Abstract
Simultaneous inhibition of histone deacetylases (HDACs) and anaplastic lymphoma kinase (ALK) could enhance therapeutic activity against ALK addicted cancer cells. Herein, a new series of 2,4-pyrimidinediamine derivatives as ALK and HDACs dual inhibitors were designed, synthesised and evaluated. Compound 12a which possessed good inhibitory potency against ALKwt and HDAC1, exhibited stronger antiproliferative activity than Ceritinib on ALK positive cancer cell lines though inducing cell apoptosis and cell cycle arrest in vitro and in vivo. In addition, the mechanism is further verified by the down-regulation of p-ALK protein, and up-regulation of Acetylated histone 3 (Ac-H3) protein in cancer cells. These results suggested that 12a would be a potential candidate for the ALK addicted cancer treatment.
Graphical Abstract
Introduction
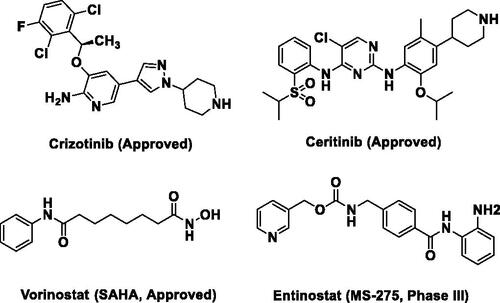
Anaplastic lymphoma kinase (ALK) is a tyrosine kinase that belongs to the insulin receptor (IR) superfamilyCitation1,Citation2. ALK alterations are often involved in the development of several types of cancers including non-small cell lung cancer (NSCLC), anaplastic large cell lymphoma (ALCL) and neuroblastomaCitation3,Citation4. For example, the echinoderm microtubule-associated protein-like 4 (EML4–ALK) fusion, as a common oncogenic gene fusion detected in NSCLC, promotes the dimerisation and phosphorylation of ALK protein, which finally leads to NSCLC occurrenceCitation5. As a consequence, small molecular ALK inhibitors such as CrizotinibCitation6 and CeritinibCitation7 () were approved for the treatment of ALK-driven NSCLC via blocking ALK and its downstream signal transduction pathways. However, the effective application of ALK inhibitors is often limited by the drug resistance that emerges following the prolonged treatment in the clinicCitation8.
Histone deacetylases (HDACs) are a class of scavenging enzyme that catalysed the removal of acetyl from lysine residues, leading to chromatin condensation and transcriptional suppression in cellsCitation9,Citation10. However, aberrant activation of HDACs often contributes to the development and progression of many cancersCitation11. Hence, inhibition of HDACs can reverse the genetic aberrations of epigenetic states associated with malignancyCitation9,Citation12. Currently, four small molecule HDACs inhibitors (HDACIs) (), Vorinostat (SAHA) Citation13, Belinostat (PXD-101)Citation14, Romidepsin (FK228)Citation15 and Panobinostat (LBH589)Citation16 targeting HDACs to suppress the growth of cancers were approved by FDA and Entinostat (MS-275)Citation17 () was being evaluated in the third stages of clinical trials.
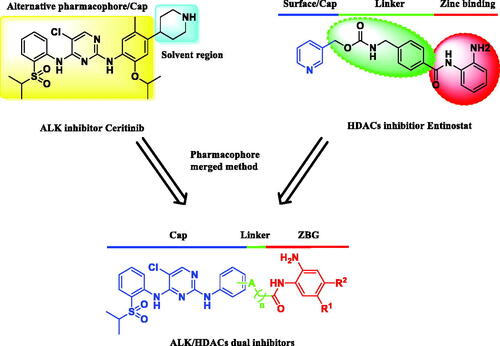
As AXL-dependent epithelial-to-mesenchymal transition (EMT) regulated by HDACs has been proved to be associated with the emergence of drug resistance to ALK inhibitors, simultaneous inhibition of HDACs could reduce the levels of H3K27ac related to AXL to decrease its gene expression, thus improving the efficacy of ALK inhibitorsCitation18. Increasing evidences have indicated that ALK inhibitors in combination with HDACIs could synergistically induce the anti-proliferative effects on ALK inhibitor resistant cells or xenografts and are more efficient in ALK positive NSCLC patientsCitation19–22. In addition to the combinational therapy methods, we conceived that ALK and HDACs dual inhibitors that can concurrently inhibit both targets would be an alternative and attractive therapeutic strategy for ALK addicted cancer.
In our previous work, we have discovered a series of ALK and HDACs novel dual inhibitors via fused pharmacophore approachCitation23. Among them, the optimal compound 10f featuring a flexible side-chain exhibited good potency on ALK-positive cancer cell lines. However, compound 10f displayed poor antitumor activities in vivo. It was speculated that the hydroxamic acid group might account for the low permeability or aqueous solubility of compound 10f. Therefore, in this work, a more effective ALK/HDACs dual inhibitor compound 12a, referring to the structure of Ceritinib and Entinostat, was identified and evaluated (. To our delight, 12a showed a remarkable antitumor efficacy against different cancer cell lines in vitro and in vivo. These results indicated that 12a would be a promising anti-NSCLC candidate which deserves further research.
Results and discussion
Chemistry
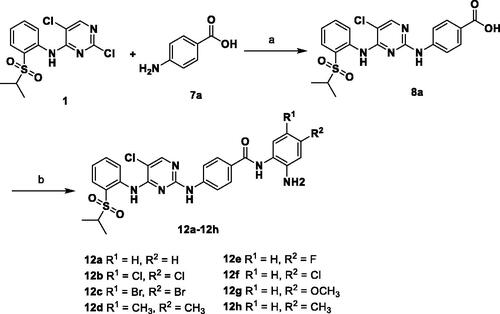
The desired compounds 6a–l and 11a–j were prepared according to the synthetic route depicted in Schemes 1 and Citation2. 2,5-dichloro-N-(2-(isopropylsulfonyl)phenyl)pyrimidin-4-amine (1) was purchased and used as the starting material. With regard to procedures in Scheme 1, m/p-phenylenediamines (2a–b) were firstly reacted with compound 1 to obtain intermediates 3a–b, respectively. Next, methyl alkanoate derivatives were activated by 1-ethyl-3–(3-dimethyllaminopropyl) carbodiimide hydrochloride (EDCI)/1-hydroxybenzotriazole (HOBT), and subsequently condensed with intermediates 3a–b to produce the key ester intermediates 4a–l in the presence of N, N-Diisopropylethylamine (DIPEA). Then 4a–l were hydrolysed by NaOH to afford carboxylic acid intermediates 5a–l, which were finally condensed with o-phenylenediamine to give the target products 6a–l under the same condensed reaction condition. In Scheme 2, compounds 11a–j were synthesised by following similar procedures from compound 1 and p/m-aminobenzoic acid (7a–b).
Scheme 1. Reagents and conditions: (a) HCl, i-PrOH, 90 °C, 6 h, 52–63%; (b) Corresponding methyl alkanoates, HOBT, EDCI, DIPEA, DMF, r.t., 12 h, 24–68%; (c) NaOH, MeOH/H2O, 70 °C, 6 h, 79–93%; and (d) HOBT, EDCI, DIPEA, DMF, r.t., 5 h, 25–89%.
Scheme 2. Reagents and conditions: (a) HCl, EtOH, 90 °C, 6 h, 68–88%; (b) Corresponding methyl aminoalkanoates, HOBT, EDCI, DIPEA, DMF, r.t., 12 h, 47–76%; (c) NaOH, MeOH/H2O, 70 °C, 6 h, 70–89%; and (d) HOBT, EDCI, DIPEA, DMF, r.t., 5 h, 36–90%.
Another series of desired compounds 12a–h were prepared as described in Scheme 3. Condensation of previously obtained 4-((5-chloro-4-((2-(isopropylsulfonyl)phenyl)amino)pyrimidin-2-yl)amino)benzoic acid (8a) with o-phenylenediamines yielded the corresponding final compounds 12a–h in moderate yields.
Biological evaluation
Anti-proliferative activities of target compounds against cancer cell lines
The in vitro antiproliferative effects of the target compounds on A549 (lung cancer cells), MDA-MB-231 (breast carcinoma cells), HepG2 (hepatocellular carcinoma cells), and SK-N-BE(2) (neuroblastoma cells) were detected via CCK-8 assay for 72 h, Ceritinib and Entinostat were selected as two positive controls. As shown in , most of the synthetic compounds displayed stronger inhibitory activity than the positive controls on selected cancer cell line, especially compound 12a, which inhibited the growth of cells with IC50 values ranging from 0.0003 to 0.01 μM, demonstrating that the HDACs inhibitory effects of these dual inhibitors may contribute to their antiproliferative capacities. Notably, SK-N-BE(2) cell line harbouring ALKwt mutation seems to be the most sensitive cancer cell line than others, indicating that our compounds may be more effective on ALK positive cancer cell lines. To further investigate the effect of linker on the antitumor activity, compounds 6a–f with different linker length (n = 2–6) were synthesised. However, it was found that increasing the linker length may be unfavourable for enhancing the anti-proliferative activity. Moreover, introducing electron-withdrawing or electron-donating groups such as F, Cl, CH3 groups on the phenyl ring (12b–h) also lead to decreased inhibition activity. Interestingly, the meta-substituted compounds (6g–l) showed good antitumor activity as well as their para-substituted analog (6a–f), indicated that the substituted position may had little effect on inhibition capacities. Finally, in comparison with 6a–l, compounds 11a–j were made as reversed amide and they exhibited parallel potency compared to compound 6a–l.
Table 1. Antiproliferative activity of compounds against cancer cell lines.
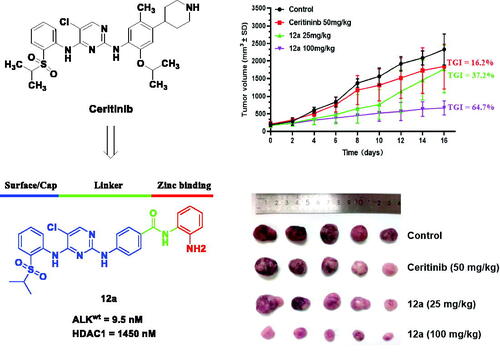
Considering the excellent antitumor efficacy, compound 12a was further selected to evaluate its potency on ALK-dependent H2228 lung cancer cells, and 12a showed an IC50 value of 11 nM against the proliferation of H2228, which was almost 10-fold potent than that of Ceritinib and Entinostat (). In addition, the binding affinity of compound 12a to ALK and HDAC1 were investigated in kinase assay. As depicted in , although less efficient than Ceritinib and Entinostat, compound 12a showed good inhibitory potency against ALKwt and HDAC1, with IC50 values of 9.5 and 1450 nM, respectively (), indicating that compound 12a was a dual ALK/HDACs inhibitor.
Table 2. In vitro inhibitory activity of compound 12a against cancer cell lines and enzymes.
Compound 12a represses cell invasion and migration in vitro
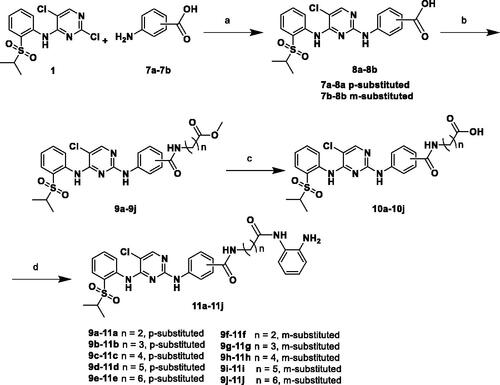
Next, we detected the effect of compound 12a on the migration and invasion ability of A549, H2228 and SK-N-BE(2) cells by cell scratch assay and Transwell method. As shown in C), the results showed that the migration capability of all cancer cells was significantly suppressed by 12a after 24 h treatment, compared with the Ceritinib group and Entinostat group. In Transwell assay, 12a also reduced the metastasis ability in A549 and SK-N-BE(2) cells at 4.0 μM or 0.4 μM concentration (). These results clearly displayed that compound 12a can prevent the cell migration and invasion in a dose-dependent manner.
Figure 3. Cell scratch and Transwell assays. Effect of compound 12a (1.0, 2.0 and 4.0 μM or 0.1, 0.2 and 0.4 μM), Ceritinib (1.0 μM) and Entinostat (1.0 μM) on A549 (A), H2228 (B) and SK-N-BE(2) (C) on cell migration ability. The distance was measured as the mean ± SD (n = 3). (D, E) Transwell assay results in SK-N-BE(2) and A549 cell lines treated with Ceritinib (1.0 μM) and compound 12a (1.0, 2.0 and 4.0 μM or 0.1, 0.2, and 0.4 μM) for 24 h. The number of cells was calculated as the mean ± SD (n = 3). Scale bar = 100 μm, *p < 0.05, **p < 0.01 and ***p < 0.001, compared with the control.
Compound 12a arrests cell cycle and induces cell apoptosis in vitro
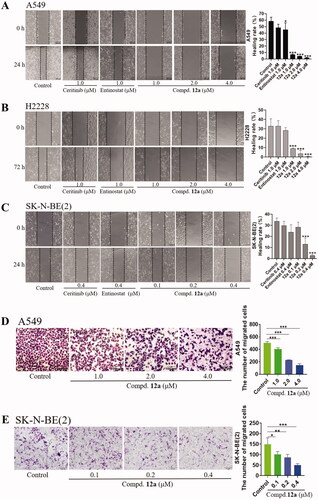
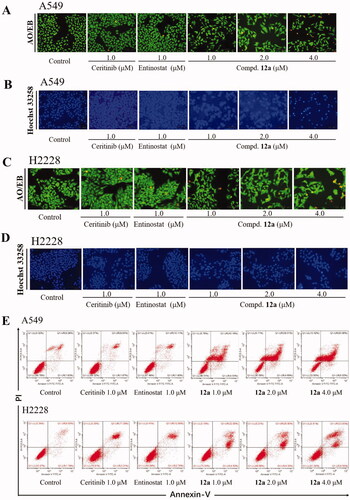
Since we had demonstrated that compound 12a is effective to inhibit proliferation of cells in vitro, the effect of compound 12a on the cell cycle was then investigated via flow cytometry to further explore the mechanism. The results of flow cytometry were illustrated in . After A549 cells were treated with 12a at different concentrations (1.0, 2.0, 4.0 μM) for 24 h, it can be observed that 12a could slightly arrest cell cycle at S phase. When H2228 cells were treated in the same manner, the percentage of cell population in G1 phase was increased from 38.95% (control) to 60.92% (1.0 μM), indicating that 12a could arrest the cell cycle at G1 phase in H2228 cells.
