Abstract
The symptoms of Alzheimer’s disease (AD) do not include only memory loss and cognitive decline but also neuropsychiatric manifestation. These AD-related symptoms are usually treated with the aid of antipsychotics; however, their effects on cognition and safety remain unexplored. The present study determines the effects of quetiapine, an atypical antipsychotic, and two imidazo[1,2-a]pyrimidine-based inhibitors of PDE10A on the activity of human cholinesterases. Quetiapine moderately inhibited BuChE (IC50 = 6.08 ± 1.64 µmol/L) but improved the anti-BuChE properties of donepezil by decreasing its IC50 value. Both PDE10A inhibitors were found to possess moderate anti-AChE properties. The combined mixtures of donepezil and imidazo[1,2-a]pyrimidine analogues produce a synergistic anti-BuChE effect which was greater than either compound alone, improving the IC50 value by approximately six times. These favourable interactions between quetiapine, PDE10A inhibitors and clinically approved donepezil, resulting in improved anti-BuChE activity, can lead to a wider variety of potent AD treatment options.
Introduction
Alzheimer’s disease (AD) is an irreversible progressive neurological disorder. It is the most common cause for dementia and imposes immense suffering on patients and their families. AD is characterised by memory loss, the retardation of thinking and reasoning, and changes in personality and behaviours. It is estimated that there are currently about 46.8 million people suffer from AD worldwide. Furthermore, the number of patients is increasing, doubling every 20 years and is predicted to reach approximately 131.5 million in 2050Citation1,Citation2.
A comprehensive review of literature and clinical trials indicates that many hypothesis have been proposed regarding AD development: the cholinergic hypothesis, amyloid hypothesis, tau propagation hypothesis, oxidative stress and mitochondrial cascade hypothesis, calcium homeostasis hypothesis, neurovascular hypothesis, inflammatory hypothesis, metal ion hypothesis, and the lymphatic system hypothesis. It is well established that AD is a complex disease involving many factors, although its ultimate aetiology remains obscure. Although many attempts have been made to develop anti-AD drugs based on these hypotheses, only five drugs have been approved by the FDA to treat AD. Nowadays, the drugs used for AD treatments can be classified as either acetylcholinesterase inhibitors (AChEIs) or N-methyl-d-aspartic acid antagonists. Hence, there is a pressing need to develop more effective drugs for AD treatmentCitation1–3.
The process of developing new applications for a drug beyond its original use or commercially approved indicationCitation3, known as drug repurposing or reprofiling, is a relatively new idea. It is believed that such drug repurposing offers greater benefits over de novo drug discovery, i.e. the method of drug discovery by searching for a new active substanceCitation4,Citation5. Moreover, it has gained increasing interest in recent years, mainly due to the fact that pharmaceutical companies seek competitive alternatives to compensate for the high costs and low success rate associated with the drug discovery processCitation4. Repurposing also allows faster identification of new therapies for diseases, particularly in those cases where preclinical safety studies have already been accomplishedCitation3. Examples of successful repurposing can be found in existing literature. For instance, Baker et al.3 mention that bupropion, a drug originally used for depression, was repurposed for smoking cessation, while thalidomide, administered for treatment for morning sickness, is now used for multiple myelomaCitation3.
Drugs have also been repurposed to treat neurodevelopmental and neurodegenerative disordersCitation6. For instance, an extensive review by Bourque et al.Citation7 examines the repurposing of sec steroids for the treatment of Parkinson’s disease (PD)Citation7. In addition, fenfluramine, an appetite suppressant, can produce a durable reduction in seizures among patients with Dravet Syndrome, a rare genetic form of epilepsyCitation6. Examples of repurposing of psychiatric drugs as anticancer agents are also givenCitation8.
Drug repurposing offers an opportunity to reinvigorate the drug discovery process for the treatment of ADCitation9–13. Drug repurposing might become a promising alternative for AD treatment, since in the last few years, fewer than 25 potential drugs for AD have entered phase II and III clinical trials, compared to over 1700 anti-cancer moleculesCitation10.
Kumar et al.Citation12 adopted a computational method based on ligand–protein interaction to explore potential antipsychotic drugs for the treatment of AD. The authors found that some antipsychotic drugs might exhibit encouraging potential against multiple targets associated with ADCitation12.
