Abstract
The TAM (Axl, Mer, and Tyro3) family is implicated in the survival and chemoresistance of tumours and has emerged as a potential therapeutic target. A novel series of 7-aryl-2-anilino-pyrrolopyrimidines were identified as potent Axl/Mer tyrosine kinase inhibitors without significant inhibition of Tyro3. A representative compound 27 exhibited IC50 values of 2 nM and 16 nM for Mer and Axl, respectively, and considerable inhibition for Mer phosphorylation in cells. Docking studies suggested that the formation of a salt bridge between the nitrogen of the aniline moiety with ASP678 of the Mer kinase domain as well as an interaction with the hinge region that most kinase inhibitors have in common would be essential to retain activity. These results could provide useful information for finding promising inhibitors of Axl/Mer for the treatment of cancer.
Introduction
The TAM (TYRO3-AXL-MER) family consists of three receptor tyrosine kinases, Axl, Mer, and Tyro3. Several endogenous ligands have been identified for TAM receptorsCitation1. GAS6 binds to all three receptors but has a higher affinity for Axl compared to Mer and Tyro3. Protein S is known to be a specific ligand for Mer and Tyro3. TAM receptors are widely distributed in many tissues, including the nervous system, and they are involved in cell proliferation, survival, and migration as well as immune responses.
Oncogenic TAM receptor signalling is involved in tumour developmentCitation1. Particularly, ectopic expression of TAM receptors has been associated with a poor prognosis in a variety of cancersCitation1. Furthermore, it has been demonstrated that blockage of TAM signalling could improve the effectiveness of immunotherapy for cancer treatmentCitation2. TAM receptors, mainly the Mer receptor, induce M2 polarisation of macrophages in tumour microenvironments, which promotes tumour progressionCitation3.
Recent studies have demonstrated that Axl and Mer are implicated in resistance to chemotherapy and targeted therapyCitation4,Citation5. Thus, Axl/Mer inhibitors could provide a significant benefit for the treatment of patients with acquired resistance. With regard to a role of Mer in tumour associated macrophages, radiation therapy induced the upregulation of Mer in macrophages without changing the expression of Axl and tyro3Citation6. Mertk knockout mice showed better overall survival than wild type mice after radiation therapy. Therefore, the Mer tyrosine kinase could be a target to prevent the resistance of tumours to radiation therapy.
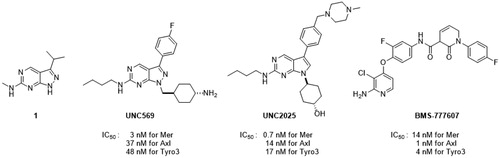
Recent studies revealed that Axl is a key molecule in hematological malignancies including multiple myelomaCitation7 and metastatic breast cancerCitation8. The combination of a pan-TAM kinase inhibitor, BMS-777607, with anti-PD1 resulted in a better anti-tumour effect than each monotherapy alone in a mouse modelCitation9. Currently, many inhibitors for multiple TAM receptors are under clinical or preclinical investigationCitation10. Representative TAM kinase inhibitors are shown in .
Pyrazolopyrimidine UNC569 was derived from an analysis of the co-crystal structure of 1 with Mer tyrosine kinaseCitation11 and showed potent inhibitory activity against the TAM family. Pyrrolopyrimidine UNC2025 showed more potent inhibitory activity against Mer than UNC569, but both exhibited strong activity against Tyro3. The MET tyrosine kinase inhibitor, BMS-777607, also showed activity as a pan- TAM inhibitor.
Basically, the development of inhibitors specific to a single TAM receptor would be difficult because of structural similarities among the tree TAM receptors. However, Tyro3 is widely expressed in the adult central nervous system (CNS)Citation12. Especially, Tyro3 is distributed in the nervous system at higher levels than Mer and Axl, indicating that inhibition of tyro3 could potentially lead to a toxicity issue even though Tyro3 could also be a therapeutic target for cancer. Mer is associated with resistance induced by Axl inhibition. Therefore, for the development of TAM kinase inhibitors, Axl/Mer inhibitors could provide an advantage over pan-TAM inhibitors. Moreover, the activation of Tyro3 could suppress retinal degeneration associated with Mer inhibitionCitation13. Therefore, it could be a plausible hypothesis that the discovery of Axl/Mer inhibitors that do not affect Tyro3 could give a better toxicity profile. Herein, we describe the identification of novel small-molecule inhibitors for Mer and Axl, and an investigation of their structure-activity relationship.
Materials and methods
Chemistry
All commercially available reagents were purchased from Sigma Aldrich®, Alfa Aesar, Tokyo Chemical Industry, Combi Blocks, Ark Pharm, Inc., or AstaTech. USP-grade solvents were purchased from Samchun Pure Chemical. HPLC grade solvents were purchased from either Fisher Scientific or J.T. Baker®. Microwave irradiation was performed using an Anton Paar Monoave 300. All reactions were monitored by thin-layer chromatography (TLC), using silica gel 60F254 from Merck and UV light visualisation. Flash chromatography was performed by Combiflash Rf+ (Teledyne Isco, USA) using silica gel (ZEOprep 60, 4063 µm, Zeochem LLC, USA) manually, a prepacked flash column Welux™ Column ultra-pure silica gel 4063 µm 60 Å (Intertechnologies Co., Ltd., Republic of Korea), or a RediSep® Rf Gold (Teledyne Isco, USA). 1H and 13C-NMR spectra were obtained using Jeol Resonance ECZ 600 R (600 MHz) or Varian Gemini 2000 (300 Mhz). Chemical shifts were reported in parts per million (ppm, δ) using tetramethylsilane (TMS) as the internal standard. Coupling constants (J) were provided in Hertz (Hz). Splitting patterns were described as follows: s, singlet; d, doublet; t, triplet; q, quartette; p, pentet; dd, doublet of doublets; dt, doublet of triplets; td, triplet of doublets; m, multiplet; br, broad signal. High-resolution mass spectra (HRMS) were obtained using a Q Exative™ Hybrid Quadropole-Orbitrap Mass Spectrometer (Thermo Scientific) with the ESI method.
N-(3-Methoxyphenyl)-7–(4-methoxyphenyl)-7H-pyrrolo[2,3-d]pyrimidin-2-amine (2)
A mixture of 3-methoxy aniline (11.18 µL 0.1 mmol), 7 (26 mg, 0.1 mmol), BINAP (2.4 mg, 0.004 mmol), Pd2(dba)3 (1.8 mg, 0.002 mmol), caesium carbonate (65 mg, 0.2 mmol) in anhydrous dioxane (2 ml) was stirred at 100 °C for 8 h. After being cooled to room temperature, the reaction mixture was filtered through celite. The filtrate was concentrated in vacuo and purified by MPLC with dichloromethane/methanol gave to 2 (11 mg, 32%).
1H-NMR (600 MHz, CDCl3) δ 8.71 (s, 1H), 7.62–7.64 (m, 3H), 7.28 (s, 1H), 7.16–7.19 (m, 2H), 7.03–7.04 (m, 3H), 6.52–6.55 (m, 2H), 3.88 (s, 3H), 3.70 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 160.25, 158.24, 156.10, 151.71, 150.98, 141.62, 130.82, 129.38, 126.31, 125.34, 114.44, 113.36, 110.67, 107.29, 103.85, 101.18, 55.57, 55.17. IR(neat): 2954, 2835, 1597, 1568, 1518, 1455, 1417, 1248, 1210, 1173, 1156, 1032, 832, 751, 733, 690 cm−1.
7–(4-Methoxyphenyl)-N-phenyl-7H-pyrrolo[2,3-d]pyrimidin-2-amine (3)
Following the procedure for 2, aniline and 7 provided the title compound 3 (12 mg, 39%).
1H-NMR (600 MHz, CDCl3) δ 8.70 (s, 1H), 7.65–7.70 (m, 4H), 7.29 (t, J = 7.9 Hz, 2H), 7.18–7.20 (m, 2H), 7.04 (dd, J = 6.9, 2.1 Hz, 2H), 6.97 (t, J = 7.6 Hz, 1H), 6.54 (d, J = 4.1 Hz, 1H), 3.88 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 158.18, 156.17, 151.72, 150.97, 140.36, 130.88, 128.83, 126.08, 124.98, 121.54, 118.37, 114.40, 113.45, 101.26, 55.61. IR(neat): 2954, 2835, 1597, 1568, 1536, 1518, 1483, 1417, 1210, 1156, 1032, 832, 766, 751, 733, 690, 596 cm−1.
N-([1,1′-Biphenyl]-3-yl)-7–(4-methoxyphenyl)-7H-pyrrolo[2,3-d]pyrimidin-2-amine (4)
Following the procedure for 2, [1,1′-biphenyl]-3-amine and 7 provided the title compound 4 (10 mg, 5%).
1H-NMR (600 MHz, CDCl3) δ 8.73 (s, 1H), 8.23 (t, J = 1.9 Hz, 1H), 7.61 (dd, J = 6.7, 2.2 Hz, 2H), 7.54–7.55 (m, 2H), 7.39–7.42 (m, 3H), 7.34–7.37 (m, 2H), 7.28 (s, 1H), 7.20–7.22 (m, 2H), 6.86 (dd, J = 6.7, 2.2 Hz, 2H), 6.56 (d, J = 3.8 Hz, 1H), 3.79 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 158.07, 156.12, 151.58, 151.08, 141.94, 141.47, 140.66, 130.74, 129.10, 128.64, 127.30, 127.15, 126.25, 125.00, 120.51, 117.29, 116.99, 114.47, 113.45, 101.23, 55.55. IR(neat): 2954, 2835, 1597, 1568, 1536, 1518, 1483, 1455, 1417, 1283, 1210, 1156, 1032, 832, 766, 751, 733, 690 cm−1.
