prete S, Vullo D, Caminiti-Segonds N, et al. Protonography and anion inhibition profile of the α-carbonic anhydrase (CruCA4) identified in the Mediterranean red coral Corallium rubrum. Bioorg Chem 2018;76:281–7.
- Khalifah RG. The carbon dioxide hydration activity of carbonic anhydrase. I. Stop-flow kinetic studies on the native human isoenzymes B and C. J Biol Chem 1971;246:2561–73.
- De Luca V, Vullo D, Del Prete S, et al. Cloning, characterization and anion inhibition studies of a γ-carbonic anhydrase from the Antarctic bacterium Colwellia psychrerythraea. Bioorg Med Chem 2016;24:835–40.
- Bossy R, Golik W, Ratkovic Z, et al. Overview of the gene regulation network and the bacteria biotope tasks in BioNLP'13 shared task. BMC Bioinformatics 2015;16:S1.
- Fu X, Yu LJ, Mao-Teng L, et al. Evolution of structure in gamma-class carbonic anhydrase and structurally related proteins. Mol Phylogenet Evol 2008;47:211–20.
- Angeli A, Ferraroni M, Supuran CT. Famotidine, an antiulcer agent, strongly inhibits Helicobacter pylori and human carbonic anhydrases. ACS Med Chem Lett 2018;9:1035–8.