Quetiapine is a psychotropic agent belonging to the group of dibenzothiazepine derivativesCitation14. Quetiapine acts as an antagonist at multiple neurotransmitter receptors in the brain: serotonin 5HT1A and 5HT2, dopamine D1 and D2, histamine H1, and adrenergic α1 and α2 receptorsCitation14. The drug effectively alleviates positive and negative symptoms, as well as cognitive impairment in schizophrenia patientsCitation14. In addition to schizophrenia, quetiapine has been approved for the treatment of bipolar disorder and as add-on treatment of major depressive disorderCitation15. The FDA extended the use of quetiapine to include generalised anxiety disorder, major depressive disorder, obsessive compulsive disorder, psychosis in PD, and treatment of behavioural and psychological symptoms in dementia, such as agitation, aggression, depression, and psychosesCitation16. As presented by Schneider et al.Citation17, quetiapine at a dose of 100 mg per day is effective for the treatment of psychotic symptoms and hostility in subjects with ADCitation17,Citation18. Takahashi et al.Citation19 claim that quetiapine may be effective in treating psychotic symptoms and disruptive behaviour in some patients with dementia with Lewy bodies. There are also examples of in vivo animal studies evaluating the effects of quetiapine on various pathological hallmarks of ADCitation19. For instance, He et al.Citation15 report that quetiapine can alleviate cognitive impairment and pathological changes in an amyloid precursor protein/presenilin double transgenic mouse model of AD15. Furthermore, it has been claimed that quetiapine may improve cognitive symptoms of schizophrenia by stimulating brain-derived neurotrophic factor (BDNF) mRNA expressionCitation20. Hence, it is possible that quetiapine may serve as effective therapy in AD patients.
Therefore, the purpose of this in vitro study was to explore the effects of quetiapine and two novel antipsychotic compounds, currently undergoing clinical trials (), on the activity of human acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE), and to establish the type of inhibition. CPL500036-01 and its dihydrochloride salt (CPL500036-02) with imidazo[1,2-a]pyrimidine scaffold are new classes of PDE10A inhibitors characterised by high activity, good metabolic stability and satisfactory PK properties. In recent years, numerous studies of novel PDE10A inhibitors as potential antipsychotic agents have been publishedCitation21.
Figure 1. The chemical structure of quetiapine and PDE10A inhibitors, CPL500036-01 and CPL500036-02.
The study also assesses the potential synergism of quetiapine, CPL500036-01, CPL500036-02, and donepezil towards both cholinesterases (ChEs). The findings provide a greater insight into the potential application of quetiapine as an effective adjuvant to clinically approved AChEIs.
Materials and methods
Materials
Quetiapine, CPL500036-01 and CPL500036-02 (which synthesis, affinity for PDE10A enzyme and metabolic stability were previously describedCitation21; ), were obtained from CelonPharma. Assuming the therapeutic plasma concentrations of quetiapine to be 4−400 ng/mL, i.e. 8−800 nmol/L (0.008−0.8 µmol/L), the compounds were examined within the concentration range 0.01−100 µmol/L.
The following reagents were used: 0.9% NaCl (0.15 mol/L; Chempur, Poland); 0.1 mol/L phosphate buffer pH = 7.0 and pH = 8.0 (disodium phosphate, monosodium phosphate [Baker, Poland]); a stock solution of 5,5′-dithiobisnitrobenzoic acid (DTNB; 0.01 mol/L [Sigma Aldrich, St. Louis, MO, USA]) prepared in phosphate buffer at pH = 7.0; a stock aqueous solution of acetyltiocholine iodide (ATC; 21.67 mg/mL; Sigma Aldrich); a stock aqueous solution of butyryltiocholine iodide (BTC; 20.50 mg/mL; Sigma Aldrich). The stock solutions of DTNB, ATC, and BTC were stored in low-volume aliquots at a temperature of −30 °C. Before each experiment the solutions were restored at 37 °C for 15 min. For the establishment of kinetic parameters and type of inhibition, decreasing concentrations of both substrates (ATC and BTC) were used (1:2 to 1:20).
Biological material
The studies using biological material were approved by the Bioethics Committee of the Medical University of Lodz (RNN/109/16/KE – for compounds CPL500036-01 and CPL500036-02 analysis and RNN/278/19/KE – for quetiapine). Blood samples were obtained from healthy donors of Blood Donation Centre in Lodz and The Voievodal Specialised Hospital in Lodz. The preparation of erythrocytes for AChE activity measurements and plasma for the assessment of BuChE activity has been described previouslyCitation22.
Inhibition of ChE
The effects of quetiapine, CPL500036-01 and CPL500036-02 on the activity of both ChEs were determined according to Ellman with modificationsCitation22,Citation23. Briefly, the experiments were conducted on a CE 2021 spectrophotometer (CECIL Cambridge, UK) with circulating thermostated water (37 °C) and a Model 300 Electronic Stirrer (Rank Brothers Ltd, England). The biological sample, consisting of a 400-fold diluted solution of haemolysed erythrocytes or 200-fold diluted plasma was incubated for 15 min (37 °C) with DTNB; the tested compound was then added in a volume of 10 µL. The enzymatic reaction was initiated by addition of a reaction substrate (ATC or BTC). The absorbance was continuously recorded at λ = 436 nm for three minutes, and the maximal velocity of the reaction was counted on the basis of changes in absorbance over time.