7–(4-Methoxyphenyl)-2–(4-methylpiperazin-1-yl)-7H-pyrrolo[2,3-d]pyrimidine (5)
A mixture of 1-methylpiperazine (11.1 µL, 0.1 mmol), 7 (26.1 mg, 0.1 mmol), and 4 M HCl in dioxane (50.0 µL, 0.2 mmol) in isopropanol (2 ml) was heated at 160 °C for 1 h in a microwave reactor. After concentration, the crude mixture was diluted with dichloromethane (100 ml) and washed with saturated NaHCO3 solution (10 ml) and water. After drying over MgSO4, the organic layer was concentrated in vacuo and purified by MPLC with dichloromethane/methanol to give the title compound 5 (5 mg, 15%).
1H-NMR (300 MHz, CDCl3) δ 8.63 (s, 1H), 7.64 (d, J = 9 Hz, 2H), 7.12 (d, J = 3.9 Hz, 1H), 7.02 (d, J = 9 Hz, 2H), 6.47 (d, J = 3.9 Hz, 1H), 3.87 (br, 7H), 2.51 (t, J = 5.1 Hz, 4H), 2.36 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 159.2, 157.7, 152.5, 150.5, 131.3, 125.2, 124.5, 114.3, 111.9, 101.0, 55.6, 55.1, 46.2, 44.3; IR(neat): 2934, 2840, 2792, 1607, 1551, 1524, 1508, 1431, 1386, 1362, 1330, 1248, 1227, 1203, 1170, 1143, 1034, 1006, 950, 830, 734 cm−1. HRMS (ESI): m/z calculated for C18H21N5O [M + H]+ 324.1819 found 324.1823.
2-Chloro-7–(4-methoxyphenyl)-7H-pyrrolo[2,3-d]pyrimidine (7)
A mixture of 2-chloro-7H-pyrrolo[2,3-d]pyrimidine 6 (768 mg, 5.0 mmol), 4-methoxy phenyl boronic acid(1519 mg, 10 mmol), anhydrous pyridine (811 µL, 10 mmol), Copper(II)acetate (1362 mg, 7.5 mmol) in dichloromethane (10 ml) was stirred at room temperature for 12 h. The mixture was filtered through celite. The filtrate was concentrated in vacuo and purified by MPLC with dichloromethane/methanol to give the title compound 7 (218 mg, 17%).
1H-NMR (300 MHz, CDCl3) δ 8.88 (s, 1H), 7.56 (d, J = 9 Hz, 2H), 7.44 (d, J = 3.6 Hz, 1H), 7.05 (d, J = 9 Hz, 2H), 6.70 (d, J = 3.6 Hz, 1H), 3.88 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 158.9, 154.1, 151.6, 151.3, 130.1, 129.6, 125.4, 118.3, 114.8, 101.0, 55.6.
2–(2-Chloro-7H-pyrrolo[2,3-d]pyrimidin-7-yl)thiazole (8)
A mixture of 2-chloro-7H-pyrrolo[2,3-d]pyrimidine 6 (150 mg, 0.977 mmol), 2-bromothiazole (436.2 µL, 4.88 mmol), copper(I) iodide (1.9 mg, 0.01 mmol), tripotassium phosphate (414.6 mg, 1.95 mmol), and 1,2-trans-cyclohexanediamine (12.0 µL, 0.098 mmol) in toluene (1 ml) was stirred at 100 °C for 24 h, and then cooled down to room temperature. The reaction mixture was concentrated and diluted with dichloromethane (100 ml). The organic layer was washed with water and brine. After the mixture was dried over MgSO4, the organic layer was concentrated in vacuo and purified by MPLC with chloroform/acetonitrile to give the title compound 8 (51 mg, 22%).
1H-NMR (300 MHz, CDCl3) δ 8.90 (s, 1H), 8.25 (d, J = 3.9 Hz, 1H), 7.63 (d, J = 3.3 Hz, 1H), 7.29 (d, J = 3.3 Hz, 1H), 6.77 (d, J = 3.9 Hz, 1H); 13C-NMR (150 MHz, CDCl3) δ 155.3, 154.6, 151.4, 150.7, 138.6, 127.6, 119.1, 116.8, 102.9.
5-Bromo-2-chloro-N-phenylpyrimidin-4-amine (10a)
A mixture of 5-bromo-2,4-dichloropyrimidine 9 (683 mg, 3.0 mmol), aniline (273 µL, 3 mmol), and triethylamine (1254 µL, 9.0 mmol) in anhydrous isopropanol (10 ml) was stirred at room temperature for 12 h. The reaction mixture was diluted with dichloromethane (100 ml) and washed with saturated NaHCO3 solution (10 ml) and water. After drying over MgSO4, the organic layer was concentrated in vacuo and purified by MPLC with dichloromethane/methanol to give the title compound 10a (584 mg, 68%).
1H-NMR (600 MHz, CDCl3) δ 8.28 (s, 1H), 7.60 (d, J = 8.3 Hz, 2H), 7.45–7.34 (m, 2H), 7.27 (d, J = 13.9 Hz, 1H), 7.23–7.16 (m, 1H); 13C-NMR (150 MHz, CDCl3) δ 159.24, 157.59, 157.16, 137.00, 129.27, 125.27, 121.30, 103.78.
5-Bromo-2-chloro-N-(4-(trifluoromethoxy)phenyl)pyrimidin-4-amine (10 b)
Following the procedure for 10a, 4-(trifluoromethoxy)aniline and 9 provided the title compound 10 b (89%).
1H-NMR (600 MHz, CDCl3) δ 8.31 (s, 1H), 7.65 (d, J = 9.0 Hz, 2H), 7.31 (s, 1H), 7.26 (s, 1H), 7.24 (s, 1H); 13C-NMR (151 MHz, CDCl3) δ 159.20, 157.86, 157.04, 145.99, 135.63, 122.50, 121.91, 120.53(q, J = 257 Hz), 103.775.
Trans-4-((5-Bromo-2-chloropyrimidin-4-yl)amino)cyclohexanol (10c)
Following the procedure for 10a, trans-4-Aminocyclohexanol and 9 provided the title compound 10c (97%).
1H-NMR (600 MHz, CDCl3) δ 8.11 (s, 1H), 5.31 (d, J = 6.9 Hz, 1H), 3.98–4.05 (m, 1H), 3.66–3.71 (m, 1H), 2.13–2.15 (m, 2H), 2.03–2.06 (m, 2H), 1.71 (s, 1H), 1.50 (ddd, J = 23.4, 13.1, 3.4 Hz, 2H), 1.30–1.37 (m, 2H); 13C-NMR (150 MHz, CDCl3) δ 159.42, 158.74, 156.38, 102.96, 69.60, 49.27, 33.67, 30.43.
Trans-4-((2-Chloro-5-((trimethylsilyl)ethynyl)pyrimidin-4-yl)amino)cyclohexanol (11c)
A mixture of trans-4-((5-Bromo-2-chloropyrimidin-4-yl)amino)cyclohexanol 10c (153 mg, 0.5 mmol), TMS acetylene (71.7 µL, 0.5 mmol), TEA (246.5 µL, 2.5 mmol), Pd(PPh3)Cl2 (7 mg, 0.01 mmol), CuI (2 mg, 0.01 mmol) in anhydrous toluene (10 ml) was stirred at 80 °C for 4 h. After being cooled to room temperature, the reaction mixture was filtered through celite. The filtrate was concentrated in vacuo and purified by MPLC with dichloromethane/methanol to give the title compound 11c (39 mg, 24%).
1H-NMR (600 MHz, CDCl3) δ 8.08 (s, 1H), 5.45 (d, J = 7.7 Hz, 1H), 3.99–4.03 (m, 1H), 3.68–3.73 (m, 1H), 2.15–2.17 (m, 2H), 2.01–2.04 (m, 2H), 1.84 (s, 1H), 1.51 (ddd, J = 23.3, 12.8, 3.3 Hz, 2H), 1.31 (ddd, J = 24.0, 12.8, 3.2 Hz, 2H), 0.27 (s, 9H); 13C-NMR (150 MHz, CDCl3) δ 162.21, 159.71, 158.21, 106.46, 101.18, 96.04, 69.69, 48.94, 33.72, 30.56.
2-Chloro-7-phenyl-7H-pyrrolo[2,3-d]pyrimidine (12a)
A mixture of 10a (584 mg, 2.05 mmol), TMS acetylene (291 µL, 2.05 mmol), (1420 µL, 10.25 mmol) Pd(PPh3)Cl2 (28.7 mg, 0.041 mmol) and CuI (7.8 mg, 0.041 mmol) in anhydrous toluene (5 ml) was stirred at 80 °C for 4 h. After being cooled to room temperature, the reaction mixture was filtered through celite. The filtrate was concentrated in vacuo and purified by MPLC with dichloromethane/methanol to give 11a. Next, a mixture of 11a (607 mg, 2.01 mmol), TBAF 1 M in THF (4.02 ml, 4.02 mmol) in THF (25 ml) was stirred at 60 °C for 12 h. After the mixture was filtered through celite, the mixture was diluted with dichloromethane (100 ml) and washed with saturated NaHCO3 solution (10 ml) and brine. After drying over Na2SO4, the organic layer was concentrated in vacuo and purified by MPLC with dichloromethane/methanol to give the title compound 12a (236 mg, 52%).