Figure 4. Effect of Compound 12a on cell cycle progression. A549 and H2228 cells were treated with, Ceritinib (1.0 μM), Entinostat (1.0 μM), compound 12a (1.0, 2.0 and 4.0 μM) and DMSO (0.8 μL) for 24 h, then stained with PI, followed by analysed via flow cytometry. The bar graph shows the percentages of cells in the G1, G2, S phases. (A) After treatment with compound 12a, the cell cycle of A549 cells was slightly blocked at S phase, compared with the positive groups or control. (B) The cell cycle was arrested at G1 phase after being treated with compound 12a, vs. control in H2228 cells.
Furthermore, AO/EB and Hoechst 33258 staining assays were utilised to evaluate whether compound 12a could induce apoptosis of cells. The AO/EB staining results were illustrated in , it can be seen that the cells in the control group showed well-distributed green fluorescence. On the contrary, after 24 h treatment of compound 12a at 4.0 μM concentration, the number of orange fluorescent cells increased and the cell morphology gradually changed in A549 and H2228 cells, indicating that the number of late apoptotic cells increased. Similarly, the results of Hoechst 33258 staining further verified that compound 12a could induce cell death. As illustrated in , the uneven blue fluorescence was emerged and enhanced in a dose-dependent manner in compound treatment groups compared with the control groups. Flow cytometry was further conducted to investigate whether the anti-proliferation effect of 12a was associated with cellular apoptosis in A549 and H2228 cells. As shown in , when treated with compound 12a, the percentage of apoptotic cells increased in a dose-dependent manner, from 10.9% (control) to 46.7% (1.0 μM), 64.2% (2.0 μM), and 66.98% (4.0 μM). Likewise, the apoptosis rate of cells treated with compound 12a also increased significantly in H2228 cells, much greater than that of control (). From these results, we conclude that 12a was able to cause apoptotic effects at 1ow concentration in cancer cell lines.
Figure 5. Compound 12a induced apoptosis in A549 and H2228 cell lines. A549 and H2228 cells were treated with Ceritinib (1.0 μM), Entinostat (1.0 μM), compound 12a (1.0, 2.0 and 4.0 μM) for 24 h, followed by stained with AO/EB (A and C) or Hoechst 33258 (B and D), and photographed. Scale bar = 100 μm. (E and F) Flow cytometry analysis results. A549 and H2228 cells (E) cells were treated with compound 12a or positive drugs for 48 h, then stained with Annexin V-FITC/PI.
Compound 12a blocks ALK and HDAC signalling pathways
Furthermore, the changes of protein expression related to apoptosis were also investigated by western blot assays in A549 and H2228 cells, the results were illustrated in . Not surprisingly, compound 12a could dose-dependently up-regulated the expression of pro-apoptotic protein Bax accompanied with a decreased expression of anti-apoptotic protein Bcl-2, which were correlated with previous outcomes.
Figure 6. Effect of compound 12a on the protein expression of ALK- and HDAC-mediated signalling pathways in A549 and H2228 cell lines. (A, B) Western blot analysis of apoptosis-associated and ALK protein expression after treated with compound 12a in A549 (A) and H2228 (B) cell lines. (C, D) Western blot analysis of Ac-H3 and H3 protein expression after treated with compound 12a in A549 (C) and H2228 (D) cell lines. All data were expressed as the mean ± SD of three independent experiments. *p < 0.05, ***p < 0.001 compared with untreated samples.
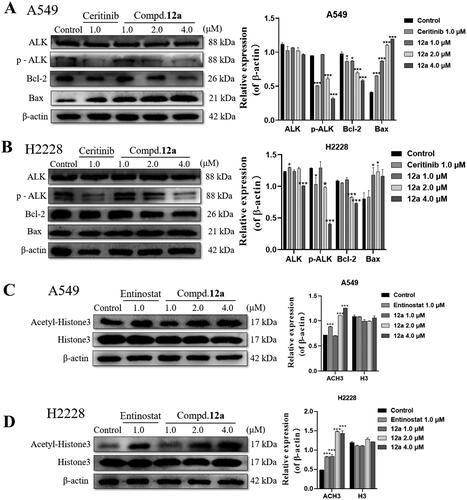
Meanwhile, to clarify the mechanism, the relative proteins involved in the ALK- or HDAC-mediated signalling pathway were also tested. It was found that the intracellular levels of p-ALK was significantly suppressed in 12a treatment groups (4.0 μM), compared to that of control group, while the expression of ALK was not changed. On the other hand, after exposure to compound 12a for 24 h, the expression levels of Acetylated histone 3 (Ac-H3) protein increased in a dose-dependent manner in drug treatment group (). Taken together, these results displayed that compound 12a could block the ALK- and HDAC- mediated signalling pathways simultaneously as a dual inhibitor.
Compound 12a inhibits the growth of SK-N-BE(2) cells in vivo
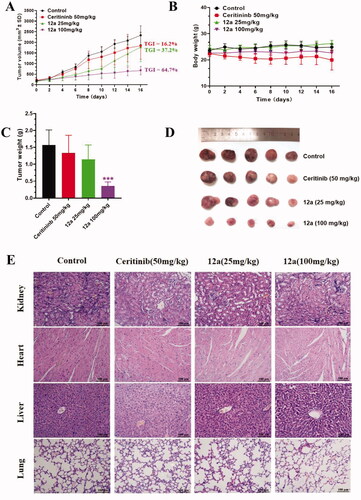
On the basis of the favourable in vitro anticancer activity, compound 12a was further selected to evaluate its preliminary antitumor efficacy in the SK-N-BE(2) xenografts in vivoCitation24. When tumours had reached an average volume of 100 mm3, mice were random divided into four groups and intraperitoneally administered with saline, 25 or 100 mg/kg compound 12a and 50 mg/kg Ceritinib every two days for 16 consecutive days, respectively. The results in showed that compound 12a could significantly suppress the tumour growth. Compared with 50 mg/kg Ceritinib, compound 12a treatment group at a dose of 25 or 100 mg/kg results in 37.2% and 64.7% TGI (tumour growth inhibition), respectively. In addition, no obvious weight loss was observed in all compound treatment groups (). Moreover, the H&E staining results showed no obvious pathological changes were observed in lung, heart and kidney, but vacuolisation in liver were observed in Ceritinib group and high-dose group, indicating that high dose of 12a may have toxic effects on liver ().
Figure 7. Effect of compound 12a on the growth of SK-N-BE(2) xenografts in vivo. Nude mice were injected with SK-N-BE(2) cells, followed by treatment with compound 12a (25 mg/kg and 100 mg/kg) and Ceritinib (50 mg/kg) for 16 days. (A) Tumour volume curves. (B) Bodyweight of mice. (C) Tumour weight. (D) Images of tumour xenografts excised from mice model. ***p < 0.005, compared with the control. (E) H&E staining of the organs from the model, control, low-dose and high-dose experimental groups.
Molecular docking studies
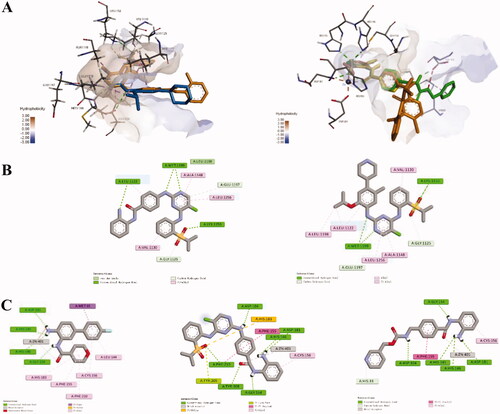
Docking studies of compound 12a with ALK and HDAC2 were performed (). Similar to Ceritinib, the pyrimidine nitrogen atom in compound 12a establish strong hydrogen bonds at the hinge area with Met1199. In addition, the phenylamine group was found to be projected towards solvent region which didn’t form key interactions with ALK (). On the other hand, from the overlap model of 12a, the original ligand and Entinostat in HDAC2, we could see that the NH2 group and carbonyl group in compound 12a can coordinate with Zn2+ to form a stable six-ring in ZBG pocket, as the original ligand and Entinostat did, which might explain the good inhibitory activity of 12a against HDAC2 (). Interestingly, it can be observed that the GAP group in compound 12a and Entinostat adopts a totally opposite orientation, respectively, which might barely affect the HDAC2 inhibitory potency.
Figure 8. (A) Overlap modelling of 12a (orange) and Ceritinib (blue) in ALK (PDB: 4MKC) (left); Overlap modelling of 12a (orange), Entinostat (green) and original ligand (yellow) in HDAC2 (PDB: 5IWG) (right). (B) 2D diagram of the interactions of 12a (left) and Ceritnib (right) with ALK. (C) 2D diagram of the interactions of original ligand (left), 12a (middle) and Entinostat (right) with HDAC2.
Conclusions
In summary, a novel and potent dual ALK and HDACs inhibitor 12a was discovered. Compound 12a exhibited good inhibitory activities against ALKwt or HDAC1 enzymes and synergistically inhibited proliferation of ALK-driven cancer cells via inducing cell apoptosis and cell cycle arrest. Western blot assays were further conducted and confirmed that compound 12a can concurrently inhibits ALK and HDACs signal pathways as an ALK/HDACs dual inhibitor. More importantly, compound 12a possessed desirable in vivo antitumor potency in a SK-N-BE(2) xenograft model. These results suggested compound 12a was expected to be a good candidate for the ALK-positive cancer treatment.
Experimental section
Chemistry
All materials used in this study were obtained from Tansoole, Macklin and Adamas without further purification after purchasing. The conventional 1H NMR and 13 C HNMR spectra were measured in CDCl3/DMSO-d6 by a Bruker spectrometer with tetramethylsilane (TMS) as internal standard. Coupling constants (J) and chemical shifts (δ) are noted in Hz and ppm separately. The melting point of compounds was detected with microscopic melting point apparatus. High-resolution mass spectra (HRMS) were recorded with Aglient 6470 Triple Quad LC-MS apparatus. Column chromatography was conducted on silica gel (200–300 mesh).
General procedure for the synthesis of intermediates 3a–b
To a solution of p-phenylenediamine (1.25 g, 11.6 mmol, 2a) in 50 mL isopropanol, was added 2,5-dichloro-N-(2-(isopropylsulfonyl)phenyl)pyrimidin-4-amine (3.48 g, 10.0 mmol, 1) and 37% HCl solution (0.8 mL). The mixture was heated and stirred at 90 °C for 11 h. After reaction completion, the solvent was evaporated in vacuo and extracted with 100 mL EtOAc twice, then the organic layer was sequentially washed with saturated sodium chloride aqueous solution twice, dried over anhydrous Na2SO4 and filtered to give desired compound 3a which can be directly used without further purification. 3b was synthesised in the manner of 3a.
General procedure for the synthesis of intermediates 4a–l
Methyl 3-((4-((5-chloro-4-((2-(isopropylsulfonyl)phenyl)amino)pyrimidin-2-yl)amino)phenyl)amino)-3-oxopropanoate (4a)
To a solution of monomethyl malonate hydrochloride (0.58 g, 4.96 mmol) in dry DMF, HOBT (0.65 g, 4.80 mmol), EDCI (0.91 g, 4.77 mmol) and DIPEA (1.24 g, 9.60 mmol) were added. The resulting solution was stirred at room temperature for 0.5 h. Then, 3a (1.07 g, 2.37 mmol) was added to the solution and stirred for another 12 h. Then the reaction mixture was extracted by ethyl acetate and washed with brine. The organic layer was dried overnight with anhydrous Na2SO4, filtered and concentrated under reduced pressure. The obtained powder was purified by column chromatography eluting on silica gel with DCM/methanol (30:1) to obtain 0.43 g of 4a as a white crystal. Yield: 35%. M.p. 175.2–177.3 °C. 1H NMR (600 MHz, DMSO-d6) δ 10.09 (s, 1H), 9.51 (s, 1H), 9.46 (s, 1H), 8.59 (s, 1H), 8.27 (s, 1H), 7.85 (dd, J = 8.0, 1.6 Hz, 1H), 7.76–7.70 (m, 1H), 7.54 (d, J = 8.5 Hz, 2H), 7.44 (d, J = 9.0 Hz, 2H), 7.41–7.38 (m, 1H), 3.66 (s, 3H), 3.46–3.42 (m, 3H), 1.16 (d, J = 6.8 Hz, 6H). MS (ESI) m/z: [M + H]+: 518.3.
Methyl 4-((4-((5-chloro-4-((2-(isopropylsulfonyl)phenyl)amino)pyrimidin-2-yl)amino)phenyl)amino)-4-oxobutanoate (4b)
Purple solid. Yield: 36%. M.p. 202.2–204.9 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.88 (s, 1H), 9.47 (d, J = 10.0 Hz, 2H), 8.59 (s, 1H), 8.27 (s, 1H), 7.85 (dd, J = 8.0, 1.5 Hz, 1H), 7.73 (t, J = 7.5 Hz, 1H), 7.50 (d, J = 8.4 Hz, 2H), 7.44 (d, J = 8.9 Hz, 2H), 7.42–7.37 (m, 1H), 3.60 (s, 3H), 3.44 (dt, J = 13.6, 6.8 Hz, 1H), 2.59 (s, 4H), 1.16 (d, J = 6.8 Hz, 6H). MS (ESI) m/z: [M + H]+: 532.3.