The method was validated, eight control tests were conducted both for AChE and BuChE. The coefficients of variability were counted (CVAChE = 7.6%, CVBuChE = 8.9%).
Kinetic parameters of enzymatic reactions
To determine the type of BuChE inhibition by quetiapine, the experiments using decreasing concentrations (2-, 3-, 5-, 10-, 20-fold) of substrate (BTC) were conducted. The inhibitor was used at the IC50 value (6 µmol/L). A three-minute absorbance recording was carried out at λ = 436 nm using a CECIL 2021 spectrophotometer (CECIL Cambridge, UK) with a thermostatic water flow (temperature 37 °C).
Due to the high level of variation in the individual concentration and activity of BuChE in human plasma, 32 biological samples were studied. The following kinetic parameters were calculated for BuChE (mean ± SD; n = 32): Km = 69.57 ± 21.34 µmol/L, Vmax = 0.235 ± 0.027 A/min.
ChE inhibition by binary mixtures of donepezil and tested compounds
The potential synergism between donepezil and quetiapine, CPL500036-01 or CPL500036-02 was estimated according to Ellman, with modificationsCitation22. Briefly, adequately diluted haemolysed erythrocytes or plasma (470 µL) were preincubated with donepezil (10 µL) and quetiapine or tested compounds (10 µL) for 15 min, before substrate addition (ATC or BTC at a final concentration of 0.75 µmol/mL). Donepezil was used at a concentration between 0.01 and 100 nmol/L for AChE inhibition, and 0.2 to 100 µmol/L for BuChE activity assessment. Quetiapine was administered at 200 ng/mL (400 nmol/L) while CPL500036-01 and CPL500036-02 were used at 25 µmol/L. The concentration of quetiapine was chosen on the basis of the mean concentration in plasma previously detected using HPLCCitation24.
Data analysis
All the experiments conducted within this article were carried out in duplicate or triplicate using at least three different biological samples. The values presented in tables and figures are expressed as mean ± standard deviation (SD).
The effects of quetiapine and compounds on ChE activity are presented as percentage of inhibition in comparison to controls, assumed to represent 100% of enzyme activity. The IC50 value, i.e. the concentration of a drug that inhibits 50% of the activity of an enzyme, was calculated using linear or logarithmic regression.
The kinetic parameters of enzymatic reactions were estimated using linear regression (Hanes-Woolf plots). The following parameters were calculated: the maximal velocity (Vmax) and the Michaelis constant (Km).
The effects of binary drug mixtures on the activity of ChEs were examined according to the median-effect principle described by ChouCitation25 a method based on plotting dose-effect curves for every single drug and their binary mixtures in different doses. The potential synergism and antagonism between the drugs at all doses were thus analysed using commercially available ComboSyn software (http://www.combosyn.com/). The results of the analysis were obtained using a combination index (CI) plot. A CI value below 1 indicates synergism, while CI above 1 indicates antagonism between the examined drugsCitation26.
Results
General ChE activity
The main objective of this study was to determine the effects of quetiapine and two novel compounds (CPL500036-01 and CPL500036-02) on the activity of human ChEs. Our findings () indicate that quetiapine administered within the concentration range 0.01−100 µmol/L inhibited AChE inhibition by up to 8.82 ± 1.68%. In addition, the maximal inhibition towards AChE inhibition was 22.32 ± 1.46% for compound 1 and 34.87 ± 1.65% for compound 2 ().
Figure 2. The effects of quetiapine (A) and CPL500036-01 (B) and CPL500036-02 (C) on AChE activity expressed as the percentage of enzyme inhibition in comparison to control (100% of activity). Each data point represents mean ± SD for at least three independent experiments conducted in duplicates.
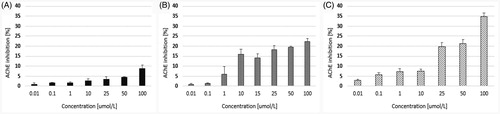
Figure 3. The effects of quetiapine (A) and CPL500036-01 (B) and CPL500036-02 (C) on BuChE activity expressed as the percentage of enzyme inhibition in comparison to control (100% of activity). Each data point represents mean ± SD for at least three independent experiments conducted in duplicates. Subsequent calculations using logarithmic equations from each conducted experiment allowed to determine the IC50 value for quetiapine.
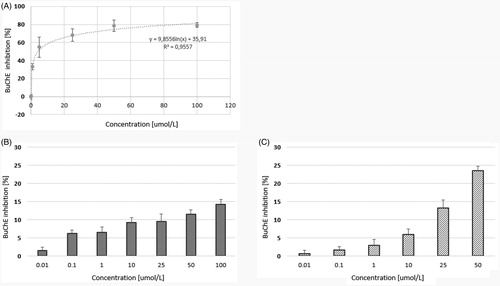
Of the tested compounds, quetiapine presented the highest level of BuChE inhibition (IC50 = 6.08 ± 1.64 µmol/L). The two other compounds moderately inhibited BuChE activity within the tested concentration range (); however, the IC50 values could not be calculated. Compared to donepezil, a commercially approved drug for the treatment of AD quetiapine demonstrated approximately half the IC50 value against BuChECitation22, and compounds CPL500036-01 and CPL500036-02 were also significantly lower ().