1H-NMR (600 MHz, CDCl3) δ 8.88 (s, 1H), 7.69 (dd, J = 8.5, 0.9 Hz, 2H), 7.51–7.55 (m, 3H), 7.40 (t, J = 7.5 Hz, 1H), 6.73 (d, J = 3.7 Hz, 1H); 13C-NMR (150 MHz, CDCl3) δ 154.34, 151.70, 151.57, 136.77, 129.78, 129.72, 127.58, 123.94, 118.66, 101.54.
2-Chloro-7–(4-(trifluoromethoxy)phenyl)-7H-pyrrolo[2,3-d]pyrimidine (12b)
Following the procedure for 12a, TMS acetylene and 10b provided the title compound 12b (28%).
1H-NMR (600 MHz, CDCl3) δ 8.90 (s, 1H), 7.76 (dd, J = 7.1, 1.9 Hz, 2H), 7.50 (d, J = 3.8 Hz, 1H), 7.40 (d, J = 8.8 Hz, 2H), 6.76 (d, J = 3.6 Hz, 1H); 13C-NMR (150 MHz, CDCl3) δ 154.53, 151.78, 151.73, 148.01, 135.23, 129.34, 125.19, 122.29, 120.50(q, J = 258.2 Hz, 118.64, 102.05.
Trans-4–(2-Chloro-7H-pyrrolo[2,3-d]pyrimidin-7-yl)cyclohexanol (12c)
A mixture of trans-4-((2-Chloro-5-((trimethylsilyl)ethynyl)pyrimidin-4-yl)amino)cyclohexanol 11c (38.7 mg, 0.12 mmol), TBAF 1 M in THF (240 µL, 0.24 mmol). in THF (10 ml) was stirred at 60 °C for 24 h. After the mixture was filtered through celite, the mixture was diluted with dichloromethane (100 ml) and washed with saturated NaHCO3 solution (10 ml) and brine. After drying over Na2SO4, the organic layer was concentrated in vacuo and purified by MPLC with dichloromethane/methanol to give the title compound 12c (25 mg, 83%).
1H-NMR (600 MHz, CDCl3) δ 8.78 (s, 1H), 7.27 (s, 1H), 6.57 (d, J = 3.7 Hz, 1H), 4.72–4.76 (m, 1H), 3.77–3.81 (m, 1H), 2.10–2.18 (m, 4H), 1.82–1.89 (m, 3H), 1.62 (ddd, J = 24.2, 13.1, 3.3 Hz, 2H); 13C-NMR (150 MHz, CDCl3) δ 153.30, 151.62, 150.96, 126.61, 117.96, 100.25, 69.71, 52.52, 34.41, 31.05.
1–(2-Methoxy-4-nitrophenyl)-4-methylpiperazine (14a)
A mixture of 2-bromo-5-nitroanisole 13 (300 mg, 1.3 mmol), 1-methylpiperazine (199 µL, 1.8 mmol), BINAP (96.5 mg, 0.16 mmol) and Pd2(dba)3 (34.8 mg, 0.04 mmol), caesium carbonate (65 mg, 0.2 mmol) in anhydrous toluene (10 ml) was stirred at 120 °C for overnight. After being cooled to room temperature, the reaction mixture was filtered through celite. The filtrate was concentrated in vacuo and purified by MPLC with dichloromethane/methanol to give the title compound 14a (232 mg, 71%).
1H-NMR (600 MHz, CDCl3) δ 7.86 (dd, J = 8.8, 2.5 Hz, 1H), 7.71 (d, J = 2.5 Hz, 1H), 6.90 (d, J = 8.8 Hz, 1H), 3.95 (s, 3H), 3.27 (s, 4H), 2.61 (s, 4H), 2.37 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 151.20, 147.38, 142.02, 117.83, 116.72, 106.54, 55.96, 54.99, 49.90, 46.13. HRMS (ESI): m/z calcd for C12H18N3O3 [M + H] + 252.1343, found 252.13525.
N-(2-Methoxy-4-nitrophenyl)-1-methylpiperidin-4-amine (14b)
Following the procedure for 14a, 4-amino-1-methylpiperidine and 13 provided the title compound 14b (40%).
1H-NMR (300 MHz, CDCl3) δ 7.89 (dd, J = 2.4, 9 Hz, 1H), 7.62 (d, J = 2.4 Hz, 1H), 6.50 (d, J = 9 Hz, 1H), 4.96 (d, J = 7.8 Hz, 1H), 3.94 (s, 3H), 3.42 (br, 1H), 2.88 (d, J = 11.4 Hz, 2H), 2.35 (s, 3H), 2.21 (t, J = 11.4 Hz, 2H), 2.02–2.10 (m, 2H), 1.59–1.70 (m, 2H); 13C-NMR (150 MHz, CDCl3) δ 145.1, 143.1, 136.8, 119.9, 106.7, 104.9, 55.9, 54.1, 48.7, 46.0, 31.8.
1–(2-Methoxy-4-nitrophenyl)-N,N-dimethylpyrrolidin-3-amine (14c)
Following the procedure for 14a, 3-dimethylaminopyrrolidine and 13 provided the title compound 14c (29%).
1H-NMR (600 MHz, CDCl3) δ 7.83 (dd, J = 9.0, 2.5 Hz, 1H), 7.65 (d, J = 2.5 Hz, 1H), 6.47 (d, J = 9.0 Hz, 1H), 3.85 (s, 3H), 3.72 (dd, J = 10.2, 7.0 Hz, 1H), 3.64–3.67 (m, 2H), 3.50 (dd, J = 10.1, 8.6 Hz, 1H), 2.76 (t, J = 9.0 Hz, 1H), 2.32 (s, 6H), 2.14–2.18 (m, 1H), 1.84–1.89 (m, 1H); 13C-NMR (150 MHz, CDCl3) δ 147.47, 144.79, 137.73, 119.47, 111.68, 107.35, 77.24, 77.03, 76.82, 65.21, 56.07, 55.26, 49.99, 44.32, 29.93.
N-(3-Methoxy-4–(4-methylpiperazin-1-yl)phenyl)-7–(4-methoxyphenyl)-7H-pyrrolo[2,3-d]pyrimidin-2-amine (16)
A mixture of 14a (25.1 mg, 0.1 mmol) and Pd/C (10 w/w%) in MeOH (10 ml) were sealed with a septum and substituted with H2 gas. The reaction mixture was stirred at room temperature for 4 h. The mixture was filtered off and the filtrate was concentrated in vacuo to give the corresponding amine 15a. Successively, a mixture of 15a, 7 (26.0 mg, 0.1 mmol), BINAP (9.3 mg, 0.0150 mmol), sodium tert-butoxide (19.2 mg, 0.2 mmol), and Pd2(dba)3 (4.6 mg, 0.005 mmol) in anhydrous toluene (4 ml) was stirred at 100 °C for 20 h. After being cooled to room temperature, the reaction mixture was concentrated and diluted with dichloromethane (100 ml). The organic layer was washed with water and brine. After the mixture was dried over MgSO4, the organic layer was concentrated in vacuo and purified by MPLC with chloroform/acetonitrile to give the title compound 16 (25 mg, 56%).
1H-NMR (300 MHz, CDCl3) δ 8.68 (s, 1H), 7.56–7.61 (m, 3H), 7.24 (br, 1H), 7.14 (d, J = 3.9 Hz, 1H), 6.98–7.04 (m, 2H), 6.93 (dd, J = 2.4, 8.7 Hz, 1H), 6.87 (d, J = 8.7 Hz, 1H), 6.53 (d, J = 3.6 Hz, 1H), 3.87 (s, 3H), 3.66 (s, 3H), 3.07 (br, 4H), 2.68 (br, 4H), 2.39 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 158.3, 156.3, 152.5, 151.9, 136.3, 135.4, 130.9, 126.4, 125.7, 118.4, 114.4, 113.0, 110.2, 102.8, 101.1, 55.6, 55.3(2 C), 50.7, 45.9; IR(neat): 2935, 2798, 1599, 1562, 1530, 1510, 1417, 1249, 1212, 1151, 1033, 1012, 832, 733 cm−1. HRMS (ESI): m/z calcd for C25H28N6O2 [M + H]+ 445.2347 found 445.2344.
2-Methoxy-N4-(7–(4-methoxyphenyl)-7H-pyrrolo[2,3-d]pyrimidin-2-yl)-N1-(1-methylpiperidin-4-yl)benzene-1,4-diamine (17)
Following the procedure for 16, 14b and 7 provided the title compound 17 (17%).
1H-NMR (300 MHz, CDCl3) δ 8.65 (s, 1H), 7.60 (d, J = 9 Hz, 2H), 7.49 (d, J = 2.4 Hz, 1H), 7.12 (d, J = 3.6 Hz, 1H), 7.08 (s, 1H), 7.00 (d, J = 9 Hz, 2H), 6.84 (dd, J = 2.4, 8.4 Hz, 1H), 6.55 (d, J = 8.4 Hz, 1H), 6.51 (d, J = 3.6 Hz, 1H), 3.87 (s, 3H), 3.67 (s, 3H), 3.23–3.30 (m, 1H), 2.86 (d, J = 11.7 Hz, 2H), 2.34 (s, 3H), 2.20 (t, J = 10.8 Hz, 2H), 2.05–2.11 (m, 2H), 1.50–1.62 (m, 2H); IR(neat): 2933, 1601, 1563, 1513, 1416, 1248, 1210, 1034, 832, 732 cm−1. HRMS (ESI): m/z calcd for C26H30N6O2 [M + H]+ 459.2503 found 459.2490.