Methyl 5-((4-((5-chloro-4-((2-(isopropylsulfonyl)phenyl)amino)pyrimidin-2-yl)amino)phenyl)amino)-5-oxopentanoate (4c)
White solid. Yield: 30%. M.p. 201.7–202.3 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.79 (s, 1H), 9.46 (d, J = 6.4 Hz, 2H), 8.59 (s, 1H), 8.26 (s, 1H), 7.85 (dd, J = 8.0, 1.5 Hz, 1H), 7.73 (t, J = 7.5 Hz, 1H), 7.50 (d, J = 8.4 Hz, 2H), 7.45 (d, J = 8.9 Hz, 2H), 7.40–7.37 (m, 1H), 3.59 (s, 3H), 3.43 (dd, J = 13.6, 6.8 Hz, 1H), 2.36 (t, J = 7.4 Hz, 2H), 2.32 (t, J = 7.4 Hz, 2H), 1.83 (p, J = 7.4 Hz, 2H), 1.16 (d, J = 6.8 Hz, 6H). MS (ESI) m/z: [M + H]+: 546.3.
Methyl 6-((4-((5-chloro-4-((2-(isopropylsulfonyl)phenyl)amino)pyrimidin-2-yl)amino)phenyl)amino)-6-oxohexanoate (4d)
Purple solid. Yield: 26%. M.p. 181.7–182.4 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.77 (s, 1H), 9.47 (d, J = 5.7 Hz, 2H), 8.60 (s, 1H), 8.27 (s, 1H), 7.85 (dd, J = 8.0, 1.5 Hz, 1H), 7.73 (t, J = 7.4 Hz, 1H), 7.50 (d, J = 8.5 Hz, 2H), 7.45 (d, J = 8.9 Hz, 2H), 7.40–7.37 (m, 1H), 3.59 (s, 3H), 3.48–3.41 (m, 1H), 2.34 (t, J = 7.0 Hz, 2H), 2.29 (t, J = 6.9 Hz, 2H), 1.61–1.55 (m, 4H), 1.16 (d, J = 6.8 Hz, 6H). MS (ESI) m/z: [M + H]+: 560.3.
Ethyl 7-((4-((5-chloro-4-((2-(isopropylsulfonyl)phenyl)amino)pyrimidin-2-yl)amino)phenyl)amino)-7-oxoheptanoate (4e)
Brown solid. Yield: 54%. M.p. 174.5–176.8 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.76 (s, 1H), 9.47 (s, 2H), 8.60 (s, 1H), 8.27 (s, 1H), 7.86 (dd, J = 8.0, 1.6 Hz, 1H), 7.73 (t, J = 7.3 Hz, 1H), 7.50 (d, J = 8.6 Hz, 2H), 7.46 (d, J = 9.0 Hz, 2H), 7.41–7.38 (m, 1H), 4.04 (t, J = 7.1 Hz, 2H), 3.49–3.41 (m, 1H), 2.28 (dt, J = 11.4, 7.4 Hz, 4H), 1.62–1.52 (m, 4H), 1.34–1.28 (m, 2H), 1.19–1.17 (m, 6H), 1.16 (d, J = 2.6 Hz, 3H). MS (ESI) m/z: [M + H]+: 588.3.
Methyl 8-((4-((5-chloro-4-((2-(isopropylsulfonyl)phenyl)amino)pyrimidin-2-yl)amino)phenyl)amino)-8-oxooctanoate (4f)
Yellow solid. Yield: 68%. M.p. 173.1–174.5 °C. 1H NMR (600 MHz, CDCl3) δ 9.62 (s, 1H), 8.55 (d, J = 7.9 Hz, 1H), 8.12 (s, 1H), 7.90 (dd, J = 7.9, 1.5 Hz, 1H), 7.64–7.60 (m, 1H), 7.46 (s, 4H), 7.25 (d, J = 5.0 Hz, 2H), 7.18 (s, 1H), 3.67 (s, 3H), 3.23 (dt, J = 13.7, 6.9 Hz, 1H), 2.30–2.36 (m, 4H), 1.77–1.72 (m, 2H), 1.66–1.62 (m, 2H), 1.43–1.36 (m, 4H), 1.31 (d, J = 6.9 Hz, 6H). MS (ESI) m/z: [M + H]+: 588.3.
Methyl 3-((3-((5-chloro-4-((2-(isopropylsulfonyl)phenyl)amino)pyrimidin-2-yl)amino)phenyl)amino)-3-oxopropanoate (4g)
White solid. Yield: 36%. M.p. 149.2–150.1 °C. 1H NMR (600 MHz, DMSO-d6) δ 10.13 (s, 1H), 9.59 (s, 1H), 9.55 (s, 1H), 8.70 (d, J = 7.5 Hz, 1H), 8.30 (d, J = 6.3 Hz, 1H), 7.86–7.81 (m, 2H), 7.72 (t, J = 7.3 Hz, 1H), 7.40–7.35 (m, 2H), 7.22–7.18 (m, 2H), 3.65 (d, J = 4.1 Hz, 3H), 3.47 (s, 2H), 3.46–3.43 (m, 1H), 1.18 (d, J = 6.8 Hz, 6H). MS (ESI) m/z: [M + H]+: 518.3.
Methyl 4-((3-((5-chloro-4-((2-(isopropylsulfonyl)phenyl)amino)pyrimidin-2-yl)amino)phenyl)amino)-4-oxobutanoate (4h)
Yellow solid. Yield: 24%. M.p. 101.2–103.5 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.92 (s, 1H), 9.54 (d, J = 7.2 Hz, 2H), 8.70 (d, J = 7.2 Hz, 1H), 8.27 (s, 1H), 7.85–7.81 (m, 2H), 7.68 (t, J = 7.4 Hz, 1H), 7.36–7.32 (m, 2H), 7.18–7.14 (m, 2H), 3.58 (s, 3H), 3.44–3.41 (m, 1H), 2.60 (d, J = 5.7 Hz, 2H), 2.57 (d, J = 5.4 Hz, 2H), 1.17 (d, J = 6.8 Hz, 6H). MS (ESI) m/z: [M + H]+: 532.3.
Methyl 5-((3-((5-chloro-4-((2-(isopropylsulfonyl)phenyl)amino)pyrimidin-2-yl)amino)phenyl)amino)-5-oxopentanoate (4i)
Yellow solid. Yield: 60%. M.p. 129.8–130.1 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.84 (s, 1H), 9.56 (s, 2H), 8.71 (d, J = 6.4 Hz, 1H), 8.28 (s, 1H), 7.89–7.82 (m, 2H), 7.68 (t, J = 7.6 Hz, 1H), 7.35 (t, J = 7.5 Hz, 2H), 7.21–7.14 (m, 2H), 3.59 (s, 3H), 3.46–3.43 (m, 1H), 2.37–2.34 (m, 4H), 1.86–1.79 (m, 2H), 1.18 (d, J = 6.8 Hz, 6H). MS (ESI) m/z: [M + H]+: 546.3.
Methyl 6-((3-((5-chloro-4-((2-(isopropylsulfonyl)phenyl)amino)pyrimidin-2-yl)amino)phenyl)amino)-6-oxohexanoate (4j)
White solid. Yield: 26%. M.p. 128.9–131.5 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.81 (s, 1H), 9.55 (s, 2H), 8.70 (d, J = 7.4 Hz, 1H), 8.29 (s, 1H), 7.85–7.82 (m, 2H), 7.68 (t, J = 7.4 Hz, 1H), 7.36 (d, J = 7.3 Hz, 1H), 7.33 (d, J = 7.8 Hz, 1H), 7.19 (d, J = 8.1 Hz, 1H), 7.17–7.14 (m, 1H), 3.58 (d, J = 4.0 Hz, 3H), 3.47–3.43 (m, 1H), 2.33 (d, J = 6.7 Hz, 2H), 2.30 (d, J = 5.1 Hz, 2H), 1.58–1.53 (m, 4H), 1.17 (d, J = 6.8 Hz, 6H). MS (ESI) m/z: [M + H]+: 560.0.
Ethyl 7-((3-((5-chloro-4-((2-(isopropylsulfonyl)phenyl)amino)pyrimidin-2-yl)amino)phenyl)amino)-7-oxoheptanoate (4k)
Yellow solid. Yield: 38%. M.p. 128.4–129.2 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.80 (s, 1H), 9.55 (d, J = 7.1 Hz, 2H), 8.71 (d, J = 6.2 Hz, 1H), 8.28 (s, 1H), 7.87–7.81 (m, 2H), 7.68 (t, J = 7.5 Hz, 1H), 7.35–7.33 (m, 2H), 7.19–7.15 (m, 2H), 4.03 (q, J = 7.0 Hz, 2H), 3.47–3.44 (m, 1H), 2.28 (t, J = 5.8 Hz, 4H), 1.57–1.54 (m, 4H), 1.36–1.23 (m, 3H), 1.17 (d, J = 6.6 Hz, 6H), 1.15 (s, 2H). MS (ESI) m/z: [M + H]+: 588.4.
Methyl 8-((3-((5-chloro-4-((2-(isopropylsulfonyl)phenyl)amino)pyrimidin-2-yl)amino)phenyl)amino)-8-oxooctanoate (4l)
White solid. Yield: 32%. M.p. 152.2–154.8 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.79 (s, 1H), 9.55 (d, J = 11.5 Hz, 2H), 8.71 (d, J = 7.7 Hz, 1H), 8.29 (s, 1H), 7.89–7.81 (m, 2H), 7.68 (t, J = 7.7 Hz, 1H), 7.38–7.30 (m, 2H), 7.21–7.12 (m, 2H), 3.58 (s, 3H), 3.45 (dt, J = 13.5, 6.8 Hz, 1H), 2.30–2.22 (m, 4H), 1.58–1.50 (m, 4H), 1.29 (dt, J = 6.9, 3.5 Hz, 4H), 1.18 (d, J = 6.8 Hz, 6H). MS (ESI) m/z: [M + H]+: 588.4.
General procedure for the synthesis of intermediates 5a–l
Sodium hydroxide (0.10 g, 2.50 mmol) was dissolved in methanol/water (80%, 10 mL) mixture and heated to 70 °C, compound 4a (0.40 g, 0.77 mmol) was slowly added to the solution and stirred under reflux for 6 h. When the reaction completed, the solvent was evaporated in vacuo, and water (40 mL) was added, after stirring for 0.5 h at room temperature. The reaction mixture was filtered, dried in an oven to give intermediate 5a which can be directly used. 5b–l were synthesised in the manner of 5a.
General procedure for the synthesis of 6a–l
N1-(2-aminophenyl)-N3-(4-((5-chloro-4-((2-(isopropylsulfonyl)phenyl)amino)pyrimidin-2-yl)amino)phenyl)malonamide (6a)
Synthesised using the preparation method of 4a using 5a (0.30 g, 0.59 mmol), o-phenylenediamine (0.06 g, 0.52 mmol), HOBT (0.17 g, 1.2 mmol), EDCI (0.23 g, 1.17 mmol) and DIPEA (0.31 g, 2.37 mmol) in 6 mL DMF, and 0.07 g of 6a was gained as white solid. Yield: 25%. M.p. 210.0–212.2 °C. 1H NMR (600 MHz, DMSO-d6) δ 10.10 (s, 1H), 9.52 (s, 1H), 9.47 (s, 1H), 9.34 (s, 1H), 8.60 (s, 1H), 8.28 (s, 1H), 7.86 (dd, J = 8.0, 1.5 Hz, 1H), 7.75 (t, J = 7.8 Hz, 1H), 7.54 (d, J = 8.4 Hz, 2H), 7.48 (d, J = 8.9 Hz, 2H), 7.40 (dd, J = 11.6, 4.4 Hz, 1H), 7.14 (dd, J = 7.8, 1.1 Hz, 1H), 6.95–6.91 (m, 1H), 6.72 (dd, J = 8.0, 1.2 Hz, 1H), 6.57–6.52 (m, 1H), 4.97 (s, 2H), 3.48–3.41 (m, 3H), 1.17 (d, J = 6.8 Hz, 6H). 13 C NMR (151 MHz, DMSO-d6) δ 166.15, 166.07, 158.15, 155.75, 155.36, 143.08, 138.51, 136.13, 135.26, 133.82, 131.41, 126.85, 126.40, 125.13, 124.65, 124.16, 123.21, 120.57, 119.98, 116.40, 115.94, 104.92, 55.33, 45.41, 15.33. HRMS m/z calcd for C28H29ClN7O4S [M + H]+: 594.1690, found: 549.1685.
N1-(2-aminophenyl)-N4-(4-((5-chloro-4-((2-(isopropylsulfonyl)phenyl)amino)pyrimidin-2-yl)amino)phenyl)succinimide (6b)
Yellow solid. Yield: 68%. M.p. 215.4–217.9 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.89 (s, 1H), 9.47 (d, J = 6.2 Hz, 2H), 9.17 (s, 1H), 8.60 (s, 1H), 8.27 (s, 1H), 7.85 (dd, J = 8.0, 1.5 Hz, 1H), 7.73 (t, J = 7.4 Hz, 1H), 7.51 (d, J = 8.2 Hz, 2H), 7.46 (d, J = 8.9 Hz, 2H), 7.38 (t, J = 7.7 Hz, 1H), 7.13 (d, J = 6.9 Hz, 1H), 6.91–6.87 (m, 1H), 6.70 (dd, J = 7.9, 1.0 Hz, 1H), 6.54–6.50 (m, 1H), 4.88 (s, 2H), 3.45 (dt, J = 13.6, 6.8 Hz, 1H), 2.64 (s, 4H), 1.16 (d, J = 6.8 Hz, 6H). 13 C NMR (151 MHz, DMSO-d6) δ 170.93, 170.60, 158.18, 155.73, 155.33, 142.71, 138.52, 135.68, 135.26, 134.31, 131.40, 126.36, 126.08, 125.00, 124.56, 124.11, 123.77, 120.59, 119.77, 116.47, 116.09, 104.86, 55.34, 32.01, 31.28, 15.33. HRMS m/z calcd for C29H31ClN7O4S [M + H]+: 608.1847, found: 608.1843.