Table 1. Effects of quetiapine and donepezil on the human erythrocyte acetylcholinesterase (AChE) and plasma butyrylcholinesterase (BuChE) activity.
Kinetic parameters of BuChE enzymatic reactions
The kinetic parameters of BuChE reactions were obtained in a series of experiments using decreasing concentrations of BTC. The curves were analysed using the Hanes-Woolf equation, in which the ratio of the initial substrate concentration [S] to the reaction velocity v is plotted against [S].
The type of inhibition was determined on the basis of Km and Vmax values of the results obtained for pure enzyme and quetiapine at IC50 concentration (, ). According to the data presented in , quetiapine exhibited mixed inhibition, as Vmax(i) (Vmax of the reactions with inhibitor) significantly decreased in comparison with Vmax while Km(i) (Km of the reaction with inhibitor) increased.
Figure 4. Determination of kinetic parameters of BuChE enzymatic reactions. Hanes-Woolf plots we used to calculate the Michaelis constant (Km) and maximal velocity (Vmax). Quetiapine was used at the concentration of 12.8 µmol/L. Presented data constitute the results of one exemplary experiment conducted in duplicates. The results of kinetic studies conducted in three independent experiments and calculated kinetic parameters are enclosed in .
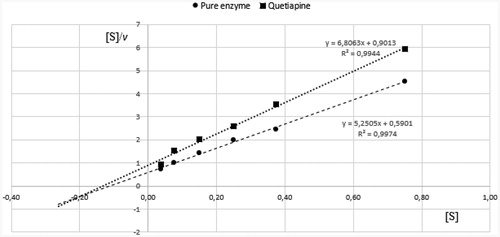
Table 2. Kinetic parameters of BuChE enzymatic reaction.
Inhibition of ChEs by donepezil and tested mixtures
In order to investigate the potential synergy between donepezil and quetiapine and the other two new compounds, several tests were performed using binary mixtures. The results of these experiments are presented in . Regarding BuChE activity, the greatest effect was demonstrated by the mixture of donepezil and compound CPL500036-01: IC50 was 2.13 ± 1.45 µmol/L, this value being about 83% lower than that of pure donepezil. In the case of 400 nmol/L quetiapine and 25 µmol/L compound CPL500036-02, the IC50 values decreased by 1.3- and 3.4-fold. Regarding AChE activity, similar IC50 values were obtained for a mixture of donepezil and quetiapine compared to pure donepezil. The mixture of donepezil with compound CPL500036-02 showed an IC50 of 21.72 ± 0.48 nmol/L, which was 15.09% lower than the IC50 of donepezil alone.
Table 3. Effects of the mixture of donepezil and quetiapine, or two new compounds on the human erythrocyte acetylcholinesterase (AChE) and plasma butyrylcholinesterase (BuChE) activity.
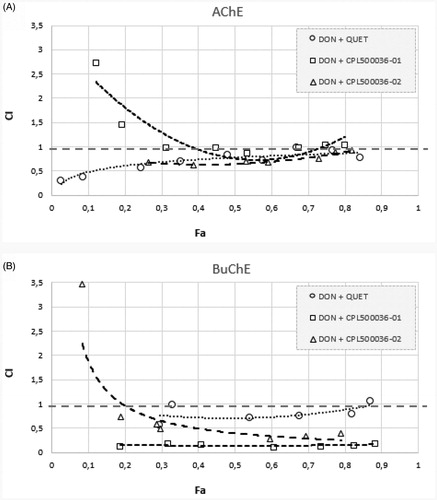
The above results were confirmed using the median-effect principle (). shows the anti-AChE activity of binary mixtures for which the CI value is in the range of 0.5–1, indicating synergy. The donepezil and CPL500036-01 mixture demonstrated CI values higher than 1 for the low Fa points, suggesting antagonistic dependency. Regarding the effects on BuChE inhibition (), all obtained Fa-CI curves are below 1, except for the donepezil and CPL500036-02 mixture at the smallest Fa, and the donepezil and quetiapine mixture at the highest Fa. The strongest anti-BuChE effect was demonstrated by donepezil and CPL500036-02 as shown by the position of the curve in the graph, i.e. close to CI = 0.