N-(4–(3-(Dimethylamino)pyrrolidin-1-yl)-3-methoxyphenyl)-7–(4-methoxyphenyl)-7H-pyrrolo[2,3-d]pyrimidin-2-amine (18)
Following the procedure for 16, 14c and 7 provided the title compound 18 (19%).
1H-NMR (600 MHz, CDCl3) δ 8.68 (s, 1H), 7.65–7.54 (m, 3H), 7.26 (s, 3H), 7.18–6.97 (m, 4H), 6.89 (dd, J = 8.6, 2.4 Hz, 1H), 6.73 (d, J = 9.0 Hz, 1H), 6.53 (d, J = 3.4 Hz, 1H), 3.88 (s, 3H), 3.66 (s, 3H), 3.50–3.36 (1H), 3.36–3.24 (1H), 3.24–3.07 (1H), 2.43 (s, 6H), 2.26–2.14 (1H), 2.01–1.87 (1H); 13C-NMR (150 MHz, CDCl3) δ 158.30, 156.49, 151.99, 151.35, 151.02, 130.95, 126.18, 125.64, 123.54, 118.21, 116.22, 114.42, 112.97, 110.55, 103.48, 101.14, 65.15, 55.62, 55.42, 49.75, 43.18, 41.04; IR(neat): 2935, 2821, 1600, 1561, 1511, 1417, 1346, 1249, 1209, 1035, 962, 832, 748, 698 cm−1.
1–(3-Methoxy-5-nitrophenyl)-4-methylpiperazine (20a)
A mixture of 3-bromo-5-anisole 19 (232 mg, 1 mmol), 1-methylpiperazine (122 µL, 1.1 mmol), caesium carbonate (651.6 mg, 2 mmol), BINAP (37.4 mg, 0.06 mmol), and Pd2(dba)3 (18.3 mg, 0.02 mmol) in anhydrous toluene (2.5 ml) was stirred at 120 °C for overnight. After being cooled to room temperature, the reaction mixture was filtered through celite. The filtrate was concentrated in vacuo and purified by MPLC with dichloromethane/methanol to give the title compound 20a (197 mg, 44%).
1H-NMR (600 MHz, CDCl3) δ 7.40 (t, J = 2.0 Hz, 1H), 7.19 (t, J = 2.0 Hz, 1H), 6.68 (t, J = 2.2 Hz, 1H), 3.85 (s, 3H), 3.28 (t, J = 5.0 Hz, 4H), 2.57 (t, J = 5.1 Hz, 4H), 2.36 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 160.85, 152.33, 150.05, 107.67, 103.53, 98.26, 55.76, 54.71, 48.27, 46.10. HRMS (ESI): m/z calcd for C12H18N3O3 [M + H] + 252.1343, found 252.13544.
1–(3-Methoxy-5-nitrophenyl)-4-(pyrrolidin-1-yl) piperidine (20b)
Following the procedure for 20a, 4-(pyrrolidinyl) piperazine and 19 provided the title compound 20b (75%).
1H-NMR (600 MHz, CDCl3) δ 7.40 (t, J = 2.1 Hz, 1H), 7.15 (t, J = 2.1 Hz, 1H), 6.69 (t, J = 2.3 Hz, 1H), 3.84 (s, 3H), 3.75–3.70 (2H), 2.92–2.83 (m, 2H), 2.65–2.55 (m, 4H), 2.18 (tt, J = 10.5, 3.8 Hz, 1H), 2.00 (d, J = 12.1 Hz, 2H), 1.86–1.75 (m, 4H), 1.71–1.59 (2H); 13C-NMR (150 MHz, CDCl3) δ 160.80, 152.28, 150.03, 107.85, 103.67, 97.73, 61.43, 55.72, 51.51, 47.54, 30.88, 23.24. HRMS (ESI): m/z calcd for C16H24N3O3 [M + H] + 306.1812, found 306.18291.
1–(3-Methoxy-5-nitrophenyl)-4-methyl-4-(pyrrolidin-1-yl) piperidine (20c)
Following the procedure for 20a, 4-methyl-4-(pyrrolidin-1-yl)piperidine and 19 provided the title compound 20c (67%).
1H-NMR (600 MHz, CDCl3) δ 7.41 (t, J = 2.1 Hz, 1H), 7.13 (t, J = 2.0 Hz, 1H), 6.69 (t, J = 2.2 Hz, 1H), 3.84 (s, 3H), 3.31–3.27 (m, 4H), 2.61 (s, 4H), 1.87 (d, J = 13.8 Hz, 2H), 1.74–1.72 (m, 4H), 1.59–1.55 (m, 2H), 0.98 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 160.77, 152.61, 150.04, 107.32, 103.30, 97.28, 55.70, 51.95, 44, 52, 44.49, 36.12, 24.10, 16.43. HRMS (ESI): m/z calcd for C17H26N3O3 [M + H] + 320.1969, found 320.19865.
1–(3-Methoxy-5-nitrophenyl)-3-(pyrrolidin-1-yl) piperidine (20d)
Following the procedure for 20a, pyrrolidine and 19 provided the title compound 20d (90%).
1H-NMR (600 MHz, CDCl3) δ 7.38 (t, J = 2.2 Hz, 1H), 7.16 (t, J = 2.1 Hz, 1H), 6.68 (t, J = 2.3 Hz, 1H), 3.92–3.86 (m, 1H), 3.85 (s, 3H), 3.65 (d, J = 12.6 Hz, 1H), 2.84 (td, J = 12.1, 2.9 Hz, 2H), 2.74 (s, 4H), 2.53–2.26 (m, 1H), 2.11 (d, J = 9.8 Hz, 1H), 1.93–1.79 (m, 5H), 1.71–1.61 (m, 1H); 13C-NMR (150 MHz, CDCl3) δ 160.89, 152.28, 150.12, 108.00, 103.88, 97.77, 60.52, 55.76, 51.59, 49.11, 23.55, 23.22. HRMS (ESI): m/z calcd for C16H24N3O3 [M + H]+ 306.1812, found 306.18253.
1–(3-Methoxy-5-nitrophenyl)-4-(oxetan-3-yl) piperazine (20e)
Following the procedure for 20a, 1-oxetan-3-yl-piperazine and 19 provided the title compound 20e (64%).
1H-NMR (600 MHz, CDCl3) δ 7.39 (t, J = 2.0 Hz, 1H), 7.20 (t, J = 1.9 Hz, 1H), 6.68 (t, J = 2.1 Hz, 1H), 4.71 (t, J = 6.6 Hz, 2H), 4.65 (t, J = 6.1 Hz, 2H), 3.86 (s, 3H), 3.62–3.49 (m, 1H), 3.30 (t, J = 5.0 Hz, 4H), 2.55–2.44 (4H); 13C-NMR (150 MHz, CDCl3) δ 160.91, 152.28, 150.09, 107.85, 103.63, 98.50, 75.44, 59.16, 55.84, 49.34, 48.09. HRMS (ESI): m/z calcd for C14H20N3O4 [M + H] + 294.1449, found 294.1461.
8–(3-Methoxy-5-nitrophenyl)-8-azaspiro[4.5]decane (20f)
Following the procedure for 20a, 8-aza-sprio[4,5]decane and 19 provided the title compound 20f (93%).
1H-NMR (600 MHz, CDCl3) δ 7.40 (t, J = 2.1 Hz, 1H), 7.14 (d, J = 4.0 Hz, 1H), 6.69 (t, J = 2.2 Hz, 1H), 3.84 (s, 3H), 3.24 (t, J = 5.7 Hz, 4H), 1.64–1.66 (m, 4H), 1.59 (t, J = 5.7 Hz, 4H), 1.46–1.48 (m, 4H); 13C-NMR (150 MHz, CDCl3) δ 160.88, 152.72, 150.14, 107.69, 103.64, 97.59, 55.78, 46.60, 40.77, 37.66, 36.92, 24.39.
8–(3-Methoxy-5-nitrophenyl)-1-oxa-8-azaspiro[4.5]decane (20g)
Following the procedure for 20a, 1-oxs-8-aza-sprio[4,5]decane-HCl and 19 provided the title compound 20g (89%).
1H-NMR (600 MHz, CDCl3) δ 7.39 (t, J = 2.1 Hz, 1H), 7.13 (t, J = 2.0 Hz, 1H), 6.67 (t, J = 2.2 Hz, 1H), 3.85 (q, J = 6.9 Hz, 5H), 3.31–3.38 (m, 4H), 1.93–1.96 (m, 2H), 1.70–1.73 (m, 6H); 13C-NMR (150 MHz, CDCl3) δ 160.93, 152.30, 150.18, 107.85, 103.83, 97.72, 79.59, 66.96, 55.79, 46.30, 36.67, 35.72, 25.53.