N1-(2-aminophenyl)-N5-(4-((5-chloro-4-((2-(isopropylsulfonyl)phenyl)amino)pyrimidin-2-yl)amino)phenyl)glutaramide (6c)
White solid. Yield: 47%. M.p. 159.3–161.2 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.82 (s, 1H), 9.47 (d, J = 6.6 Hz, 2H), 9.10 (s, 1H), 8.60 (s, 1H), 8.27 (s, 1H), 7.85 (dd, J = 8.0, 1.5 Hz, 1H), 7.73 (t, J = 7.4 Hz, 1H), 7.46–7.52 (m, 4H), 7.41–7.37 (m, 1H), 7.18 (dd, J = 7.8, 1.0 Hz, 1H), 6.91–6.86 (m, 1H), 6.71 (dd, J = 8.0, 1.2 Hz, 1H), 6.56–6.51 (m, 1H), 4.83 (s, 2H), 3.42–3.46 (m, 1H), 2.40–2.33 (m, 4H), 1.94–1.88 (m, 2H), 1.16 (d, J = 6.8 Hz, 6H). 13 C NMR (151 MHz, DMSO-d6) δ 171.23, 170.92, 158.18, 155.74, 155.34, 142.38, 138.52, 135.70, 135.25, 134.32, 131.41, 126.19, 125.85, 125.01, 124.66, 124.13, 123.96, 120.52, 119.86, 116.59, 116.31, 104.84, 55.33, 36.06, 35.50, 21.73, 15.33. HRMS m/z calcd for C30H33ClN7O4S [M + H]+: 622.2003, found: 622.2008.
N1-(2-aminophenyl)-N6-(4-((5-chloro-4-((2-(isopropylsulfonyl)phenyl)amino)pyrimidin-2-yl)amino)phenyl)adipamide (6d)
White solid. Yield: 34%. M.p. 209.8–211.1 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.80 (s, 1H), 9.48 (s, 1H), 9.46 (s, 1H), 9.11 (s, 1H), 8.59 (s, 1H), 8.27 (s, 1H), 7.84 (dd, J = 8.0, 1.4 Hz, 1H), 7.73 (t, J = 7.6 Hz, 1H), 7.50 (d, J = 8.1 Hz, 2H), 7.46 (d, J = 8.9 Hz, 2H), 7.38 (t, J = 7.6 Hz, 1H), 7.16–7.13 (m, 1H), 6.90–6.87 (m, 1H), 6.71 (dd, J = 7.9, 1.0 Hz, 1H), 6.54–6.51 (m, 1H), 4.83 (s, 2H), 3.48–3.40 (m, 1H), 2.35 (s, 2H), 2.32 (s, 2H), 1.64 (d, J = 3.1 Hz, 4H), 1.16 (d, J = 6.8 Hz, 6H). 13 C NMR (151 MHz, DMSO-d6) δ 171.50, 171.18, 158.19, 155.73, 155.32, 142.38, 138.52, 135.70, 135.24, 134.34, 131.40, 126.19, 125.81, 124.95, 124.49, 124.08, 124.02, 120.56, 119.87, 116.65, 116.37, 104.85, 55.35, 36.69, 36.15, 25.58, 25.43, 15.33. HRMS m/z calcd for C31H35ClN7O4S [M + H]+: 636.2160, found: 636.2157.
N1-(2-aminophenyl)-N7-(4-((5-chloro-4-((2-(isopropylsulfonyl)phenyl)amino)pyrimidin-2-yl)amino)phenyl)heptanediamide (6e)
White solid. Yield: 40%. M.p. 215.4–216.9 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.77 (s, 1H), 9.47 (d, J = 4.5 Hz, 2H), 9.08 (s, 1H), 8.60 (s, 1H), 8.27 (s, 1H), 7.85 (dd, J = 8.0, 1.6 Hz, 1H), 7.73 (t, J = 7.4 Hz, 1H), 7.50 (d, J = 8.5 Hz, 2H), 7.46 (d, J = 9.0 Hz, 2H), 7.41–7.36 (m, 1H), 7.15 (dd, J = 7.8, 1.2 Hz, 1H), 6.90–6.86 (m, 1H), 6.71 (dd, J = 8.0, 1.3 Hz, 1H), 6.58–6.49 (m, 1H), 4.81 (s, 2H), 3.45 (dt, J = 13.6, 6.8 Hz, 1H), 2.39–2.29 (m, 4H), 1.67–1.60 (m, 4H), 1.40–1.33 (m, 2H), 1.17 (d, J = 6.8 Hz, 6H). 13 C NMR (151 MHz, DMSO-d6) δ 171.60, 171.26, 158.19, 155.73, 155.32, 142.35, 138.52, 135.67, 135.24, 134.36, 131.40, 126.15, 125.76, 124.97, 124.53, 124.08, 124.05, 120.55, 119.84, 116.64, 116.36, 104.86, 55.34, 36.70, 36.14, 28.84, 25.61, 25.48, 15.33. HRMS m/z calcd for C32H37ClN7O4S [M + H]+: 650.2316, found: 650.2312.
N1-(2-aminophenyl)-N8-(4-((5-chloro-4-((2-(isopropylsulfonyl)phenyl)amino)pyrimidin-2-yl)amino)phenyl)octanediamide (6f)
Yellow solid. Yield: 60%. M.p. 201.2–203.8 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.76 (s, 1H), 9.46 (s, 2H), 9.08 (s, 1H), 8.59 (s, 1H), 8.26 (s, 1H), 7.84 (dd, J = 8.0, 1.6 Hz, 1H), 7.73 (t, J = 7.3 Hz, 1H), 7.42–7.55 (m, 4H), 7.41–7.35 (m, 1H), 7.14 (dd, J = 7.8, 1.2 Hz, 1H), 6.91–6.84 (m, 1H), 6.70 (dd, J = 8.0, 1.3 Hz, 1H), 6.59 − 6.50 (m, 1H), 4.80 (s, 2H), 3.41–4.46 (m, 1H), 2.23–2.35 (m, 4H), 1.64–1.54 (m, 4H), 1.34 (dd, J = 6.9, 3.4 Hz, 4H), 1.16 (d, J = 6.8 Hz, 6H). 13 C NMR (151 MHz, DMSO-d6) δ 171.61, 171.27, 158.18, 155.74, 155.33, 142.35, 138.52, 135.66, 135.24, 134.36, 131.40, 126.15, 125.75, 125.00, 124.54, 124.10, 124.06, 120.54, 119.83, 116.65, 116.36, 104.85, 55.33, 36.79, 36.23, 29.02, 29.00, 25.71, 25.60, 15.33. HRMS m/z calcd for C33H39ClN7O4S [M + H]+: 664.2473, found: 664.2477.
N1-(2-aminophenyl)-N3-(3-((5-chloro-4-((2-(isopropylsulfonyl)phenyl)amino)pyrimidin-2-yl)amino)phenyl)malonamide (6g)
White solid. Yield: 54%. M.p. 135.4–137.2 °C. 1H NMR (600 MHz, DMSO-d6) δ 10.14 (s, 1H), 9.60 (s, 1H), 9.55 (s, 1H), 9.35 (s, 1H), 8.70 (d, J = 6.7 Hz, 1H), 8.30 (s, 1H), 7.84 (d, J = 8.2 Hz, 2H), 7.73 (t, J = 7.8 Hz, 1H), 7.42–7.34 (m, 2H), 7.25 (d, J = 8.0 Hz, 1H), 7.20 (t, J = 8.0 Hz, 1H), 7.14 (d, J = 7.8 Hz, 1H), 6.93 (t, J = 7.7 Hz, 1H), 6.72 (d, J = 7.9 Hz, 1H), 6.54 (t, J = 7.5 Hz, 1H), 4.96 (s, 2H), 3.48 (s, 2H), 3.46–4.43 (m, 1H), 1.17 (d, J = 6.8 Hz, 6H). 13 C NMR (151 MHz, DMSO-d6) δ 166.36, 166.12, 158.15, 155.59, 155.25, 143.08, 140.80, 139.47, 138.47, 135.44, 131.39, 129.06, 126.85, 126.39, 124.50, 124.09, 123.96, 123.18, 116.40, 115.94, 115.82, 113.84, 111.55, 105.47, 55.39, 45.47, 15.33. HRMS m/z calcd for C28H29ClN7O4S [M + H]+: 594.1690, found: 594.1684.
N1-(2-aminophenyl)-N4-(3-((5-chloro-4-((2-(isopropylsulfonyl)phenyl)amino)pyrimidin-2-yl)amino)phenyl)succinimide (6h)
Yellow solid. Yield: 39%. M.p. 135.9–138.6 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.93 (s, 1H), 9.55 (d, J = 1.5 Hz, 2H), 9.16 (s, 1H), 8.70 (d, J = 7.2 Hz, 1H), 8.29 (s, 1H), 7.84–7.81 (m, 2H), 7.71 (t, J = 7.6 Hz, 1H), 7.36 (d, J = 7.5 Hz, 1H), 7.33 (d, J = 8.2 Hz, 1H), 7.22 (d, J = 8.1 Hz, 1H), 7.16 (t, J = 8.0 Hz, 1H), 7.13 (d, J = 7.7 Hz, 1H), 6.88 (dd, J = 11.1, 4.2 Hz, 1H), 6.69 (d, J = 7.9 Hz, 1H), 6.51 (dd, J = 11.0, 4.1 Hz, 1H), 4.86 (s, 2H), 3.45 (dt, J = 13.6, 6.8 Hz, 1H), 2.67–2.63 (m, 4H), 1.18–1.16 (m, 6H). 13 C NMR (151 MHz, DMSO-d6) δ 170.90, 170.88, 158.19, 155.61, 155.21, 142.67, 140.67, 139.88, 138.49, 135.42, 131.36, 128.92, 126.31, 126.00, 124.40, 124.04, 123.91, 123.77, 116.44, 116.08, 115.51, 113.83, 111.61, 105.37, 55.41, 32.05, 31.16, 15.34. HRMS m/z calcd for C29H31ClN7O4S [M + H]+: 608.1847, found: 608.1845.
N1-(2-aminophenyl)-N5-(3-((5-chloro-4-((2-(isopropylsulfonyl)phenyl)amino)pyrimidin-2-yl)amino)phenyl)glutaramide (6i)
Yellow solid. Yield: 43%. M.p. 200.6–201.7 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.87 (s, 1H), 9.56 (s, 2H), 9.11 (s, 1H), 8.71 (d, J = 6.9 Hz, 1H), 8.29 (s, 1H), 7.87 (s, 1H), 7.84–7.81 (m, 1H), 7.69 (t, J = 7.6 Hz, 1H), 7.37–7.34 (m, 2H), 7.23 (d, J = 8.1 Hz, 1H), 7.20–7.17 (m, 2H), 6.91–6.87 (m, 1H), 6.72 (dd, J = 8.0, 1.2 Hz, 1H), 6.56–6.51 (m, 1H), 4.84 (s, 2H), 3.47–3.45 (m, 1H), 2.40–2.37 (m, 4H), 1.93–1.87 (m, 2H), 1.17 (d, J = 6.8 Hz, 6H). 13 C NMR (151 MHz, DMSO-d6) δ 171.23, 158.19, 155.62, 155.20, 142.37, 140.66, 139.90, 138.51, 135.35, 131.37, 128.89, 126.19, 125.84, 124.38, 124.00, 123.97, 123.87, 116.60, 116.32, 115.54, 113.90, 111.71, 105.37, 55.42, 36.14, 35.51, 21.71, 15.34. HRMS m/z calcd for C30H33ClN7O4S [M + H]+: 622.2003, found: 622.2001.
N1-(2-aminophenyl)-N6-(3-((5-chloro-4-((2-(isopropylsulfonyl)phenyl)amino)pyrimidin-2-yl)amino)phenyl)adipamide (6j)
White solid. Yield: 39%. M.p. 145.8–147.6 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.83 (s, 1H), 9.55 (d, J = 4.2 Hz, 2H), 9.10 (s, 1H), 8.71 (d, J = 7.4 Hz, 1H), 8.29 (s, 1H), 7.86–7.82 (m, 2H), 7.69 (t, J = 7.6 Hz, 1H), 7.36 (d, J = 7.5 Hz, 1H), 7.33 (d, J = 8.5 Hz, 1H), 7.20 (d, J = 8.1 Hz, 1H), 7.18–7.14 (m, 2H), 6.90–6.87 (m, 1H), 6.71 (dd, J = 7.9, 1.0 Hz, 1H), 6.59–6.50 (m, 1H), 4.81 (s, 2H), 3.45 (dd, J = 13.6, 6.8 Hz, 1H), 2.34 (d, J = 5.4 Hz, 4H), 1.63 (s, 4H), 1.18 (d, J = 6.8 Hz, 6H). 13 C NMR (151 MHz, DMSO-d6) δ 171.46, 158.19, 155.62, 155.19, 142.37, 140.66, 139.89, 138.51, 135.35, 131.37, 128.90, 126.18, 125.78, 124.37, 124.00, 123.86, 116.63, 116.35, 115.53, 113.88, 111.70, 105.36, 55.41, 36.74, 36.13, 32.01, 29.90, 25.56, 25.38, 15.34. HRMS m/z calcd for C31H35ClN7O4S [M + H]+: 636.2160, found: 636.2161.