Figure 5. Analysis of potential synergism between donepezil and quetiapine, CPL500036-01, and CPL500036-02 by the median effect principle. Data from the AChE (A) and BuChE (B) inhibitory activities assay were analysed by means of the Chou–Talalay method. The results are depicted using Fa-CI plots, where Fa is Fraction affected, and CI is combination index.
Discussion
One of the most important challenges faced by contemporary science is the alleviation of neuropsychiatric symptoms associated with AD. As reviewed by Silva et al.Citation27, some non-pharmacological strategies, such as music, aromatherapy, and pet therapy, have been applied to alleviate neuropsychiatric symptoms; however, their efficacy is disputableCitation27. Regarding pharmacological interventions, a number of drug classes can be used: benzodiazepines, anticholinesterases, antidepressants such as selective serotonin re-uptake inhibitors (SSRI), mood stabilisers, anticonvulsants and antipsychoticsCitation28. For instance, SSRIs were found to be effective in the control of anxiety and depression; however, no significant difference was found between the efficacy of citalopram and risperidone for the treatment of either agitation or psychotic symptoms in subjects suffering from dementia. The authors conclude that these results need to be further confirmed before citalopram or other SSRI can be recommended as an option to antipsychotics for the treatment of agitation or psychotic symptoms associated with dementiaCitation27,Citation28. In turn, Cummings et al.Citation29 claim that results of numerous clinical trials of cholinesterase inhibitors and memantine confirm that the treatment offers behavioural benefitsCitation29.
The treatment of agitation and aggressiveness during the course of AD is usually carried out with the aid of antipsychoticsCitation30,Citation31. According to McGrathCitation31, up to 45% of patients in residential or nursing homes are treated with antipsychotics. Currently, interest is growing in the atypical antipsychotics, with over 20 clinical trials testing their efficacyCitation30,Citation31. For instance, a review of several clinical trials suggests that risperidone and olanzapine are effective in reducing aggression, and that risperidone reduces psychosis, but their administration is associated with serious adverse cerebrovascular events and extra-pyramidal symptoms. Therefore, the authors conclude that neither risperidone nor olanzapine should be used for a long period of time to treat dementia patients with aggression or psychosisCitation29–33.
Although there is some clinical evidence that atypical antipsychotics exert moderate activity in the treatment of agitation and aggressiveness, a number of uncertainties still remain regarding their effects on cognition. An observational study of McShane et al.Citation32 showed a doubling in the rate of cognitive decline in patients with dementia taking typical antipsychotics. Ballard et al.Citation33 reported that administration of quetiapine does not result in significant improvement in agitation in patients with dementia compared with placebo and is associated with a greater decline in cognitive function. This has been attributed to the suppression of BDNF, accelerating the accumulation of the core pathological substrates of ADCitation33, or to its antimuscarinic activityCitation34,Citation35. In contrast, Fleming et al.Citation36 report that quetiapine offers potential cognitive benefits in people with schizophrenia. Lafuente and ThiamwongCitation37 indicate quetiapine to be well-tolerated in patients with AD, and that it decreases clinical signs and symptoms of agitation; in addition, the use of quetiapine appears to be associated with cognitive stabilityCitation37. De Deyn et al.Citation38 report that treatment with quetiapine leads to improvement in the symptoms of psychosis and agitation, without any significant change in cognitionCitation38. Similarly, Roca et al.Citation39 found that quetiapine administration in AD patients is not associated with any cognitive changesCitation39.
Since one of the most important pathophysiological feature of AD is a deficit in cholinergic transmission, which can potentially influence all aspects of cognition, processing of information and behaviour, the present study evaluates the effects of quetiapine on the activity of two main human ChEs: AChE and BuChE. Most of the medicines currently registered for AD treatment act as AChEIs: they increase acetylcholine (ACh) levels in the synaptic cleft and partially ameliorate cognitive symptoms, and enhance quality of life in patients with mild to severe ADCitation40.
Our present findings indicate that at 100 µmol/L, quetiapine exerts a maximal inhibitory effect of 8.82 ± 1.68% against AChE. This is the first report of the effects of quetiapine on the activity of human ChEs. This result is also of vital importance in view of the potential interactions between AChEIs and antipsychotic drugs. Since Mehrpouya et al.Citation41 claim that co-prescription of AChEIs and dopamine D2 receptor blockers may induce an ACh/dopamine imbalance in the striatum, leading to extrapyramidal syndrome and extrapyramidal adverse effects, the present study examines the potential synergy between donepezil and quetiapine with regard to AChE inhibition. The findings () indicate that quetiapine does not alter the AChE activity of donepezil, an approved drug for AD treatment. Therefore, we presume that the therapeutic potential of donepezil is not influenced by co-administration of quetiapine.