3-Methoxy-5-nitrobenzaldehyde (23)
A mixture of 3-methoxy-5-nitrobenzonitirle 22 (359 mg, 2.0 mmol) and 1.0 M diisobutyl aluminium hydride in THF (5.0 ml, 5.0 mmol) in anhydrous toluene (20 ml) was stirred at 0 °C for 3 h. The reaction mixture was concentrated and diluted with dichloromethane (100 ml). The organic layer was washed with water and brine. After the mixture was dried over Na2SO4, the organic layer was concentrated in vacuo and purified by MPLC with hexanes/ethyl acetate to give the title compound 23 (84 mg, 23%).
1H-NMR (600 MHz, CDCl3) δ 10.04 (s, 1H), 8.28 (s, 1H), 7.97 (t, J = 2.2 Hz, 1H), 7.71 (t, J = 1.2 Hz, 1H), 3.96 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 189.73, 160.94, 149.78, 138.30, 119.26, 117.17, 114.64, 56.46.
1–(3-Methoxy-5-nitrobenzyl)-4-methylpiperazine (24)
A mixture of 23 (83 mg, 0.458 mmol), 1-methylpiperazine (66 µL, 0.596 mmol), acetic acid (26 µL, 0.458 mmol), and NaBH(OAc)3 (291 mg, 1.375 mmol) in 1,2-dichloroethane (5 ml) was stirred at room temperature for 12 h. The mixture was diluted with dichloromethane (100 ml) and washed with saturated NaHCO3 solution (10 ml) and brine. After drying over Na2SO4, the organic layer was concentrated in vacuo and purified by MPLC with hexane/ethyl acetate to give the title compound 24 (40 mg, 33%).
1H-NMR (600 MHz, CDCl3) δ 7.82 (s, 1H), 7.61 (t, J = 2.1 Hz, 1H), 7.23 (s, 1H), 3.89 (s, 3H), 3.55 (s, 2H), 2.41 (d, J = 113.6 Hz, 11H); 13 C-NMR (150 MHz, CDCl3) δ 160.08, 149.26, 141.81, 121.49, 116.13, 106.77, 77.24, 77.03, 76.82, 62.03, 55.88, 55.03, 53.00, 45.97.
N-(3-Methoxy-5–(4-methylpiperazin-1-yl)phenyl)-7–(4-methoxyphenyl)-7H-pyrrolo[2,3-d]pyrimidin-2-amine (25)
A mixture of 20a (25.1 mg, 0.1 mmol) and Pd/C (10 w/w%) in MeOH (10 ml) was sealed with a septum and substituted with H2 gas. The reaction mixture was stirred at room temperature for 4 h. The mixture was filtered off and the filtrate was concentrated in vacuo to give compound 21a. Successively, a mixture of 21a, 7 (26.0 mg, 0.1 mmol), 4 M HCl in dioxane (50 µL, 0.2 mmol) in anhydrous isopropanol (2 ml) was heated at 160 °C for 1 h in a microwave reactor. After being cooled to room temperature, the reaction mixture was concentrated and diluted with dichloromethane (100 ml). The organic layer was washed with water and brine. After the mixture was dried over MgSO4, the organic layer was concentrated in vacuo and purified by MPLC with chloroform/acetonitrile to give the title compound 25 (13 mg, 29%).
1H-NMR (300 MHz, CDCl3) δ 8.69 (s, 1H), 7.59 (d, J = 9 Hz, 2H), 7.25 (s, 1H), 7.14 (d, J = 3.6 Hz, 1H), 7. 02 (d, J = 9 Hz, 2H), 6.99 (t, J = 2.1 Hz, 1H), 6.80 (t, J = 2.1 Hz, 1H), 6.54 (d, J = 3.6 Hz, 1H), 6.11 (t, J = 2.1 Hz, 1H), 3.88 (s, 3H), 3.70 (s, 3H), 3.10 (t, J = 5.1 Hz, 4H) 2.49 (t, J = 5.1 Hz, 4H), 2.34 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 160.9, 158.2, 156.2, 152.8, 151.7, 151.0, 142.0, 130.9, 126.5, 125.7, 114.5, 113.2, 101.1, 98.7, 96.2, 95.2, 55.5, 55.2, 55.1, 48.9, 46.1; IR(neat): 2934, 2837, 1593, 1568, 1536, 1518, 1483, 1452, 1418, 1248, 1209, 1161, 1032, 1003, 831, 734, 699 cm−1; HRMS (ESI): m/z calcd for C25H28N6O2 [M + H]+ 445.2347 found 445.2345.
N-(3-Methoxy-5-((4-methylpiperazin-1-yl)methyl)phenyl)-7–(4-methoxyphenyl)-7H-pyrrolo[2,3-d]pyrimidin-2-amine (26)
Following the procedure for 25, 24 and 7 provided the title compound 26 (51%).
1H-NMR (300 MHz, CDCl3) δ 8.69 (s, 1H), 7.59 (d, J = 9 Hz, 2H), 7.25 (s, 1H), 7.14 (d, J = 3.6 Hz, 1H), 7. 02 (d, J = 9 Hz, 2H), 6.99 (t, J = 2.1 Hz, 1H), 6.80 (t, J = 2.1 Hz, 1H), 6.54 (d, J = 3.6 Hz, 1H), 6.11 (t, J = 2.1 Hz, 1H), 3.88 (s, 3H), 3.70 (s, 3H), 3.10 (t, J = 5.1 Hz, 4H) 2.49 (t, J = 5.1 Hz, 4H), 2.34 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 160.9, 158.2, 156.2, 152.8, 151.7, 151.0, 142.0, 130.9, 126.5, 125.7, 114.5, 113.2, 101.1, 98.7, 96.2, 95.2, 55.5, 55.2, 55.1, 48.9, 46.1; IR(neat): 2934, 2836, 2800, 1599, 1569, 1537, 1518, 1456, 1417, 1349, 1248, 1209, 1160, 1064, 832, 734, 699 cm−1. HRMS (ESI): m/z calcd for C25H28N6O2 [M + H]+ 445.2347 found 445.2345.
N-(3-Methoxy-5–(4-(pyrrolidin-1-yl)piperidin-1-yl)phenyl)-7–(4-methoxyphenyl)-7H-pyrrolo[2,3-d]pyrimidin-2-amine (27)
Following the procedure for 25, 20b and 7 provided the title compound 27 (10%).
1H-NMR (300 MHz, CDCl3) δ: 8.69 (s, 1H), 7.60 (d, J = 9 Hz, 2H), 7.17 (s, 1H), 7.15 (d, J = 3.6 Hz, 1H), 7.02 (d, J = 9 Hz, 2H), 6.94 (t, J = 1.8 Hz, 1H), 6.81 (t, J = 1.8 Hz, 1H), 6.54 (d, J = 3.6 Hz, 1H), 6.12 (t, J = 1.8 Hz, 1H), 3.88 (s, 3H), 3.70 (s, 3H), 3.60 (d, J = 12.3 Hz, 2H), 2.80–2.89 (m, 4H), 2.62 (t, J = 12.3 Hz, 2H), 1.94–2.12 (m, 1H), 1.66–2.02 (m, 8H); 13C-NMR (150 MHz, CDCl3) δ 160.9, 158.3, 156.1, 152.1, 151.7, 150.9, 142.0, 131.0, 126.7, 125.6, 114.6, 113.2, 101.2, 99.4, 97.3, 95.9, 62.0, 55.9, 55.2, 50.2, 48.5, 27.5, 23.6; IR(neat): 2954, 1592, 1568, 1537, 1519, 1418, 1248, 1209, 1034, 1157, 831, 734 cm−1. HRMS (ESI): m/z calcd for C29H34N6O2 [M + H]+ 499.2816 found 499.2812.
N-(3-Methoxy-5–(4-methyl-4-(pyrrolidin-1-yl)piperidin-1-yl)phenyl)-7–(4-methoxyphenyl)-7H-pyrrolo[2,3-d]pyrimidin-2-amine (28)
Following the procedure for 25, 20c and 7 provided the title compound 28 (22%).
1H-NMR (600 MHz, CDCl3) δ 8.70 (s, 1H), 7.61 (dd, J = 6.9, 2.0 Hz, 2H), 7.35 (s, 1H), 7.26 (s, 1H), 7.14 (d, J = 3.6 Hz, 1H), 7.01 (dd, J = 6.9, 1.9 Hz, 2H), 6.90 (s, 1H), 6.84 (d, J = 1.6 Hz, 1H), 6.52 (d, J = 3.6 Hz, 1H), 6.14 (t, J = 2.0 Hz, 1H), 3.85 (s, 3H), 3.69 (s, 3H), 3.18–3.22 (m, 2H), 3.01–3.05 (m, 2H), 2.60 (s, 4H), 1.76–1.80 (m, 2H), 1.72 (d, J = 5.9 Hz, 4H), 1.50–1.54 (m, 2H), 0.96 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 160.96, 158.27, 156.48, 153.26, 151.83, 151.06, 141.97, 131.00, 126.44, 125.64, 114.50, 113.17, 101.17, 98.75, 96.18, 94.59, 55.57, 55.24, 52.25, 45.23, 44.52, 36.24, 24.14, 16.50; IR(neat): 2958, 2835, 1603, 1569, 1536, 1519, 1484, 1458, 1419, 1349, 1248, 1211, 1156, 1071, 1034 cm−1.
N-(3-Methoxy-5–(3-(pyrrolidin-1-yl)piperidin-1-yl)phenyl)-7–(4-methoxyphenyl)-7H-pyrrolo[2,3-d]pyrimidin-2-amine (29)
Following the procedure for 25, 20d and 7 provided the title compound 29 (11%).