N1-(2-aminophenyl)-N7-(3-((5-chloro-4-((2-(isopropylsulfonyl)phenyl)amino)pyrimidin-2-yl)amino)phenyl)heptanediamide (6k)
Yellow solid. Yield: 30%. M.p. 108.4–109.8 °C. 1H NMR (600 MHz, CDCl3) δ 9.52 (s, 1H), 8.50 (d, J = 8.3 Hz, 1H), 8.07 (s, 1H), 8.02 (s, 1H), 7.87 (s, 1H), 7.79 (d, J = 7.9 Hz, 1H), 7.62 (s, 1H), 7.50–7.45 (m, 2H), 7.28 (d, J = 7.3 Hz, 1H), 7.12 (t, J = 7.2 Hz, 1H), 7.09–7.04 (m, 3H), 6.88 (t, J = 7.6 Hz, 1H), 6.63–6.59 (m, 2H), 5.22 (s, 2H), 3.15–3.13 (m, 1H), 2.29 (t, J = 6.6 Hz, 2H), 2.22 (t, J = 6.5 Hz, 2H), 1.61–1.58 (m, 4H), 1.30 (d, J = 4.9 Hz, 2H), 1.21 (d, J = 6.7 Hz, 6H). 13 C NMR (151 MHz, DMSO-d6) δ 171.58, 171.55, 158.19, 155.62, 155.19, 142.30, 140.64, 139.93, 138.51, 135.35, 131.37, 128.89, 126.12, 125.73, 124.35, 124.07, 123.98, 123.84, 116.65, 116.35, 115.50, 113.87, 111.68, 105.36, 55.41, 36.73, 36.13, 28.83, 25.61, 25.43, 15.34. HRMS m/z calcd for C32H37ClN7O4S [M + H]+: 650.2316, found: 650.2309.
N1-(2-aminophenyl)-N8-(3-((5-chloro-4-((2-(isopropylsulfonyl)phenyl)amino)pyrimidin-2-yl)amino)phenyl)octanediamide (6l)
Yellow solid. Yield: 89%. M.p. 105.2–107.3 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.80 (s, 1H), 9.55 (d, J = 7.9 Hz, 2H), 9.09 (s, 1H), 8.71 (d, J = 7.5 Hz, 1H), 8.29 (s, 1H), 7.86–7.81 (m, 2H), 7.68 (t, J = 7.6 Hz, 1H), 7.38–7.32 (m, 2H), 7.20–7.13 (m, 3H), 6.90–6.86 (m, 1H), 6.71 (dd, J = 8.0, 1.2 Hz, 1H), 6.55–6.51 (m, 1H), 4.86 (s, 2H), 3.47–3.43 (m, 1H), 2.34–2.26 (m, 4H), 1.63–1.58 (m, 4H), 1.35–1.32 (m, 4H), 1.17 (d, J = 6.8 Hz, 6H). 13 C NMR (151 MHz, DMSO-d6) δ 171.67, 171.62, 158.19, 155.63, 155.19, 142.31, 140.63, 139.90, 138.50, 135.35, 131.38, 128.91, 126.19, 125.77, 124.34, 124.06, 123.98, 123.86, 116.71, 116.40, 115.54, 113.91, 111.76, 105.35, 55.43, 36.82, 36.21, 32.00, 28.99, 25.70, 25.54, 15.32. HRMS m/z calcd for C33H39ClN7O4S [M + H]+: 664.2473, found: 664.2477.
General procedure for the synthesis of intermediates 8a–b
Synthesised using the preparation method of 3a using 1 (1.50 g, 4.30 mmol), p-aminobenzoic acid (0.89 g, 6.50 mmol) in 40 mL absolute ethanol followed by the addition of the HCl solution (37%, 0.8 mL), and 1.70 g of 8a was gained as white solid. 8b was synthesised in the manner of 8a.
General procedure for the synthesis of intermediates 9a–j
Methyl 3–(4-((5-chloro-4-((2-(isopropylsulfonyl)phenyl)amino)pyrimidin-2-yl)amino)benzamido)propanoate (9a)
Compounds 9a–j was synthesised according to the preparation procedure of 4a using 8a (1.00 g, 2.24 mmol), methyl 3-aminopropionate (0.47 g, 3.36 mmol), HOBT (0.66 g, 4.89 mmol), EDCI (0.92 g, 4.80 mmol) and DIPEA (1.22 g, 9.46 mmol) in 10 mL DMF, and 0.91 g of 9a was gained as white solid. Yield: 77%. M.p. 155.3–157.2 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.82 (s, 1H), 9.48 (s, 1H), 8.56 (d, J = 7.3 Hz, 1H), 8.38 (t, J = 5.5 Hz, 1H), 8.35 (s, 1H), 7.88 (dd, J = 8.0, 1.3 Hz, 1H), 7.83–7.80 (m, 1H), 7.72 (d, J = 8.9 Hz, 2H), 7.69 (d, J = 9.0 Hz, 2H), 7.44 (t, J = 7.6 Hz, 1H), 3.61 (s, 3H), 3.49–3.45 (m, 3H), 2.59 (t, J = 7.0 Hz, 2H), 1.16 (d, J = 6.8 Hz, 6H). MS (ESI) m/z: [M + H]+: 532.3.
Methyl 4–(4-((5-chloro-4-((2-(isopropylsulfonyl)phenyl)amino)pyrimidin-2-yl)amino)benzamido)butanoate (9b)
White solid. Yield: 47%. M.p. 195.2–196.0 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.81 (s, 1H), 9.48 (s, 1H), 8.56 (d, J = 7.3 Hz, 1H), 8.35 (s, 1H), 8.30 (t, J = 5.6 Hz, 1H), 7.88 (dd, J = 7.9, 1.5 Hz, 1H), 7.85–7.79 (m, 1H), 7.73 (s, 1H), 7.72 (s, 1H), 7.70 (s, 1H), 7.68 (s, 1H), 7.44 (t, J = 7.6 Hz, 1H), 3.58 (s, 3H), 3.50–3.42 (m, 1H), 3.28–3.23 (m, 2H), 2.36 (t, J = 7.4 Hz, 2H), 1.80–1.75 (m, 2H), 1.16 (d, J = 6.8 Hz, 6H). MS (ESI) m/z: [M + H]+: 546.3.
Methyl 5–(4-((5-chloro-4-((2-(isopropylsulfonyl)phenyl)amino)pyrimidin-2-yl)amino)benzamido)pentanoate (9c)
Yellow solid. Yield: 68%. M.p. 167.4–169.2 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.81 (s, 1H), 9.49 (s, 1H), 8.57 (d, J = 6.7 Hz, 1H), 8.35 (s, 1H), 8.28 (t, J = 5.6 Hz, 1H), 7.88 (dd, J = 7.9, 1.2 Hz, 1H), 7.84–7.80 (m, 1H), 7.73 (d, J = 8.8 Hz, 2H), 7.70 (d, J = 8.7 Hz, 2H), 7.44 (t, J = 7.6 Hz, 1H), 3.58 (s, 3H), 3.49–3.42 (m, 1H), 3.25 (dd, J = 12.4, 6.4 Hz, 2H), 2.34 (t, J = 7.2 Hz, 2H), 1.60–1.55 (m, 2H), 1.55–1.50 (m, 2H), 1.17 (d, J = 6.8 Hz, 6H). MS (ESI) m/z: [M + H]+: 560.3.
Methyl 6–(4-((5-chloro-4-((2-(isopropylsulfonyl)phenyl)amino)pyrimidin-2-yl)amino)benzamido)hexanoate (9d)
White solid. Yield: 59%. M.p. 181.3–182.6 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.81 (s, 1H), 9.49 (s, 1H), 8.57 (d, J = 7.2 Hz, 1H), 8.36 (s, 1H), 8.26 (t, J = 5.6 Hz, 1H), 7.89 (dd, J = 8.0, 1.5 Hz, 1H), 7.84–7.80 (m, 1H), 7.74 (s, 1H), 7.72 (s, 1H), 7.71 (s, 1H), 7.69 (s, 1H), 7.47–7.43 (m, 1H), 3.58 (s, 3H), 3.50–3.43 (m, 1H), 3.25–3.21 (m, 2H), 2.31 (t, J = 7.4 Hz, 2H), 1.59–1.54 (m, 2H), 1.54–1.49 (m, 2H), 1.35–1.28 (m, 2H), 1.17 (d, J = 6.8 Hz, 6H). MS (ESI) m/z: [M + H]+: 574.3.
Methyl 7–(4-((5-chloro-4-((2-(isopropylsulfonyl)phenyl)amino)pyrimidin-2-yl)amino)benzamido)heptanoate (9e)
White solid. Yield: 76%. M.p. 154.1–156.8 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.81 (s, 1H), 9.49 (s, 1H), 8.57 (d, J = 7.1 Hz, 1H), 8.36 (s, 1H), 8.25 (t, J = 5.6 Hz, 1H), 7.89 (dd, J = 7.9, 1.3 Hz, 1H), 7.85–7.80 (m, 1H), 7.73 (d, J = 8.8 Hz, 2H), 7.70 (d, J = 8.7 Hz, 2H), 7.45 (t, J = 7.6 Hz, 1H), 3.58 (s, 3H), 3.49–3.46 (m, 1H), 3.25–3.21 (m, 2H), 2.30 (t, J = 7.4 Hz, 2H), 1.55–1.51 (m, 4H), 1.30 (t, J = 3.5 Hz, 4H), 1.17 (d, J = 6.8 Hz, 6H). MS (ESI) m/z: [M + H]+: 588.3.
Methyl 3–(3-((5-chloro-4-((2-(isopropylsulfonyl)phenyl)amino)pyrimidin-2-yl)amino)benzamido)propanoate (9f)
White solid. Yield: 49%. M.p. 152.0–153.7 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.69 (s, 1H), 9.57 (s, 1H), 8.65 (d, J = 6.4 Hz, 1H), 8.46 (t, J = 5.5 Hz, 1H), 8.32 (d, J = 6.4 Hz, 1H), 8.03 (s, 1H), 7.84 (dd, J = 8.0, 1.5 Hz, 1H), 7.77 (d, J = 7.6 Hz, 1H), 7.68 (t, J = 7.6 Hz, 1H), 7.41 (d, J = 7.8 Hz, 1H), 7.38–7.34 (m, 2H), 3.60 (s, 3H), 3.48 (dd, J = 10.7, 5.1 Hz, 2H), 3.47–3.43 (m, 1H), 2.57 (t, J = 7.0 Hz, 2H), 1.17 (d, J = 6.8 Hz, 6H). MS (ESI) m/z: [M + H]+: 532.3.
Methyl 4–(3-((5-chloro-4-((2-(isopropylsulfonyl)phenyl)amino)pyrimidin-2-yl)amino)benzamido)butanoate (9g)
White solid. Yield: 35%. M.p. 149.3–150.8 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.69 (s, 1H), 9.57 (s, 1H), 8.66 (d, J = 6.3 Hz, 1H), 8.40 (t, J = 5.6 Hz, 1H), 8.33 (s, 1H), 8.04 (s, 1H), 7.84 (dd, J = 8.0, 1.5 Hz, 1H), 7.77 (d, J = 7.6 Hz, 1H), 7.67 (t, J = 7.6 Hz, 1H), 7.43 (d, J = 7.7 Hz, 1H), 7.37–7.33 (m, 2H), 3.58 (s, 3H), 3.47–3.44 (m, 1H), 3.28–3.25 (m, 2H), 2.36 (t, J = 7.4 Hz, 2H), 1.76 (p, J = 7.2 Hz, 2H), 1.17 (d, J = 6.8 Hz, 6H). MS (ESI) m/z: [M + H]+: 546.3.
Methyl 5–(3-((5-chloro-4-((2-(isopropylsulfonyl)phenyl)amino)pyrimidin-2-yl)amino)benzamido)pentanoate (9h)
Yellow solid. Yield: 47%. M.p. 75.4–77.1 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.68 (s, 1H), 9.57 (s, 1H), 8.66 (d, J = 6.3 Hz, 1H), 8.37 (t, J = 5.6 Hz, 1H), 8.32 (s, 1H), 8.03 (s, 1H), 7.84 (dd, J = 7.9, 1.4 Hz, 1H), 7.76 (d, J = 7.5 Hz, 1H), 7.67 (t, J = 7.7 Hz, 1H), 7.43 (d, J = 7.7 Hz, 1H), 7.35 (dd, J = 8.6, 4.8 Hz, 1H), 7.33 (t, J = 5.9 Hz, 1H), 3.58 (s, 3H), 3.47–3.44 (m, 1H), 3.26–3.23 (m, 2H), 2.33 (t, J = 7.2 Hz, 2H), 1.59–1.53 (m, 2H), 1.53–1.49 (m, 2H), 1.17 (d, J = 6.8 Hz, 6H). MS (ESI) m/z: [M + H]+: 560.3.
Methyl 6–(3-((5-chloro-4-((2-(isopropylsulfonyl)phenyl)amino)pyrimidin-2-yl)amino)benzamido)hexanoate (9i)
Yellow solid. Yield: 60%. M.p. 99.6–101.5 °C. 1H NMR (600 MHz, CDCl3) δ 9.66 (s, 1H), 8.58 (d, J = 8.3 Hz, 1H), 8.14 (s, 1H), 7.91 (s, 1H), 7.89–7.86 (m, 1H), 7.73 (dd, J = 8.0, 1.4 Hz, 1H), 7.64 (s, 1H), 7.56 (t, J = 7.8 Hz, 1H), 7.43 (d, J = 7.7 Hz, 1H), 7.33 (t, J = 7.9 Hz, 1H), 7.22 (t, J = 7.6 Hz, 1H), 6.49 (d, J = 5.0 Hz, 1H), 3.65 (s, 3H), 3.42–3.39 (m, 2H), 3.26–3.23 (m, 1H), 2.32 (t, J = 7.4 Hz, 2H), 1.67–1.64 (m, 2H), 1.62–1.56 (m, 2H), 1.41–1.37 (m, 2H), 1.30 (d, J = 6.9 Hz, 6H). MS (ESI) m/z: [M + H]+: 574.3.