Another important target in AD therapy is BuChE. In contrast to AChE, which predominates in the healthy brain, BuChE is considered to play a minor role in controlling ACh levels. Notwithstanding, it has been found that BuChE level progressively increases in patients suffering from ADCitation42. Therefore, both enzymes constitute potential therapeutic targets to alleviate a lack of ACh, one of the pathological hallmarks of the cognitive decline observed in AD. Our present findings indicate that against BuChE quetiapine demonstrated an IC50 value of 6.08 ± 1.64 µmol/L, which was roughly half that of the reference drug donepezil. However, it has to be noted that donepezil is an AChE-selective agent with approximately 500-fold greater affinity for AChE than BuChE.
A key finding of this study is that quetiapine supplements the anti-BuChE properties of donepezil, resulting in a 1.3-fold lower IC50 value. It is therefore vital to identify the potential synergism between recommended AChE inhibitors, such as donepezil, with antipsychotics which could lead to a wider variety of more potent treatment options in future.
The study also examines two novel compounds which primarily act by the inhibition of phosphodiesterase PDE10A. PDEs participate in many signalling processes, as they hydrolyse two crucial signalling molecules: cAMP and cGMPCitation43. During the past 30 years, the issue of PDE distribution, substrate specificity, regulatory mechanisms and inhibition has constituted an active field of research. DeNinnoCitation43 describes the major advances in the field of PDEs which include identification of subtypes, and isoforms of PDEs, and understanding of their functionCitation43. Interest in PDEs has grown since the approval of moderately selective PDE5 inhibitors such as sildenafil, and tadalafilCitation43,Citation44. Numerous studies indicate that PDE inhibitors present a wide range of pharmacological activities, including anti-inflammatory, anti-oxidant, vasodilator, anti-cancer, and neuroprotective properties, suggesting that they can be used as potential drugs for the treatment of respiratory tract diseases, cardiovascular system diseases, depression, dementia, and PDCitation44–47.
There is also great interest in the development of PDE inhibitors as potential anti-AD agents, since it has been found that AD is associated with the increased (CNS) expression of numerous PDE mRNA speciesCitation46. In addition, inhibition of PDE5A decreases the level of phosphorylated Tau. Furthermore, inhibition of PDE10A was shown to have a positive regulatory effect on basal ganglia function, making it a potential target for the treatment of psychosis and schizophreniaCitation47. The development of multitarget-directed ligand targeting ChEs and PDE is a promising approach to countering the multifactorial characteristics of ADCitation46,Citation47.
Our present findings show that both novel PDE10A inhibitors are characterised by greater potential for AChE inhibition than the reference drug, quetiapine, since its maximal inhibition by compound 1 and 2 reached approximately 22.32%, and 34.87%, respectively. The opposite situation was observed in the case of BuChE. In this case, the most effective compound was quetiapine (IC50 = 6.08 ± 1.64 µmol/L).
Our most significant result regarding the novel tested compounds is that they improved the anti-BuChE properties of donepezil: the IC50 value for the mixture of donepezil and CPL500036-01 was approximately sixfold lower than for donepezil alone (). The combination of donepezil and CPL500036-01, a PDE10A inhibitor, yielded a greater anti-BuChE effect than that for either compound alone. The observed synergistic effects might provide an additional rationale for any clinical benefit for patients suffering simultaneously from psychotic diseases and AD.
Conclusions
It has been estimated that approximately 90% of demented patients, including those suffering from AD, develop neuropsychiatric symptoms such as delusion, aggressiveness and agitationCitation27. The treatment of AD-related neuropsychiatric symptoms has been an area of extensive research in recent years. Some authors indicate that atypical antipsychotics exert moderate activity in the treatment of agitation and aggressiveness associated with AD, but their effects on cognition and ChE activity, the characteristic features of AD, remain unexplored. Therefore, our study examines the effects of quetiapine, an atypical antipsychotic, and two novel inhibitors of PDE10ACitation21 on the activity of human ChEs.
Quetiapine was found to exert weak effects on AChE activity, but moderately inhibited BuChE activity (IC50 = 6.08 ± 1.64 µmol/L). Importantly, quetiapine improves anti-BuChE properties of donepezil, demonstrated by a 1.3-fold lower IC50 value. Although these findings suggest that possible beneficial interplay may exist between quetiapine and a clinically approved ChE inhibitor (donepezil), these preliminary results require further examination to rationalise the potential beneficial effects of combined therapy on the symptomatic treatment of AD with concomitant psychosis.
Both imidazo[1,2-a]pyrimidine analogues inhibiting PDE10A were found to possess greater anti-AChE properties than the reference drug, quetiapine; however, in contrast, their anti-BuChE effects were lower than those of quetiapine. The key finding of this article is the observation that both PDE10A inhibitors are capable of ameliorating the inhibitory properties of donepezil towards BuChE, which were manifested by ca. sixfold lower IC50 value.