1H-NMR (600 MHz, CDCl3) δ 8.68 (s, 1H), 7.60–7.62 (m, 2H), 7.15 (d, J = 3.6 Hz, 1H), 7.10 (s, 1H), 7.01–7.03 (m, 2H), 6.98 (s, 1H), 6.76 (s, 1H), 6.53 (d, J = 3.7 Hz, 1H), 6.13 (t, J = 2.0 Hz, 1H), 3.86 (s, 3H), 3.84–3.77 (1H), 3.68 (s, 3H), 3.55–3.45 (1H), 2.63–2.66 (m, 5H), 2.32 (s, 1H), 2.06 (d, J = 12.5 Hz, 1H), 1.81 (s, 5H), 1.72 (d, J = 6.7 Hz, 2H), 1.59–1.67 (m, 1H); 13C-NMR (150 MHz, CDCl3) δ 161.14, 158.38, 156.44, 151.92, 151.00, 142.05, 131.07, 126.39, 125.83, 125.54, 114.61, 113.36, 101.17, 99.54, 97.05, 95.19, 60.95, 55.60, 55.36, 55.26, 51.66, 51.53, 49.90, 24.02, 23.33; IR(neat): 2937, 1594, 1567, 1537, 1519, 1484, 1461, 1418, 1356, 1249, 1207, 1162, 1030, 832, 735 cm−1.
N-(3-Methoxy-5–(4-(oxetan-3-yl)piperazin-1-yl)phenyl)-7–(4-methoxyphenyl)-7H-pyrrolo[2,3-d]pyrimidin-2-amine (30)
Following the procedure for 25, 20e and 7 provided the title compound 30 (27%).
1H-NMR (600 MHz, CDCl3) δ 8.70 (s, 1H), 7.89–7.76 (0H), 7.76–7.65 (0H), 7.59 (d, J = 8.7 Hz, 2H), 7.47–7.37 (0H), 7.34 (s, 1H), 7.25–7.19 (0H), 7.14 (d, J = 3.4 Hz, 1H), 7.07 (s, 1H), 7.01 (d, J = 8.7 Hz, 2H), 6.82–6.77 (0H), 6.73 (s, 1H), 6.53 (d, J = 3.4 Hz, 1H), 6.10 (s, 1H), 4.66–4.71 (m, 4H), 3.88 (d, J = 12.8 Hz, 3H), 3.71 (d, J = 12.8 Hz, 3H), 3.53 (q, J = 6.3 Hz, 1H), 3.11 (s, 4H), 2.39 (d, J = 4.1 Hz, 4H); 13C-NMR (150 MHz, CDCl3) δ 160.86, 158.19, 156.25, 152.75, 151.67, 150.99, 142.03, 130.90, 126.56, 125.77, 114.46, 113.15, 101.11, 98.73, 96.22, 95.21, 75.47, 59.25, 55.53, 55.18, 49.63, 48.66; IR(neat): 2951, 2877, 2834, 1592, 1569, 1538, 1519, 1484, 1453, 1418, 1316, 1248, 1212, 1161, 1028 cm−1.
N-(3-Methoxy-5–(8-azaspiro[4.5]decan-8-yl)phenyl)-7–(4-methoxyphenyl)-7H-pyrrolo[2,3-d]pyrimidin-2-amine (31)
Following the procedure for 25, 20g and 7 provided the title compound 31 (12%).
1H-NMR (600 MHz, CDCl3) δ 8.69 (s, 1H), 7.60 (dd, J = 6.8, 2.0 Hz, 2H), 7.19 (s, 1H), 7.14 (d, J = 3.6 Hz, 1H), 7.01–7.02 (m, 2H), 6.92 (s, 1H), 6.82 (d, J = 1.7 Hz, 1H), 6.53 (d, J = 3.6 Hz, 1H), 6.14 (d, J = 2.0 Hz, 1H), 3.86 (s, 3H), 3.70 (s, 3H), 3.06 (t, J = 5.6 Hz, 4H), 1.62–1.64 (m, 4H), 1.52 (t, J = 5.6 Hz, 4H), 1.43 (t, J = 7.1 Hz, 4H); 13C-NMR (150 MHz, CDCl3) δ 160.86, 158.22, 156.31, 153.41, 151.75, 150.95, 141.83, 130.89, 126.44, 125.61, 114.44, 113.12, 101.09, 99.02, 96.44, 94.79, 55.48, 55.17, 47.21, 40.73, 37.67, 37.32, 24.34; IR(neat): 2935, 1592, 1568, 1537, 1519, 1484, 1462, 1418, 1248, 1210, 1160, 1137, 1033, 831, 734 cm−1.
N-(3-Methoxy-5–(1-oxa-8-azaspiro[4.5]decan-8-yl)phenyl)-7–(4-methoxyphenyl)-7H-pyrrolo[2,3-d]pyrimidin-2-amine (32)
Following the procedure for 25, 20h and 7 provided the title compound 32 (31%).
1H-NMR (600 MHz, CDCl3) δ 8.69 (s, 1H), 7.60 (d, J = 8.6 Hz, 2H), 7.22 (s, 1H), 7.14 (d, J = 3.6 Hz, 1H), 7.02 (d, J = 8.6 Hz, 2H), 6.87 (d, J = 15.8 Hz, 2H), 6.53–6.53 (m, 1H), 6.14 (s, 1H), 3.86 (t, J = 6.7 Hz, 5H), 3.69 (s, 3H), 3.13–3.23 (m, 4H), 1.92–1.97 (m, 2H), 1.64–1.71 (m, 7H); 13C-NMR (150 MHz, CDCl3) δ 160.90, 158.23, 156.31, 152.87, 151.75, 150.97, 141.89, 130.89, 126.43, 125.61, 114.46, 113.15, 101.09, 99.20, 96.62, 94.93, 79.98, 66.70, 55.50, 55.17, 47.07, 36.26, 36.08, 25.48; IR(neat): 2938, 1592, 1568, 1538, 1519, 1484, 1462, 1418, 1347, 1300, 1248, 1209, 1161, 1133, 1034 cm−1.
N-(3-Methoxy-5–(4-(pyrrolidin-1-yl)piperidin-1-yl)phenyl)-7–(4-(trifluoromethoxy)phenyl)-7H-pyrrolo[2,3-d]pyrimidin-2-amine (33)
Following the procedure for 25, 20b and 12b provided the title compound 33 (43%).
1H-NMR (600 MHz, CDCl3) δ 8.71 (s, 1H), 7.82 (dd, J = 7.0, 2.0 Hz, 2H), 7.36 (d, J = 8.6 Hz, 2H), 7.25 (s, 1H), 7.20 (d, J = 3.6 Hz, 1H), 6.92 (d, J = 1.5 Hz, 1H), 6.76 (s, 1H), 6.58 (d, J = 3.8 Hz, 1H), 6.16 (t, J = 2.0 Hz, 1H), 3.71 (s, 3H), 3.61 (d, J = 12.4 Hz, 2H), 2.68 (td, J = 12.2, 2.0 Hz, 2H), 2.60 (s, 4H), 2.10 (s, 1H), 1.93 (d, J = 12.4 Hz, 2H), 1.80 (s, 4H), 1.59–1.66 (m, 2H); 13C-NMR (150 MHz, CDCl3) δ 160.96, 156.48, 153.03, 151.78, 151.34, 147.14, 141.58, 136.43, 125.39, 124.98, 121.91, 121.85, 120.48 (q, J = 258.2 Hz), 113.34, 102.26, 99.29, 96.79, 95.60, 61.88, 55.14, 51.39, 48.47, 31.27, 23.27; IR(neat): 2952, 2800, 1596, 1572, 1540, 1511, 1483, 1461, 1415, 1379, 1355, 1256, 1206, 1161, 831 cm−1.
N-(3-Methoxy-5–(4-(pyrrolidin-1-yl)piperidin-1-yl)phenyl)-7-phenyl-7H-pyrrolo[2,3-d]pyrimidin-2-amine (34)
Following the procedure for 25, 20 b and 12a provided the title compound 34 (17%).
1H-NMR (600 MHz, CDCl3) δ 8.71 (s, 1H), 7.75 (d, J = 7.6 Hz, 2H), 7.50–7.52 (m, 2H), 7.37 (t, J = 7.4 Hz, 1H), 7.28 (s, 1H), 7.23 (d, J = 3.7 Hz, 1H), 6.88 (dd, J = 21.9, 1.7 Hz, 2H), 6.57 (d, J = 3.7 Hz, 1H), 6.13 (t, J = 2.0 Hz, 1H), 3.69 (s, 3H), 3.60 (d, J = 12.6 Hz, 2H), 2.75 (s, 4H), 2.63 (td, J = 12.3, 1.9 Hz, 2H), 2.29 (s, 1H), 1.95 (d, J = 12.4 Hz, 2H), 1.88 (s, 4H), 1.68–1.70 (m, 2H); 13C-NMR (150 MHz, CDCl3) δ 160.87, 156.30, 152.85, 151.67, 151.10, 141.84, 137.84, 129.34, 126.56, 126.02, 123.98, 113.41, 101.65, 99.26, 96.87, 95.27, 61.93, 55.23, 51.17, 48.43, 30.50, 23.32; IR(neat): 2956, 1593, 1568, 1538, 1517, 1500, 1482, 1460, 1416, 1353, 1270, 1208, 1157, 1070, 759 cm−1.