Methyl 7–(3-((5-chloro-4-((2-(isopropylsulfonyl)phenyl)amino)pyrimidin-2-yl)amino)benzamido)heptanoate (9j)
Yellow solid. Yield: 59%. M.p. 128.1–129.4 °C. 1H NMR (600 MHz, CDCl3) δ 9.66 (s, 1H), 8.57 (t, J = 8.1 Hz, 1H), 8.15 (d, J = 6.3 Hz, 1H), 7.92–7.87 (m, 2H), 7.73 (dd, J = 8.1, 1.2 Hz, 1H), 7.59–7.57 (m, 1H), 7.51 (s, 1H), 7.41 (d, J = 7.8 Hz, 1H), 7.34 (t, J = 7.9 Hz, 1H), 7.23 (t, J = 7.6 Hz, 1H), 6.31 (t, J = 5.5 Hz, 1H), 3.66 (s, 3H), 3.41–3.38 (m, 2H), 3.27–3.21 (m, 1H), 2.31 (t, J = 7.5 Hz, 2H), 1.65–1.62 (m, 2H), 1.60–1.57 (m, 2H), 1.37 (dd, J = 9.7, 6.1 Hz, 4H), 1.31 (d, J = 6.9 Hz, 6H). MS (ESI) m/z: [M + H]+: 588.5.
General procedure for the synthesis of intermediates 10a–j
Synthesised using the preparation method of 5a using 9a (0.91 g, 1.70 mmol), NaOH (0.22 g, 5.50 mmol) in MeOH/H2O solution (80%, 10 mL), and 0.79 g of 10a was gained as white solid. 10b–j were synthesised in the manner of 10a.
General procedure for the synthesis of 11a–j
N-(3-((2-aminophenyl)amino)-3-oxopropyl)-4-((5-chloro-4-((2-(isopropylsulfonyl)phenyl)amino)pyrimidin-2-yl)amino)benzamide (11a)
Compounds 11a–j was synthesised according to the preparation procedure of 4a, using 10a (0.78 g, 1.50 mmol), o-phenylenediamine (0.17 g, 1.50 mmol), HOBT (0.40 g, 3.00 mmol), EDCI (0.58 g, 3.00 mmol) and DIPEA (0.77 g, 6.00 mmol) in 10 mL DMF, and 0.50 g of 11a was gained as white solid. Yield: 55%. M.p. 215.2–217.7 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.82 (s, 1H), 9.49 (s, 1H), 9.17 (s, 1H), 8.56 (d, J = 6.9 Hz, 1H), 8.43 (t, J = 5.5 Hz, 1H), 8.35 (s, 1H), 7.88 (dd, J = 7.9, 1.3 Hz, 1H), 7.83–7.79 (m, 1H), 7.75 (d, J = 8.7 Hz, 2H), 7.70 (d, J = 8.6 Hz, 2H), 7.43 (t, J = 7.6 Hz, 1H), 7.16 (d, J = 6.9 Hz, 1H), 6.92–6.88 (m, 1H), 6.71 (dd, J = 7.9, 0.9 Hz, 1H), 6.55–6.51 (m, 1H), 4.86 (s, 2H), 3.58–3.55 (m, 2H), 3.47–3.44 (m, 1H), 2.62 (t, J = 7.0 Hz, 2H), 1.16 (d, J = 6.8 Hz, 6H). 13 C NMR (151 MHz, DMSO-d6) δ 170.05, 166.42, 157.72, 155.67, 155.56, 143.24, 142.65, 138.30, 135.39, 131.45, 128.24, 127.74, 126.38, 126.09, 125.61, 124.99, 124.56, 123.70, 118.47, 116.50, 116.23, 105.95, 55.30, 36.57, 36.46, 15.32. HRMS m/z calcd for C29H31ClN7O4S [M + H]+: 608.1847, found: 608.1845.
N-(4-((2-aminophenyl)amino)-4-oxobutyl)-4-((5-chloro-4-((2-(isopropylsulfonyl)phenyl)amino)pyrimidin-2-yl)amino)benzamide (11b)
White solid. Yield: 36%. M.p. 158.9–160.3 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.82 (s, 1H), 9.49 (s, 1H), 9.13 (s, 1H), 8.56 (d, J = 7.2 Hz, 1H), 8.38–8.33 (m, 2H), 7.88 (dd, J = 8.0, 1.4 Hz, 1H), 7.84–7.80 (m, 1H), 7.75 (d, J = 8.8 Hz, 2H), 7.70 (d, J = 8.6 Hz, 2H), 7.44 (t, J = 7.6 Hz, 1H), 7.15 (dd, J = 7.8, 1.0 Hz, 1H), 6.91–6.87 (m, 1H), 6.72 (dd, J = 8.0, 1.1 Hz, 1H), 6.55–6.51 (m, 1H), 4.97 (s, 2H), 3.50–3.42 (m, 1H), 3.31 (d, J = 6.8 Hz, 2H).2.38 (t, J = 7.4 Hz, 2H), 1.89–1.83 (m, 2H), 1.16 (d, J = 6.8 Hz, 6H). 13 C NMR (151 MHz, DMSO-d6) δ 171.40, 166.30, 157.73, 155.69, 155.57, 143.19, 142.43, 138.31, 135.41, 131.46, 128.25, 127.84, 126.23, 125.94, 125.60, 124.99, 124.58, 123.95, 118.43, 116.61, 116.30, 105.94, 55.28, 39.21, 33.77, 26.00, 15.32. HRMS m/z calcd for C30H33ClN7O4S [M + H]+: 622.2003, found: 622.2005.
N-(5-((2-aminophenyl)amino)-5-oxopentyl)-4-((5-chloro-4-((2-(isopropylsulfonyl)phenyl)amino)pyrimidin-2-yl)amino)benzamide (11c)
White solid. Yield: 52%. M.p. 184.3–186.2 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.81 (s, 1H), 9.49 (s, 1H), 9.10 (s, 1H), 8.56 (d, J = 7.0 Hz, 1H), 8.36 (s, 1H), 8.31 (t, J = 5.6 Hz, 1H), 7.88 (dd, J = 8.0, 1.4 Hz, 1H), 7.84–7.80 (m, 1H), 7.74 (d, J = 8.7 Hz, 2H), 7.70 (d, J = 8.6 Hz, 2H), 7.44 (t, J = 7.6 Hz, 1H), 7.17 (d, J = 7.0 Hz, 1H), 6.91–6.87 (m, 1H), 6.73–6.70 (m, 1H), 6.55–6.51 (m, 1H), 4.83 (s, 2H), 3.48–3.44 (m, 1H), 3.31–3.28 (m, 2H), 2.36 (t, J = 7.3 Hz, 2H), 1.68–1.62 (m, 2H), 1.60–1.55 (m, 2H), 1.17 (d, J = 6.8 Hz, 6H). 13 C NMR (151 MHz, DMSO-d6) δ 171.55, 166.15, 157.74, 155.68, 155.56, 143.14, 142.34, 138.31, 135.39, 131.45, 128.20, 127.93, 126.15, 125.75, 125.57, 124.96, 124.56, 124.02, 118.45, 116.61, 116.33, 105.93, 55.28, 35.98, 29.46, 23.41, 22.57, 15.32. HRMS m/z calcd for C31H35ClN7O4S [M + H]+: 636.2160, found: 636.2158.
N-(6-((2-aminophenyl)amino)-6-oxohexyl)-4-((5-chloro-4-((2-(isopropylsulfonyl)phenyl)amino)pyrimidin-2-yl)amino)benzamide (11d)
White solid. Yield: 59%. M.p. 198.2–199.1 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.82 (s, 1H), 9.50 (s, 1H), 9.09 (s, 1H), 8.57 (d, J = 6.5 Hz, 1H), 8.36 (s, 1H), 8.29 (t, J = 5.5 Hz, 1H), 7.89 (dd, J = 7.9, 1.4 Hz, 1H), 7.84–7.80 (m, 1H), 7.75 (d, J = 8.7 Hz, 2H), 7.70 (d, J = 8.6 Hz, 2H), 7.44 (dd, J = 11.3, 4.0 Hz, 1H), 7.15 (dd, J = 7.8, 1.0 Hz, 1H), 6.91–6.87 (m, 1H), 6.72 (dd, J = 7.9, 1.1 Hz, 1H), 6.55–6.49 (m, 1H), 4.81 (s, 2H), 3.51–3.43 (m, 1H), 3.29–3.24 (m, 2H), 2.33 (t, J = 7.4 Hz, 2H), 1.67–1.61 (m, 2H), 1.59–1.55 (m, 2H), 1.40–1.36 (m, 2H), 1.17 (d, J = 6.8 Hz, 6H). 13 C NMR (151 MHz, DMSO-d6) δ 171.60, 166.13, 157.74, 155.67, 155.55, 143.12, 142.36, 138.32, 135.38, 131.45, 128.19, 127.96, 126.15, 125.77, 125.55, 124.93, 124.53, 124.04, 118.45, 116.63, 116.35, 105.94, 55.29, 40.53, 36.23, 29.61, 26.72, 25.61, 15.32. HRMS m/z calcd for C32H37ClN7O4S [M + H]+: 650.2316, found: 650.2307.
N-(7-((2-aminophenyl)amino)-7-oxoheptyl)-4-((5-chloro-4-((2-(isopropylsulfonyl)phenyl)amino)pyrimidin-2-yl)amino)benzamide (11e)
White solid. Yield: 42%. M.p. 163.2–165.6 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.81 (s, 1H), 9.49 (s, 1H), 9.09 (s, 1H), 8.57 (d, J = 6.9 Hz, 1H), 8.36 (s, 1H), 8.26 (t, J = 5.5 Hz, 1H), 7.88 (dd, J = 7.9, 1.2 Hz, 1H), 7.84–7.80 (m, 1H), 7.73 (d, J = 8.7 Hz, 2H), 7.69 (d, J = 8.7 Hz, 2H), 7.44 (t, J = 7.6 Hz, 1H), 7.15 (d, J = 7.2 Hz, 1H), 6.88 (t, J = 7.1 Hz, 1H), 6.71 (d, J = 7.2 Hz, 1H), 6.53 (t, J = 7.1 Hz, 1H), 4.81 (s, 2H), 3.51–3.43 (m, 1H), 3.26–3.22 (m, 2H), 2.32 (t, J = 7.4 Hz, 2H), 1.64–1.58 (m, 2H), 1.56–1.51 (m, 2H), 1.35 (d, J = 8.3 Hz, 4H), 1.17 (d, J = 6.8 Hz, 6H). 13 C NMR (151 MHz, DMSO-d6) δ 171.62, 166.12, 157.74, 156.28, 155.68, 155.56, 143.11, 142.35, 138.32, 135.38, 131.46, 128.18, 127.97, 126.15, 125.74, 125.58, 124.95, 124.55, 124.07, 118.45, 116.65, 116.37, 105.93, 55.29, 36.23, 29.68, 28.96, 26.82, 25.78, 15.32. HRMS m/z calcd for C33H39ClN7O4S [M + H]+: 664.2473, found: 664.2476.
N-(3-((2-aminophenyl)amino)-3-oxopropyl)-3-((5-chloro-4-((2-(isopropylsulfonyl)phenyl)amino)pyrimidin-2-yl)amino)benzamide (11f)
White solid. Yield: 76%. M.p. 143.2–145.0 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.69 (s, 1H), 9.56 (s, 1H), 9.17 (s, 1H), 8.66 (s, 1H), 8.49 (t, J = 5.5 Hz, 1H), 8.32 (s, 1H), 8.05 (s, 1H), 7.84 (dd, J = 8.0, 1.5 Hz, 1H), 7.79 (d, J = 7.7 Hz, 1H), 7.70 (t, J = 7.6 Hz, 1H), 7.45 (d, J = 7.7 Hz, 1H), 7.37–7.33 (m, 2H), 7.16 (dd, J = 7.8, 1.2 Hz, 1H), 6.91–6.87 (m, 1H), 6.71 (dd, J = 8.0, 1.2 Hz, 1H), 6.55–6.51 (m, 1H), 4.86 (s, 2H), 3.58–3.54 (m, 2H), 3.47–3.43 (m, 1H), 2.62 (t, J = 7.0 Hz, 2H), 1.17 (d, J = 6.8 Hz, 6H). 13 C NMR (151 MHz, DMSO-d6) δ 169.95, 166.99, 158.09, 155.69, 155.31, 142.62, 140.56, 138.42, 135.67, 135.43, 131.39, 128.77, 126.36, 126.08, 124.60, 124.09, 124.02, 123.70, 122.78, 120.92, 119.60, 116.50, 116.22, 105.65, 55.37, 36.65, 36.24, 15.34. HRMS m/z calcd for C29H31ClN7O4S [M + H]+: 608.1847, found: 608.1848.
N-(4-((2-aminophenyl)amino)-4-oxobutyl)-3-((5-chloro-4-((2-(isopropylsulfonyl)phenyl)amino)pyrimidin-2-yl)amino)benzamide (11g)
White solid. Yield: 76%. M.p. 179.3–182.1 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.69 (s, 1H), 9.58 (s, 1H), 9.12 (s, 1H), 8.66 (s, 1H), 8.43 (t, J = 5.5 Hz, 1H), 8.33 (s, 1H), 8.07 (s, 1H), 7.83 (dd, J = 7.9, 1.2 Hz, 1H), 7.78 (d, J = 7.3 Hz, 1H), 7.68 (t, J = 7.8 Hz, 1H), 7.46 (d, J = 7.7 Hz, 1H), 7.36 (d, J = 8.1 Hz, 1H), 7.34 (d, J = 8.1 Hz, 1H), 7.16 (d, J = 7.2 Hz, 1H), 6.91–6.87 (m, 1H), 6.72 (d, J = 7.2 Hz, 1H), 6.53 (t, J = 7.2 Hz, 1H), 4.86 (s, 2H), 3.46–3.44 (m, 1H), 3.34–3.31 (m, 2H), 2.38 (t, J = 7.4 Hz, 2H), 1.84 (p, J = 7.2 Hz, 2H), 1.17 (d, J = 6.8 Hz, 6H). 13 C NMR (151 MHz, DMSO-d6) δ 171.34, 166.95, 158.11, 155.73, 155.29, 142.48, 140.52, 138.44, 135.80, 135.41, 131.40, 128.74, 126.23, 125.90, 124.55, 124.01, 123.97, 123.92, 122.76, 120.98, 119.69, 116.57, 116.28, 105.62, 55.39, 39.38, 33.79, 25.87, 15.33. HRMS m/z calcd for C30H33ClN7O4S [M + H]+: 622.2003, found: 622.2006.