Although anti-psychotics are still widely used for the treatment of the serious psychotic symptoms associated with AD, their use is associated with a number of uncertainties regarding their effects on cognition and possible adverse cerebrovascular events. Our findings reinforce the need to identify the potential synergism between recommended AChE inhibitors, such as donepezil, and antipsychotics; such knowledge could open the door to a wider variety of potent treatment options, or exclude adverse drug combinations resulting in impairments in ACh levels.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Liu P, Xie Y, Meng XY, Kang JS. History and progress of hypotheses and clinical trials for Alzheimer’s disease. Signal Transduct Target Ther 2019;4:29.
- Du X, Wang X, Geng M. Alzheimer’s disease hypothesis and related therapies. Transl Neurodegener 2018;7:2.
- Baker NC, Ekins S, Williams AJ, et al. A bibliometric review of drug repurposing. Drug Discov Today 2018;23:661–72.
- Langedijk J, Mantel-Teeuwisse AK, Slijkerman DS, et al. Drug repositioning and repurposing: terminology and definitions in literature. Drug Discov Today 2015;20:1027–34.
- Oprea TJ, Bauman JE, Bologa CG, et al. Drug repurposing from an academic perspective. Drug Discov Today Ther Strateg 2011;8:61–9.
- Tranfaglia MR, Thibodeaux C, Mason DJ, et al. Repurposing available drugs for neurodevelopmental disorders: the fragile X experience. Neuropharmacology 2019;147:74–86.
- Bourque M, Morissette M, Di Paolo T. Repurposing sex steroids and related drugs as potential treatment for Parkinson’s disease. Neuropharmacology 2019;147:37–54.
- Huang J, Zhao D, Liu Z, et al. Repurposing psychiatric drugs as anti-cancer agents. Cancer Lett 2018;419:257–65.
- Madhusoodanan S, Ting MB. Pharmacological management of behavioral symptoms associated with dementia. World J Psychiatry 2014;4:72–9.
- Cummings J, Lee G, Ritter A, Zhong K. Alzheimer’s disease drug development pipeline: 2018 Alzheimer’s Dement. Transl Res Clin Interv 2018;4:195–214.
- Socias SB, González-Lizárraga F, Avila CL, et al. Exploiting the therapeutic potential of ready-to-use drugs: repurposing antibiotics against amyloid aggregation in neurodegenerative diseases. Prog Neurobiol 2018;162:17–36.
- Kumar S, Chowdhury S, Kumar S. In silico repurposing of antipsychotic drugs for Alzheimer’s disease. BMC Neurosci 2017;18:16.
- Saavedra JM. Beneficial effects of Angiotensin II receptor blockers in brain disorders. Pharmacol Res 2017;125:91–103.
- Seroquel (Quetiapine fumarate) TABLETS, 2. pp. 1–31. Available from: https://www.rxlist.com/seroquel-drug.htm [last accessed 04 Jan 2020].
- He J, Luo H, Yan B, et al. Beneficial effects of quetiapine in a transgenic mouse model of Alzheimer’s disease. Neurobiol Aging 2009;30:1205–16.
- Gareri P, Gallelli L, Pirritano D, et al. Role of quetiapine in the treatment of Alzheimer’s disease. J Gerontol Geriatric Res 2015;4:197.
- Schneider L, Yeung L, Sweitzer P, et al. Effects of Seroquel (quetiapine) on reducing hostility and psychosis in patients with Alzheimer’s disease (Poster). Washington, DC: American Psychiatric Association; 1999.
- Sharma T. Quetiapine-efficacy in different domains. Eur Neuropsychopharmacol 2001;11:S385–S90.
- Takahashi H, Yoshida K, Sugita T, et al. Quetiapine treatment of psychotic symptoms and aggressive behavior in patients with dementia with Lewy bodies: a case series. Prog Neuro-Psychopharmacology Biol Psychiatry 2003;27:549–53.
- Park SW, Lee SK, Kim JM, et al. Effects of quetiapine on the brain-derived neurotrophic factor expression in the hippocampus and neocortex of rats. Neurosci Lett 2006;402:25–9.
- Moszczyński-Pętkowski R, Majer J, Borkowska M, et al. Synthesis and characterization of novel classes of PDE10A inhibitors - 1H-1,3-benzodiazoles and imidazo[1,2-a]pyrimidines. Eur J Med Chem 2018;155:96–116.
- Markowicz-Piasecka M, Sikora J, Mateusiak Ł, et al. Metformin and its sulfenamide prodrugs inhibit human cholinesterase activity. Oxid Med Cell Longev 2017;2017:7303096.
- Kuźma Ł, Wysokińska H, Sikora J, et al. Taxodione and extracts from Salvia austriaca roots as human cholinesterase inhibitors. Phytother Res 2016;30:234–42.
- Mandrioli R, Fanali S, Ferranti A, et al. HPLC analysis of the novel antipsychotic drug quetiapine in human plasma. J Pharm Biomed Anal 2002;30:969–77.