N-(3-Methoxy-5–(4-(pyrrolidin-1-yl)piperidin-1-yl)phenyl)-7-(thiazol-2-yl)-7H-pyrrolo[2,3-d]pyrimidin-2-amine (35)
Following the procedure for 25, 20 b and 8 provided the title compound 35 (11%).
1H-NMR (600 MHz, DMSO-D6) δ 9.56 (s, 1H), 8.84 (s, 1H), 7.97 (d, J = 3.8 Hz, 1H), 7.93–7.76 (1H), 7.70 (d, J = 3.4 Hz, 1H), 7.34–7.09 (1H), 6.98 (s, 1H), 6.78 (d, J = 3.8 Hz, 1H), 6.16 (s, 1H), 3.74 (s, 5H), 3.33 (s, 5H), 2.68 (s, 2H), 1.96 (d, J = 120.2 Hz, 9H); 13C-NMR (150 MHz, DMSO-D6) δ 160.20, 156.38, 154.85, 151.79, 150.21, 141.47, 140.53, 138.59, 123.16, 116.82, 112.72, 103.86, 100.17, 96.47, 96.00, 60.94, 54.93, 50.49, 47.59, 40.02, 39.97, 39.93, 39.85, 39.71, 39.57, 39.43, 39.29, 39.15, 39.01, 38.89, 28.90, 22.60; IR(neat): 3403, 2917, 2360, 1594, 1576, 1542, 1523, 1508, 1483, 1452, 1409, 1354, 1221, 1157, 764 cm1.
(1r,4r)-4–(2-((3-Methoxy-5–(4-(pyrrolidin-1-yl)piperidin-1-yl)phenyl)amino)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)cyclohexanol (36)
Following the procedure for 25, 20 b and 12c provided the title compound 36 (16%).
1H-NMR (600 MHz, DMSO-D6) δ 9.21 (s, 1H), 8.65 (s, 1H), 7.36 (d, J = 3.4 Hz, 1H), 7.33 (s, 1H), 6.97 (s, 1H), 6.43 (d, J = 3.4 Hz, 1H), 6.09 (s, 1H), 4.54 (q, J = 5.0 Hz, 1H), 3.73 (s, 3H), 3.67 (d, J = 9.0 Hz, 2H), 3.58 (s, 1H), 2.74 (t, J = 11.0 Hz, 5H), 2.00 (t, J = 10.7 Hz, 4H), 1.88–1.92 (m, 4H), 1.74 (s, 4H), 1.58 (s, 2H), 1.32–1.37 (m, 2H), 1.23 (s, 1H); 13C-NMR (150 MHz, DMSO-D6) δ 160.06, 155.47, 150.42, 150.34, 142.64, 123.55, 111.92, 99.49, 98.35, 95.18, 94.75, 67.61, 60.89, 54.62, 51.76, 50.70, 47.48, 34.47, 30.25, 22.75; IR(neat): 3377, 2936, 1593, 1570, 1537, 1487, 1453, 1422, 1381, 1199, 1158, 1070, 1026 cm−1.
Kinase assay
All kinase assays were carried out at Km ATP by Eurofins Discovery's Kinase Screening and Profiling services (France).
Cell culture
MKN28 cells were obtained from the Korea Institute of Science and Technology (KIST). Cells were cultured in Roswell Park Memorial Institute (RPMI) 1640 medium containing 10% fetal bovine serum and 1% penicillin/streptomycin at 37 °C with 5% CO2 under a humidified atmosphere.
Western blot
MKN 28 cells (500,000 cells/2 ml) were seeded in each well of 6-well plates and incubated for 24 h. Then, the cells were treated with 20 µL of DMSO stock solution of the corresponding compounds and incubated for a further 1.5 h. After that, the cells were washed with cold Dulbecco's Phosphate-Buffered Saline (DPBS) twice and lysed with RIPA buffer supplemented with protease inhibitor and phosphatase inhibitor cocktails on ice. Equal amounts of protein samples were boiled with 5× SDS-PAGE loading buffer and separated by 10% SDS-PAGE gels, then transferred onto polyvinylidene difluoride (PVDF) membranes. The membranes were blocked with 5% BSA in 1× TBST (1× TBS with 0.1% Tween-20) for 1 h and incubated at 4 °C overnight with a 1:1000 dilution of the following primary antibodies in blocking buffer: pMer (Tyr749 + 753 + 754, ab14921), Mer (CST, # 4319), and ß-actin (SC, # SC-47778). After three washes with 1× TBST, the membranes were incubated with a 1:2000 dilution of the following secondary antibodies at RT for 1 h followed by extensive washing three times. Secondary antibodies: anti-rabbit IgG, HRP-linked antibody (CST, # 7074S); anti-mouse IgG, HRP-linked antibody (CST, # 7076S). Antibody binding was visualised by an enhanced chemiluminescent (ECL) system (Bio-Rad, Clarity Western ECL Substrate, # 1705061) and VILBER FUSION SOLO X. Antibodies were purchased from Abcam, Cell Signalling Technology (CST), and Santa Cruz Biotechnology (SC). ß-actin was used as a loading control.
Results and discussion
To find the novel Mer and Axl inhibitors, we screened our in-house chemical library for the TAM family. As shown in , 7-aryl pyrrolopyrimidines 2 and 3 were found to be hits, with IC50 of 39 nM and 95 nM against Mer, respectively, and little activity for Tyro3. 3-phenyl aniline 4 and piperazine 5 did not have activity against the TAM family. Thus, derivatives of compound 2 were synthesised to explore the structure-activity relationship.
Table 1. Activity for TAM kinases of methoxy phenyl pyrrolopyrimidines.
The synthetic routes for the derivatives are illustrated in Schemes 1 and 2. As shown in Scheme 1, 2-chloro-pyrrolo[2,3-d]pyrimidine intermediates with various substituents were prepared by reacting commercially available 2-chloro-7H-pyrrolo[2,3-d]pyrimidine 6 with boronic acids using the Chan–Lam coupling reactionCitation14. Thiazole 8 was synthesised by the Ullmann coupling reactionCitation15. For the synthesis of further derivatives, intermediates 10a–c were synthesised by nucleophilic substitution of amine with 9. Sonogashira coupling of 10a–c with TMS acetylene followed by intramolecular cyclisation gave the desired intermediates 12a–c.
Scheme 1. Reagents and conditions: (a) (OH)2B-Ar, Cu(OAc)2, pyridine, 4 Å MS, CH2Cl2, rt, 6–24 h; (b) 2-bromothiazole, CuI, K3PO4, 1,2-trans-cyclohexanediamine, THF, 110 °C, 24 h; (c) BINAP, Pd2(dba)3, Cs2CO3, dioxane, 100 °C, 8 h; (d) HCl, i-PrOH, MW 160 °C, 1 h; (e) 4-(trifluoromethoxy)aniline, TEA, i-PrOH; (f) ethynyltrimethylsilane, Pd(PPh3)2, CuI, TEA, toluene, 80 °C, 4 h; (g) TBAF, THF, 60 °C, 4 h.
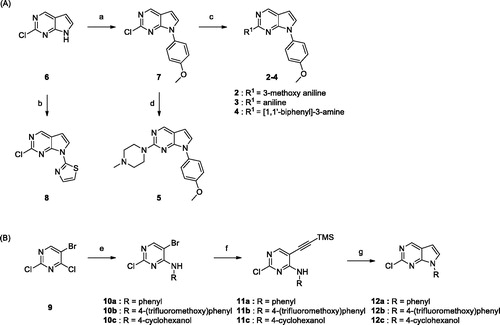
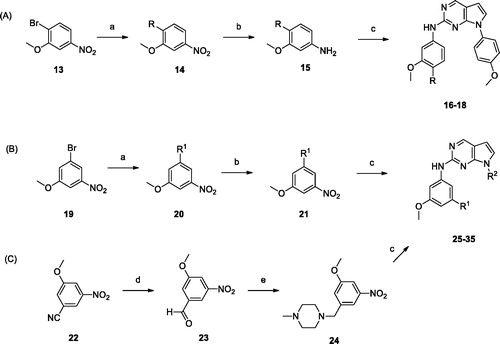
Commercially available 1-bromo-2-methoxy-4-nitrobenzene 13 was coupled with amines by the Buchwald–Hartwig coupling reaction. The resulting alkylamino compound 14 was hydrogenated to yield aniline 15, followed by coupling with 2-chloropyrrolopyrimidines (7, 8, and 12a-c) to give the desired compounds 16–18. Synthesis of meta-substituted derivatives started from 3-bromo-5-methoxy nitrobenzene. Compounds 22–32 were obtained using the same synthetic methods as those used to prepare 16–18. Derivative 23 was synthesised from 3-cyano-5-methoxy nitrobenzene, as outlined in Scheme 2(c).
Scheme 2. Reagents and conditions: (a) amines, Pd2(dba)3, BINAP, Toluene, Cs2CO3, 100 °C, 6 − 24 h; (b) H2, Pd/C, MeOH, rt, 3 − 9 h; (c) 7, 8, or 12, HCl, i-PrOH, MW 160 °C, 1 h; (d) DIBAL, toluene, 0 °C, 3 h; (e) 1-methylpiperazine, NaBH(OAc)3, AcOH, DCE, rt, 12 h.