N-(5-((2-aminophenyl)amino)-5-oxopentyl)-3-((5-chloro-4-((2-(isopropylsulfonyl)phenyl)amino)pyrimidin-2-yl)amino)benzamide (11h)
Yellow solid. Yield: 90%. M.p. 165.8–169.3 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.68 (s, 1H), 9.57 (s, 1H), 9.09 (s, 1H), 8.66 (s, 1H), 8.40 (t, J = 5.6 Hz, 1H), 8.32 (s, 1H), 8.04 (s, 1H), 7.83 (dd, J = 8.0, 1.5 Hz, 1H), 7.76 (d, J = 7.3 Hz, 1H), 7.68 (t, J = 7.8 Hz, 1H), 7.44 (d, J = 7.7 Hz, 1H), 7.37–7.34 (m, 1H), 7.33 (dd, J = 8.7, 4.2 Hz, 1H), 7.16 (dd, J = 7.8, 1.2 Hz, 1H), 6.90–6.86 (m, 1H), 6.71 (dd, J = 8.0, 1.2 Hz, 1H), 6.55–6.54 (m, 1H), 4.82 (s, 2H), 3.49–3.41 (m, 1H), 3.27–3.24 (m, 2H), 2.35 (t, J = 7.3 Hz, 2H), 1.68–1.61 (m, 2H), 1.58–1.54 (m, 2H), 1.17 (d, J = 6.8 Hz, 6H). 13 C NMR (151 MHz, DMSO-d6) δ 171.52, 166.81, 158.11, 155.73, 155.29, 142.32, 140.49, 138.46, 138.43, 135.87, 135.40, 131.39, 128.74, 126.15, 125.73, 124.55, 124.01, 123.97, 122.72, 120.95, 119.68, 116.62, 116.33, 105.60, 55.37, 39.53, 35.94, 29.29, 23.37, 15.33. HRMS m/z calcd for C31H35ClN7O4S [M + H]+: 636.2160, found: 636.2161.
N-(6-((2-aminophenyl)amino)-6-oxohexyl)-3-((5-chloro-4-((2-(isopropylsulfonyl)phenyl)amino)pyrimidin-2-yl)amino)benzamide (11i)
Yellow solid. Yield: 68%. M.p. 132.4–135.5 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.68 (s, 1H), 9.57 (s, 1H), 9.08 (s, 1H), 8.66 (s, 1H), 8.36 (t, J = 5.5 Hz, 1H), 8.32 (s, 1H), 8.03 (s, 1H), 7.84 (dd, J = 7.9, 1.5 Hz, 1H), 7.76 (d, J = 7.2 Hz, 1H), 7.68 (t, J = 7.6 Hz, 1H), 7.43 (d, J = 7.7 Hz, 1H), 7.35 (t, J = 7.7 Hz, 1H), 7.32 (t, J = 7.9 Hz, 1H), 7.16–7.13 (m, 1H), 6.90–6.86 (m, 1H), 6.72–6.69 (m, 1H), 6.54–6.51 (m, 1H), 4.81 (s, 2H), 3.47–3.43 (m, 1H), 3.27–3.24 (m, 2H), 2.32 (t, J = 7.4 Hz, 2H), 1.65–1.59 (m, 2H), 1.56–1.53 (m, 2H), 1.38–1.34 (m, 2H), 1.17 (d, J = 6.8 Hz, 6H). 13 C NMR (151 MHz, DMSO-d6) δ 171.58, 166.78, 158.11, 155.73, 155.29, 142.35, 140.49, 138.44, 135.90, 135.40, 131.40, 128.73, 126.14, 125.76, 124.54, 124.05, 124.01, 123.96, 122.70, 120.94, 119.67, 116.64, 116.35,105.60, 55.37, 36.22, 31.76, 29.43, 26.69, 25.58, 15.34. HRMS m/z calcd for C32H37ClN7O4S [M + H]+: 650.2316, found: 650.2314.
N-(7-((2-aminophenyl)amino)-7-oxoheptyl)-3-((5-chloro-4-((2-(isopropylsulfonyl)phenyl)amino)pyrimidin-2-yl)amino)benzamide (11j)
Yellow solid. Yield: 84%. M.p. 101.5–103.4 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.68 (s, 1H), 9.57 (s, 1H), 9.10 (s, 1H), 8.66 (d, J = 6.1 Hz, 1H), 8.35 (t, J = 5.5 Hz, 1H), 8.32 (s, 1H), 8.03 (s, 1H), 7.85–7.82 (m, 1H), 7.76 (d, J = 7.4 Hz, 1H), 7.67 (t, J = 7.5 Hz, 1H), 7.43 (d, J = 7.7 Hz, 1H), 7.35 (dd, J = 8.9, 4.9 Hz, 1H), 7.33 (t, J = 6.0 Hz, 1H), 7.17–7.14 (m, 1H), 6.91–6.86 (m, 1H), 6.72 (dd, J = 7.9, 1.0 Hz, 1H), 6.55–6.51 (m, 1H), 4.83 (s, 2H), 3.46–3.43 (m, 1H), 3.26–3.24 (m, 2H), 2.32 (t, J = 7.4 Hz, 2H), 1.63–1.57 (m, 2H), 1.53–1.49 (m, 2H), 1.34 (s, 4H), 1.17 (d, J = 6.8 Hz, 6H). 13 C NMR (151 MHz, DMSO-d6) δ 171.64, 166.80, 158.12, 155.72, 155.28, 142.33, 140.47, 138.44, 135.91, 135.39, 131.40, 128.74, 126.16, 125.74, 124.50, 124.07, 123.98, 123.93, 122.72, 120.97, 119.68, 116.68, 116.39, 105.60, 55.39, 39.56, 36.23, 29.51, 28.94, 26.81, 25.77, 15.33. HRMS m/z calcd for C33H39ClN7O4S [M + H]+: 664.2473, found: 664.2464.
General procedure for the synthesis of 12a–h
N-(2-aminophenyl)-4-((5-chloro-4-((2-(isopropylsulfonyl)phenyl)amino)pyrimidin-2-yl)amino)benzamide (12a)
Compounds 12a-j was synthesised according to the preparation procedure of 4a, using 8a (1.00 g, 2.24 mmol), o-phenylenediamine (0.25 g, 2.12 mmol), HOBT (0.60 g, 4.40 mmol), EDCI (0.86 g, 4.49 mmol) and DIPEA (1.19 g, 9.20 mmol) in 10 mL DMF, and 0.22 g of 12a was gained as white solid. Yield: 30%. M.p. 207.6–209.3 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.87 (s, 1H), 9.50 (s, 2H), 8.58 (d, J = 7.9 Hz, 1H), 8.37 (s, 1H), 7.90–7.84 (m, 4H), 7.76 (d, J = 8.6 Hz, 2H), 7.45 (t, J = 7.5 Hz, 1H), 7.16 (d, J = 7.3 Hz, 1H), 6.97 (t, J = 7.6 Hz, 1H), 6.79 (d, J = 8.1 Hz, 1H), 6.61 (t, J = 7.5 Hz, 1H), 4.86 (s, 2H), 3.49– 3.45 (m, 1H), 1.18 (d, J = 6.8 Hz, 6H). 13 C NMR (151 MHz, DMSO-d6) δ 165.25, 157.71, 155.69, 155.61, 143.64, 143.56, 138.30, 135.45, 131.48, 128.91, 127.63, 127.15, 126.81, 125.04, 124.61, 124.09, 118.38, 116.77, 116.63, 109.49, 106.05, 55.29, 15.33. HRMS m/z calcd for C26H26ClN6O3S [M + H]+: 537.1476, found: 537.1479.
N-(2-amino-4,5-dichlorophenyl)-4-((5-chloro-4-((2-(isopropylsulfonyl)phenyl)amino)pyrimidin-2-yl)amino)benzamide (12b)
Pink solid. Yield: 31%. M.p. 218.4–220.5 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.87 (s, 1H), 9.55 (s, 1H), 9.52 (s, 1H), 8.60 (d, J = 7.8 Hz, 1H), 8.33 (s, 1H), 7.91 (s, 1H), 7.90 (s, 1H), 7.87 (dd, J = 8.0, 1.2 Hz, 1H), 7.85–7.78 (m, 1H), 7.80 (s, 1H), 7.78 (s, 1H), 7.46 (s, 1H), 7.42 (t, J = 7.6 Hz, 1H), 7.00 (s, 1H), 5.38 (s, 2H), 3.44–3.41 (m, 1H), 1.17 (d, J = 6.8 Hz, 6H). 13 C NMR (151 MHz, DMSO-d6) δ 165.62, 157.68, 155.66, 155.61, 143.97, 143.84, 138.31, 135.44, 131.48, 129.08, 128.23, 128.07, 127.14, 125.64, 125.00, 124.60, 123.96, 118.35, 116.59, 116.48, 106.15, 55.30, 15.33. HRMS m/z calcd for C26H24Cl3N6O3S [M + H]+: 605.0696, found: 605.0695.
N-(2-amino-4,5-dibromophenyl)-4-((5-chloro-4-((2-(isopropylsulfonyl)phenyl)amino)pyrimidin-2-yl)amino)benzamide (12c)
Yellow solid. Yield: 27%. M.p. 218.6–219.3 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.88 (s, 1H), 9.50 (s, 2H), 8.58 (d, J = 8.0 Hz, 1H), 8.37 (s, 1H), 7.91–7.87 (m, 2H), 7.87–7.84 (m, 2H), 7.77 (s, 1H), 7.76 (s, 1H), 7.55 (s, 1H), 7.45 (t, J = 7.6 Hz, 1H), 7.15 (s, 1H), 5.38 (s, 2H), 3.50–3.44 (m, 1H), 1.18 (d, J = 6.8 Hz, 6H). 13 C NMR (151 MHz, DMSO-d6) δ 165.59, 157.67, 155.67, 155.62, 144.56, 143.83, 138.29, 135.45, 131.48, 131.00, 129.07, 127.12, 125.68, 125.07, 124.70, 124.64, 120.67, 119.71, 118.34, 107.77, 106.13, 55.30, 15.33. HRMS m/z calcd for C26H24Br2ClN6O3S [M + H]+: 692.9686, found: 692.9684.
N-(2-amino-4,5-dimethylphenyl)-4-((5-chloro-4-((2-(isopropylsulfonyl)phenyl)amino)pyrimidin-2-yl)amino)benzamide (12d)
Brown solid. Yield: 44%. M.p. 192.6–194.5 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.88 (s, 1H), 9.50 (s, 1H), 9.45 (s, 1H), 8.58 (d, J = 7.0 Hz, 1H), 8.37 (s, 1H), 7.92–7.88 (m, 2H), 7.87–7.83 (m, 2H), 7.76 (s, 1H), 7.75 (s, 1H), 7.45 (t, J = 7.4 Hz, 1H), 6.92 (s, 1H), 6.59 (s, 1H), 4.59 (s, 2H), 3.49–3.45 (m, 1H), 2.12 (s, 3H), 2.09 (s, 3H)., 1.17 (d, J = 6.8 Hz, 6H). 13 C NMR (151 MHz, DMSO-d6) δ 165.08, 157.72, 155.68, 155.60, 143.47, 141.16, 138.31, 135.44, 134.28, 131.47, 128.83, 127.97, 127.71, 125.64, 125.03, 124.60, 124.13, 121.83, 118.39, 118.15, 106.02, 55.29, 19.61, 18.88, 15.33. HRMS m/z calcd for C28H30ClN6O3S [M + H]+: 565.1789, found: 565.1791.
N-(2-amino-4-fluorophenyl)-4-((5-chloro-4-((2-(isopropylsulfonyl)phenyl)amino)pyrimidin-2-yl)amino)benzamide (12e)
Yellow solid. Yield: 29%. M.p. 196.7–198.3 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.88 (d, J = 6.9 Hz, 1H), 9.50 (s, 1H), 9.45 (s, 1H), 8.58 (s, 1H), 8.42–8.33 (m, 1H), 7.95–7.84 (m, 4H), 7.76 (d, J = 7.1 Hz, 2H), 7.49–7.42 (m, 1H), 7.11 (d, J = 6.5 Hz, 1H), 6.60–6.51 (m, 1H), 6.41–6.32 (m, 1H), 5.20 (s, 2H), 3.53–3.43 (m, 1H), 1.17 (d, J = 6.7 Hz, 6H). 13 C NMR (151 MHz, DMSO-d6) δ 165.52, 162.22, 160.64, 157.71, 155.68, 155.61, 146.02, 145.94, 143.57, 138.30, 135.45, 131.47, 129.05, 128.98, 128.92, 127.51, 125.02, 124.61, 120.02, 118.35, 106.05, 55.29, 15.33. HRMS m/z calcd for C26H25ClFN6O3S [M + H]+: 555.1381, found: 555.1381.
N-(2-amino-4-chlorophenyl)-4-((5-chloro-4-((2-(isopropylsulfonyl)phenyl)amino)pyrimidin-2-yl)amino)benzamide (12f)
White solid. Yield: 36%. M.p. 193.5–196.8 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.88 (s, 1H), 9.50 (s, 1H), 9.48 (s, 1H), 8.58 (d, J = 7.2 Hz, 1H), 8.37 (s, 1H), 7.89 (d, J = 8.0 Hz, 2H), 7.87–7.84 (m, 2H), 7.77 (s, 1H), 7.75 (s, 1H), 7.45 (t, J = 7.6 Hz, 1H), 7.16 (d, J = 8.4 Hz, 1H), 6.82 (d, J = 2.2 Hz, 1H), 6.59 (dd, J = 8.3, 2.2 Hz, 1H), 5.21 (s, 2H), 3.49–3.47 (m, 1H), 1.17 (d, J = 6.8 Hz, 6H). 13 C NMR (151 MHz, DMSO-d6) δ 165.52, 162.22, 160.64, 157.71, 155.68, 155.61, 146.02, 145.94, 143.57, 138.30, 135.45, 131,47, 129.05, 128.98, 128.92, 127.51, 125.03, 124.61, 120.02, 118.35, 106.05, 55.29, 15.33. HRMS m/z calcd for C26H25Cl2N6O3S [M + H]+: 571.1086, found: 571.1084.