- Chou TC, Motzer RJ, Tong Y, Bosl GJ. Computerized quantitation of synergism and antagonism of taxol, topotecan, and cisplatin against human teratocarcinoma cell growth: a rational approach to clinical protocol design. J Natl Cancer Inst 1994;86:1517–24.
- Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev 2006;58:621–81.
- da Silva EM, Braga RCOP, Avelino-Silva TJ, Gil Junior LA. Antipsychotics in Alzheimer’s disease: a critical analysis. Dement Neuropsychol 2011;5:38–43.
- Pollock BG, Mulsant BH, Rosen J, et al. A double-blind comparison of citalopram and risperidone for the treatment of behavioral and psychotic symptoms associated with dementia. Am J Geriatr Psychiatry 2007;15:942–52.
- Cummings JL, Mackell J, Kaufer D. Behavioral effects of current Alzheimer’s disease treatments: a descriptive review. Alzheimers Dement 2008;4:49–60.
- Ballard C, Waite J. The effectiveness of atypical antipsychotics for the treatment of aggression and psychosis in Alzheimer’s disease. Cochrane Database Syst Rev 2006;1:CD003476.
- McGrath AM, Jackson GA. Survey of prescribing in residents of nursing homes in Glasgow. BMJ 1996;314:611–2.
- McShane R, Keene J, Gedling K, et al. Do neuroleptic drugs hasten cognitive decline in dementia? Prospective study with necropsy follow up. BMJ 1997;314:266–70.
- Ballard C, Margallo-Lana M, Juszczak E, et al. Quetiapine and rivastigmine and cognitive decline in Alzheimer’s disease: randomised double blind placebo controlled trial. BMJ 2005;330:874.
- Coffey ET, Akerman KE, Courtney MJ. Brain derived neurotrophic factor induces a rapid upregulation of synaptophysin and tau proteins via the neurotrophin receptor TrkB in rat cerebellar granule cells. Neurosci Lett 1997;227:177–80.
- Perry EK, Kilford L, Lees AJ, et al. Increased Alzheimer pathology in Parkinson’s disease related to antimuscarinic drugs. Ann Neurol 2003;54:235–8.
- Fleming K, Thyrum P, Yeh C, et al. Cognitive improvements in psychotic subjects treated with “Seroquel” (quetiapine fumarate): an exploratory study. J Clin Psychopharmacol 2001;21:527–9.
- Lafuente S, Thiamwong L. The effects of seroquel on agitation and cognition in Alzheimer’s patients: a limited integrative literature review. OAJ Gerontol Geriatric Med 2017;1:555574.
- De Deyn PP, Eriksson H, Svensson H, Study 115 investigators. Tolerability of extended-release quetiapine fumarate compared with immediate-release quetiapine fumarate in older patients with Alzheimer’s disease with symptoms of psychosis and/or agitation: a randomised, double-blind, parallel-group study. Int J Geriatr Psychiatry 2012;27:296–304.
- Rocca P, Marino F, Montemagni C, et al. Risperidone, olanzapine and quetiapine in the treatment of behavioral and psychological symptoms in patients with Alzheimer’s disease: preliminary findings from a naturalistic, retrospective study. Psychiatry Clin Neurosci 2007;61:622–9.
- Ferreira-Vieira TH, Guimaraes IM, Silva FR, et al. Alzheimer’s disease: targeting the cholinergic system. Curr Neuropharmacol 2016;14:101–15.
- Mehrpouya M, Ataei S, Nili-Ahmadabadi A. Potential drug interactions with cholinesterase inhibitors in Alzheimer patients: a guideline for neurologists. J App Pharm Sci 2017;7:223–6.
- Lockridge O. Review of human butyrylcholinesterase structure, function, genetic variants, history of use in the clinic, and potential therapeutic uses. Pharmacol Ther 2015;148:34–46.
- DeNinno MP. Future directions in phosphodiesterase drug discovery. Bioorg Med Chem Lett 2012;22:6794–800.
- Rotella D. Phosphodiesterase 5 inhibitors: current status and potential applications. Nat Rev Drug Discov 2002;1:674–82.
- Knott EP, Assi M, Rao SN, et al. Phosphodiesterase inhibitors as a therapeutic approach to neuroprotection and repair. Int J Mol Sci 2017;18:696–734.
- Shafiee-Nick R, Afshari AR, Mousavi SH, et al. A comprehensive review on the potential therapeutic benefits of phosphodiesterase inhibitors on cardiovascular diseases. Biomed Pharmacother 2017;94:541–56.
- Zhou L, Zhu Y, Jiang Y, et al. Design, synthesis and biological evaluation of dual Acetylcholinesterase and phosphodiesterase 5A inhibitors in treatment for Alzheimer’s disease. Bioorg Med Chem Lett 2017;27:4180–4.