Introduction of an alkyl amino group at the para-position resulted in an improvement of activity over compound 2 with weak activity against Tyro3 (). N-Methyl piperazinyl derivative 16 showed 2.6-fold and 12-fold decreased IC50 for Mer and Axl, respectively, compared to 2. Compound 17 also had IC50 of 10 nM for Mer and approximately 200 nM for Axl without considerable inhibition of Tyro3. Dimethylaminopyrrolidine 18 showed a slight decrease in activity against Mer and improved activity against Axl.
Next, the effects of meta-substitution were explored. Interestingly, substitution of N-methyl piperazine (25) at the meta-position (R3) led to great potency for Mer with an IC50 of 2 nM. The one-carbon extended N-methyl piperazine derivative 26 was also highly potent, but it showed relatively high activity for Tyro3 compared to the other derivatives. 4-Pyrrolidinyl piperidine derivative 27 was also equipotent to the known compound (UNC569) for Mer and Axl but still showed weak activity against Tyro3. Methylated derivative 28 displayed 2–3-fold weaker activity than 27. 3-Pyrrolidinyl piperidine 29 had moderate activity for Mer and Axl whilst 4-oxetanyl piperazine 27 showed decreased activity for Axl. Interestingly, the introduction of an azaspirodecanyl group (31) led to complete loss of activity for all TAMs. However, the insertion of oxygen (32) resulted in the recovery of activity similar to 30. These data suggest that a heteroatom at an appropriate distance from the aniline ring might be necessary to achieve good activity for Mer and Axl. A docking study was carried out to further understand the binding mode of the described compounds.
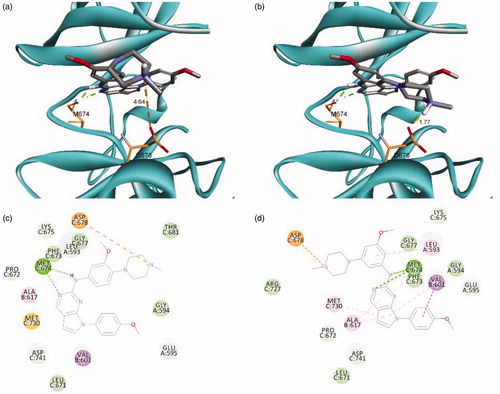
As shown in , the docking model showed that N1 and NH of 16 and 25 interact with MET674 of the hinge region through hydrogen bonding, and the nitrogen atom in the piperazine moiety forms a salt bridge with ASP678. The distances between ASP678 and the nitrogen atom in the piperazine moiety of para-derivative 16 and meta-derivative 25 were calculated as 4.64 Å and 1.77 Å, respectively. This suggests that the piperazine moiety at the meta-position could be placed closer to ASP678 than when it is at the para-position, which may induce stronger binding of 25 than that of 16. In addition, this docking model provided a reasonable explanation for the complete loss of activity of 31, which cannot interact with ASP678 owing to the absence of a nitrogen atom. The addition of an oxygen atom at the spiro-ring in 32 led to recovered activity, which also supported the docking model. These data indicate that the formation of a salt bridge with ASP678 is important for retaining activity against TAM family kinases.
Figure 2. Predicted docking orientation of 16 and 25 with the Mer kinase domain (PDB ID: 3TCP). Docking mode of (a) 16 and (b) 25 with Mer. 2 D-interaction diagram of the binding model of (c) 16 and (d) 25. Estimated binding energies were −7.52 kcal/mol and −8.26 kcal/mol for 16 and 25, respectively. Hydrogen bonds and a salt bridge between the ligand and the backbone are shown in dashed lines. The docking study was performed by AutoDock Vina.
Next, a brief structure-activity relationship investigation was carried out for R4, as shown in . According to the predicted docking model for Mer, it was postulated that R4 as a 4-methoxyphenyl group is positioned in the hydrophobic pocket and is involved in a π–alkyl interaction with Val601. As expected, trifluoromethyl phenyl compound 33 and phenyl compound 34 showed excellent activity for Mer and Axl. Thiazole derivative 35 also retained activity, although its activity was less than those of 33 and 34. However, the introduction of a cycloalkyl group, a trans-4-hydroxycyclohexyl group, in 36 significantly decreased the activity for Mer and Axl. This suggests that the described compounds may interact with Mer in a different manner than UNC569. The data indicate that N7-substituents with an aromatic group may be suitable to bind Mer or Axl.
Table 2. Activities of 3-methoxy aniline derivatives of compound 3.
Table 3. Inhibitory activity of R4 derivatives.
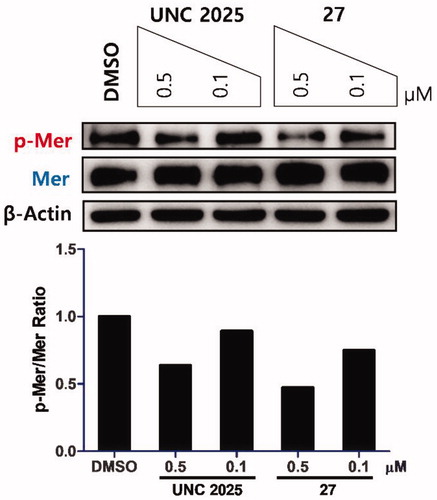
To determine the inhibitory activity of compounds on Mer phosphorylation in cells, western blot analysis was carried out. A representative compound 27 was used to treat a Mer-overexpressed human gastric cancer cell line, MKN28. A potent Mer inhibitor, UNC2025, was used as a positive control (). Compound 27 showed a better effect on blocking phosphorylation than UNC2025 at the indicated concentrations.
Figure 3. Inhibitory effect of 27 on Mer phosphorylation in a Mer-overexpressed human gastric cancer cell line, MKN28. Cells were treated with the indicated compounds at 0.5 and 0.1 μM for 1.5 h. UNC2025 and β-actin were used as a positive control and a loading control, respectively. Western blot analysis for phosphorylated and total Mer from a representative experiment is shown. Bar graph represents the relative intensities of the total and phosphorylated Mer as determined by band densitometry using image analysis software.
In summary, we report here the discovery of 7-aryl-2-anilino-pyrrolopyrimidine derivatives as potent inhibitors of Axl and Mer kinases without considerable inhibition of Tyro3. The most potent compound 27 had IC50 values of 2 nM and 16 nM for Mer and Axl, respectively, but just 40% inhibition of Tyro3 at 1 µM. In addition, compound 27 exhibited considerable inhibition for Mer phosphorylation in a cancer cell line. Structure-activity relationship and docking studies showed that forming a salt bridge and an aromatic group at the N7 position are essential for its Axl and Mer kinase inhibition activity. This work could provide useful information for the molecular design of Axl/Mer kinase inhibitors.
Disclosure statement
No potential conflict of interest was reported by the authors.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Graham DK, DeRyckere D, Davies KD, Earp HS. The tam family: phosphatidylserine sensing receptor tyrosine kinases gone awry in cancer. Nat Rev Cancer 2014;14:769–85.
- Akalu YT, Rothlin CV, Ghosh S. Tam receptor tyrosine kinases as emerging targets of innate immune checkpoint blockade for cancer therapy. Immunol Rev 2017;276:165–77.
- Myers KV, Amend SR, Pienta KJ. Targeting tyro3, axl and mertk (tam receptors): implications for macrophages in the tumor microenvironment. Mol Cancer 2019;18:94.
- Vouri M, Hafizi S. Tam receptor tyrosine kinases in cancer drug resistance. Cancer Res 2017;77:2775–8.
- Wong KM, Horton KJ, Coveler AL, et al. Targeting the tumor stroma: the biology and clinical development of pegylated recombinant human hyaluronidase (pegph20). Curr Oncol Rep 2017;19:47.
- Crittenden MR, Baird J, Friedman D, et al. Mertk on tumor macrophages is a therapeutic target to prevent tumor recurrence following radiation therapy. Oncotarget 2016;7:78653–66.
- Yan S, Vandewalle N, De Beule N, et al. Axl receptor tyrosine kinase as a therapeutic target in hematological malignancies: focus on multiple myeloma. Cancers (Basel) 2019;11:1727.
- Holland SJ, Pan A, Franci C, et al. R428, a selective small molecule inhibitor of axl kinase, blocks tumor spread and prolongs survival in models of metastatic breast cancer. Cancer Res 2010;70:1544–54.
- Kasikara C, Davra V, Calianese D, et al. Pan-tam tyrosine kinase inhibitor bms-777607 enhances anti-pd-1 mab efficacy in a murine model of triple-negative breast cancer. Cancer Res 2019;79:2669–83.
- Agha A, Tarhini AA. Adjuvant therapy for melanoma. Curr Oncol Rep 2017;19:36.
- Liu J, Yang C, Simpson C, et al. Discovery of novel small molecule mer kinase inhibitors for the treatment of pediatric acute lymphoblastic leukemia. ACS Med Chem Lett 2012;3:129–34.
- Tondo G, Perani D, Comi C. Tam receptor pathways at the crossroads of neuroinflammation and neurodegeneration. Dis Markers 2019;2019:2387614.
- Vollrath D, Yasumura D, Benchorin G, et al. Tyro3 modulates mertk-associated retinal degeneration. PLoS Genet 2015;11:e1005723.
- Lam SC, Saubern G, Adams S, et al. A. New aryl/heteroaryl c-n bond cross-coupling reactions via arylboronic acid/cupric acetate arylation. Tetrahedron Lett 1998;39:2941–4.
- Monnier F, Taillefer M. Catalytic C-C, C-N, and C-O ullmann-type coupling reactions. Angew Chem Int Ed Engl 2009;48:6954–71.