N-(2-amino-4-methoxyphenyl)-4-((5-chloro-4-((2-(isopropylsulfonyl)phenyl)amino)pyrimidin-2-yl)amino)benzamide (12g)
Brown solid. Yield: 29%. M.p. 205.6–207.7 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.87 (s, 1H), 9.50 (s, 1H), 9.39 (s, 1H), 8.58 (d, J = 7.0 Hz, 1H), 8.37 (s, 1H), 7.89 (d, J = 6.9 Hz, 2H), 7.88–7.84 (m, 2H), 7.76 (s, 1H), 7.74 (s, 1H), 7.45 (t, J = 7.6 Hz, 1H), 7.00 (d, J = 8.5 Hz, 1H), 6.37 (d, J = 2.7 Hz, 1H), 6.19 (dd, J = 8.5, 2.5 Hz, 1H), 4.89 (s, 2H), 3.69 (s, 3H), 3.51–3.43 (m, 1H), 1.17 (d, J = 6.8 Hz, 6H). 13 C NMR (151 MHz, DMSO-d6) δ 164.29, 157.52, 156.64, 154.61, 154.52, 144.07, 142.36, 137.23, 134.37, 130.39, 127.75, 127.32, 126.65, 124.58, 123.93, 123.51, 117.28, 116.24, 104.93, 101.33, 100.20, 54.24, 54.21, 14.25. HRMS m/z calcd for C27H28ClN6O4S [M + H]+: 567.1581, found: 567.1579.
N-(2-amino-4-methylphenyl)-4-((5-chloro-4-((2-(isopropylsulfonyl)phenyl)amino)pyrimidin-2-yl)amino)benzamide (12h)
White solid. Yield: 25%. M.p. 202.7–204.1 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.88 (d, J = 4.7 Hz, 1H), 9.50 (s, 1H), 9.45 (s, 1H), 8.58 (d, J = 7.3 Hz, 1H), 8.37 (s, 1H), 7.89 (dd, J = 7.8, 1.3 Hz, 2H), 7.86 (d, J = 8.7 Hz, 2H), 7.76 (s, 1H), 7.75 (s, 1H), 7.45 (t, J = 7.6 Hz, 1H), 7.02 (d, J = 7.9 Hz, 1H), 6.60 (d, J = 1.1 Hz, 1H), 6.42 (d, J = 6.9 Hz, 1H), 4.79 (s, 2H), 3.51–3.43 (m, 1H), 2.19 (d, J = 9.4 Hz, 3H), 1.17 (d, J = 6.8 Hz, 6H). 13 C NMR (151 MHz, DMSO-d6) δ 165.23, 157.72, 155.69, 155.61, 143.48, 143.45, 138.30, 135.82, 135.45, 131.47, 128.86, 127.69, 127.08, 124.61, 121.66, 118.37, 117.62, 117.04, 106.03, 56.50, 55.29, 21.30, 19.03, 15.33. HRMS m/z calcd for C27H28ClN6O3S [M + H]+: 551.1632, found: 551.1633.
Cell culture
Human cancer cells A549, HepG2, MDA-MB-231, H2228 and SK-N-BE(2) were purchased from Chinese Cell bank of Sciences Academy (Shanghai, China). The tumour cells were cultured with 10% foetal bovine serum (FBS) (Gibco, US) DMEM or RPMI-1640 growth medium supplemented with 1% streptomycin and 1% penicillin in incubator at 37 °C with 5% CO2.
Cell counting kit-8 (CCK-8) assay
Cell counting kit-8 (CCK-8) assay was employed to evaluate antiproliferative effects of target compounds. Briefly, cells were seeded in 96-well plates (4 × 103 cells/well) and cultured overnight, then treated with compounds or positive control (Ceritinib, Entinostat) for 72 h. After that, the medium was removed and 10 μL freshly prepared CCK-8 solution was added. After incubation at 37 °C for 2 h, the absorbance (OD450 nm) was determined through a microplate reader (BioTek, US). The IC50 values of different compounds were calculated by SPSS 17.0 software.
Enzyme inhibitory activity assay
The enzyme inhibitory activity assay was conducted with a fluorescent assay kit as we previously reportedCitation23.
Migration assay
For Transwell method, the tumour cells were cultured in serum-free DMEM/RPMI-1640 medium for 24 h, then seeded into the upper chamber at a density of 3 × 105 cells (100 μL/well), and DMEM/RPMI-1640 medium (750 μL/well) with 10% FBS was added to the lower chamber of 24-well plate. The cells of upper chamber were treated with the drug containing medium or DMSO control (100 μL/well) for 24 h at 37 °C. Finally, the migrated cells were fixed with 4% formaldehyde, dyed with crystal violet, and washed by PBS. The selected area was photographed by inverted microscope (Nikon, Japan) and determined using ImageJ software.
For wound healing method, A549, SK-N-BE(2) and H2228 cells were seeded in 6-well flat-bottomed plates and incubated overnight. Cells were scratched with a pipette tip to generate a clean-wound area in the cell layer, following washed with PBS and treated with compound 12a, positive control (Ceritinib, Entinostat), then replenished with serium-free DMEM/RPMI-1640 medium. The cells were photographed by inverted microscope at 0 h, 24 h or 72 h. Quantification of the area of scratch were measured by ImageJ software.
Flow cytometric analysis
Flow cytometric (FCM) was performed to detect cell cycle and apoptosis. Briefly, cancer cells (5 × 105 cells/well) were grown in 6-well plate overnight and then incubated with compound 12a, positive control (Ceritinib, Entinostat) for 24 h, then washed with PBS. In terms of cell cycle detection, resuspended with ice-cold ethanol (70%) for 24 h and stained with propidium iodine (PI) and Annexin V-FITC for 30 min. Finally, the samples were detected by FCM (CytoFLEX, Beckman Coulter, US). For apoptosis analysis, the cells were gathered with cold PBS, and then stained with PI and RNase A. Subsequently, the treated samples were tested with FCM.
Western blot analysis
The cancer cells were seeded and treated with compound 12a, positive control (Ceritinib, Entinostat) for 24 h, cells in each well were washed and decomposed in lysis buffer. Proteins were detached by SDS-PAGE and transferred to PVDF membrance. After being blocked by 5% BSA, washed and incubated with primary antibodies (1:1000 diluted) (Changzhou affinity Biosciences, China) at 4 °C, followed by incubated with horseradish peroxidase (HRP) conjugated secondary antibodies (1:5000 diluted) (Shanghai Beyotime institute of biotechnology, China). Finally, the enhanced chemiluminescence reagent was conducted to detect the bands. Immunoblotting was analysed by densitometry using the software ImageJ.
Hoechst 33258 and AO/EB staining analysis
The tumour cells were stained with AO/EB or Hoechst 33258 to preliminarily identify apoptotic morphological changes. In short, H2228 or A549 cells were seeded into 6-well plates and treated with compound 12a, positive control (Ceritinib, Entinostat) for 24 h. For AO/EB staining assay, after washing with PBS, cells were gathered and dyed with AO/EB for 15 min. Then cells were photographed using an inverted fluorescence microscope. For Hoechst 33258 staining assay, the cells were fixed with 4% polyformaldehyde for 10 min and stained with Hoechst 33258 solution after washing cells with PBS for twice. Finally, an anti-fluorescence quencher was added in each well for observation by an inverted fluorescence microscope.
SK-N-BE(2) xenograft model assay
All animal studies were conducted according to the guidelines of the Animal Experimental Ethics Committee of Chongqing Medical University (ID. SCXK2018-0003). Female BALB/C nude mice aged 4 weeks, were utilised to establish the SK-N-BE(2) xenograft model for determining the antitumor effect of compound 12a in vivo. To put it briefly, a 100 μL suspension of 5 × 106 SK-N-BE(2) cells was injected subcutaneously into the one flank region of nude mouse (Chengdu Yaokang Bio-Technology Co., LTD, Chengdu, China). Once the size of the tumours reached approximately 100–150 mm3, the mice were randomly divided into four groups and intraperitoneally (i.p.) administrated with 0.9% NaCl, compound 12a (25 and 100 mg/kg) or Ceritinib (100 mg/kg) once every two days for 16 days. The tumour volume was calculated with the formula (length × width2)/2. The tumour volume and body weight of each mouse were determined using calliper every 2 days. On day 16, the mice were sacrificed, xenograft tumour were dissected and the weight was measured in each group. The organs were further examined by H&E staining to observe drug toxicity.
Molecular docking
The Molecular docking studies were conducted with AMDOCK software as previously reportedCitation25. (PDB Code: 4MKC) and (PDB Code: 5IWG) were used and obtained from the Protein Data Bank.
Statistical analysis
The data were analysed using GraphPad Prism 6 software and expressed as mean ± SD of three independent experiments. The statistical analyses were performed using t-test and the statistical differences were measured using a one-way ANOVA with p < 0.05, which was considered as statistically significant.
Supplemental Material
Download PDF (1 MB)Disclosure Statement
No potential conflict of interest was reported by the author(s)
Additional information
Funding
References
- Chiarle R, Voena C, Ambrogio C, et al. Inghirami, The anaplastic lymphoma kinase in the pathogenesis of cancer. Nat Rev Cancer 2008;8:11–23.
- Morris SW, Kirstein MN, Valentine MB, et al. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin’s lymphoma. Science 1995;267:316–7.
- Rikova K, Guo A, Zeng Q, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell 2007;131:1190–203.
- Zhu M, Li W, Zhao T, et al. Fragment-based modification of 2,4-diarylaminopyrimidine derivatives as ALK and ROS1 dual inhibitors to overcome secondary mutants. Bioorg Med Chem 2020;28:115719.
- Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007;448:561–6.
- Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 2010;363:1693–703.
- Gristina V, La Mantia M, Iacono F, et al. The emerging therapeutic landscape of ALK inhibitors in non-small cell lung cancer. Pharmaceuticals (Basel) 2020;13:474.
- Doebele RC, Pilling AB, Aisner DL, et al. Mechanisms of resistance to crizotinib in patients with ALK gene rearranged non-small cell lung cancer. Clin Cancer Res 2012;18:1472–82.
- Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov 2006;5:769–84.
- Ververis K, Hiong A, Karagiannis TC, et al. Histone deacetylase inhibitors (HDACIs): multitargeted anticancer agents. Biologics 2013;7:47–60.
- Ropero S, Esteller M. The role of histone deacetylases (HDACs) in human cancer. Mol Oncol 2007;1:19–25.
- Li Y, Seto E. HDACs and HDAC inhibitors in cancer development and therapy. Cold Spring Harb Perspect Med 2016;6:a026831.
- Makena MR, Nguyen TH, Koneru B, et al. Vorinostat and fenretinide synergize in preclinical models of T-cell lymphoid malignancies. Cancer Res 2021;32:34–43.
- Marampon F, Di Nisio V, Pietrantoni I, et al. Pro-differentiating and radiosensitizing effects of inhibiting HDACs by PXD-101 (Belinostat) in in vitro and in vivo models of human rhabdomyosarcoma cell lines. Cancer Lett 2019;461:90–101.
- Paillas S, Then CK, Kilgas S, et al. The histone deacetylase inhibitor romidepsin spares normal tissues while acting as an effective radiosensitizer in bladder tumors in vivo. Int J Radiat Oncol Biol Phys 2020;107:212–21.
- Prince HM, Bishton MJ, Johnstone RW. Panobinostat (LBH589): a potent pan-deacetylase inhibitor with promising activity against hematologic and solid tumors. Future Oncol 2009;5:601–12.
- Liu T, Guan F, Wang Y, et al. MS-275 combined with cisplatin exerts synergistic antitumor effects in human esophageal squamous cell carcinoma cells. Toxicol Appl Pharmacol 2020;395:114971.
- Yun MR, Lim SM, Kim SK, et al. Enhancer remodeling and microRNA alterations are associated with acquired resistance to ALK inhibitors. Cancer Res 2018;78:3350–62.
- Stockhammer P, Ho CSL, Hegedus L, et al. HDAC inhibition synergizes with ALK inhibitors to overcome resistance in a novel ALK mutated lung adenocarcinoma model. Lung Cancer 2020;144:20–9.
- Fukuda K, Takeuchi S, Arai S, et al. Epithelial-to-mesenchymal transition is a mechanism of ALK inhibitor resistance in lung cancer independent of ALK mutation status. Cancer Res 2019;79:1658–70.
- Fukuda K, Takeuchi S, Katayama R, et al. HDAC inhibition overcomes crizotinib-resistance by mesenchymal-epithelial transition (MET) in EML4-ALK lung cancer cells. J Thorac Oncol 2017;12:S382–S383.
- Hagiwara K, Tokunaga T, Iida H, et al. Combined inhibition of ALK and HDAC induces synergistic cytotoxicity in neuroblastoma cell lines. Anticancer Res 2019;39:3579–84.
- Pan T, Dan Y, Guo D, et al. Discovery of 2,4-pyrimidinediamine derivatives as potent dual inhibitors of ALK and HDAC. Eur J Med Chem 2021;224:113672.
- Masanas M, Masiá N, Suárez‐Cabrera L, et al. The oral KIF11 inhibitor 4SC‐205 exhibits antitumor activity and potentiates standard and targeted therapies in primary and metastatic neuroblastoma models. Clin Trans Med 2021;11:e523.
- Wu G, Robertson DH, Brooks CL, 3rd, et al. Detailed analysis of grid-based molecular docking: A case study of CDOCKER-A CHARMm-based MD docking algorithm. J Comput Chem 2003;24:1549–62.