Abstract
In this study, a library of phthalimide Schiff base linked to 1,4-disubstituted-1,2,3-triazoles was designed, synthesised, and characterised by different spectral analyses. All analogues have been introduced for in vitro assay of their antiviral activity against COVID-19 virus using Vero cell as incubator with different concentrations. The data revealed most of these derivatives showed potent cellular anti-COVID-19 activity and prevent viral growth by more than 90% at two different concentrations with no or weak cytotoxic effect on Vero cells. Furthermore, in vitro assay was done against this enzyme for all analogues and the results showed two of them have IC50 data by 90 µM inhibitory activity. An extensive molecular docking simulation was run to analyse their antiviral mechanism that found the proper non-covalent interaction within the Mpro protease enzyme. Finally, we profiled two reversible inhibitors, COOH and F substituted analogues that might be promising drug candidates for further development have been discovered.
Introduction
The ongoing COVID-19 pandemic has highlighted the urgent need for effective antiviral agentsCitation1. One promising class of compounds that has attracted considerable attention in this context is 1,2,3-triazole-carrying scaffoldsCitation2. These compounds possess diverse pharmacological activities and have demonstrated potential as antiviral agents. Their unique structural features, such as the ability to form hydrogen bonds and effectively interact with biological targets, make them an attractive scaffold for drug design and developmentCitation2.
The rational design and synthesis of novel 1,2,3-triazole derivatives present a reasonable rationale for the discovery of new anti-COVID agents. By leveraging the structural versatility of the triazole moiety, researchers have been able to fine-tune the physicochemical and pharmacokinetic properties of these compounds, thereby enhancing their bioactivity and therapeutic potentialCitation3–8. The aims to explore the design, synthesis, and biological evaluation of 1,2,3-triazole derivatives with the specific goal of developing potent antiviral agents targeting COVID-19. Also, the work highlights the structure–activity relationships (SAR) of these derivatives, shedding light on the crucial structural motifs that confer potent antiviral effects.
Therefore, novel small molecule therapeutics exhibiting good oral bioavailability are urgently neededCitation9. Results from two anti-SARS-CoV-2 oral clinical trials were recently published. In a phase-3 clinical trials, it was discovered that Moldupiravir (), an inhibitor of viral RNA-dependent RNA polymerase (RdRp), reduced the risk of death or hospitalisation in adult patients with mild-to-moderate COVID-19 by 30% who were not hospitalizedCitation10. However, an 89% decrease in hospitalisation or mortality was observed when ritonavir and Nirmatrelvir () were used in conjunction as viral protease inhibitorsCitation11. These findings suggested that in order to prevent future pandemics, other mechanisms for oral anti-SARS-CoV-2 medications must be developedCitation12–14.
Moreover, click chemistry-a low-cost method of catalysing the reaction between azides and alkynes – allows for the simple synthesis of 1,2,3-triazoles. Click chemistry is commonly employed not only to create derivatives of triazoles but also to fuse two or more chemical groups together to form a single hybrid molecule with enhanced biological activityCitation15–18. The phthalimide group, being a derivative of triazoles, has demonstrated its utility in forming hybrid compounds that have antiviral action against HIVCitation19, CMV, and varicella-zosterCitation20. In addition, the importance of similar molecules was highlighted employing DFT theory for quantum computations and drug-like charactersCitation21 (ref Citation1 in the reviewer comments). Aromatic Schiff bases with azo linkage have also been emerged as an important scaffold in both medical and non-medical applicationsCitation8,Citation22(ref Citation2 in the reviewer comments). Crucially, compounds of phthalimide and triazole both exhibit significant anti-inflammatory efficacyCitation23,Citation24. As a result, 1,2,3-triazole-phthalimide hybrids may be able to treat the severe form of COVID-19, which is characterised by a powerful inflammatory process known as a "cytokine storm," in addition to preventing viral replicationCitation13,Citation25,Citation26.
Enzyme inhibition serves as a crucial strategy for curing several diseases including viral and bacterial infections. Several enzymes such as carbonic anhydrase and acetylcholinesterase disrupting key biological processes essential for treatment of wide range of diseases.Citation28 Targeted inhibition of specific enzymes involved in vital pathways can impede the infectious agents, offering a promising avenue for the development of antiviral and antibacterial therapeutics. Similarly, SARS-CoV-2 enzyme inhibitors, especially Mpro, the key protease, was proved as one of the fundamental strategies to impact this virus.Citation29
Our study team forecast the design and synthesis of newer 1,2,3-triazole hybrid molecules within our research scope as continuation of our interest in this area and based on those reported in the literatureCitation17,Citation18,Citation30–45. Thus, we have anticipated on the synthesis of novel Schiff base tethering active phthalimide entity and lateral acetylenic side chain as an alkyne starting material for further cycloaddition with a series of organic azides, resulting in a novel array of 1,4-disubstituted-1,2,3-triazoles molecular hybrids.
Work design
Our previous investigation introduced efficient anti-SARS-CoV-2 analogues of molecular hybrids of rigid terminal scaffold bonded through heterocyclic basic triazole ring and ended with lipophilic-substituted fragments ()Citation46. Chemically, this compound and its congeners composed of phenylpyrazolone scaffold connected to lipophilic aryl moiety (4-acetamidobenzoic acid for active derivative) through 1,2,3-triazole linker. This compound inhibits SARS-CoV-2 growth in Vero 6 cells by 63.39 ± 0.48% at a concentration of 10 µM. In addition, a preliminary mechanistic study revealed that the scaffold linked to triazole entity could impacts SARS-CoV-2 by inhibition of the main protease Mpro machinery with IC50 3.16 ± 1.2 µM with very low cytotoxic effect at higher concentrationsCitation46.
Figure 2. (A) docking pose of compound 11 in complex with Mpro (PBD ID 5R80) (left) and the 2D interaction map (right) showing the fundamental interactions of certain moieties in 11 with the protein; (B) The suggested modification aiming to improve its binding with the protein and enhance its anti-Covid activity.
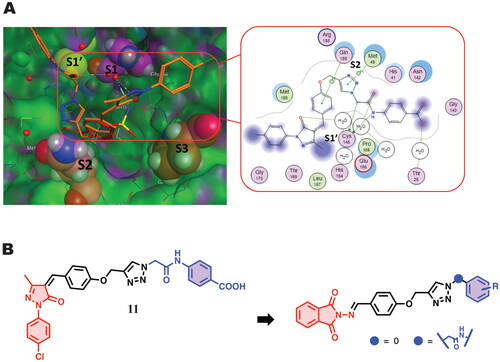
In addition, docking studies showed prominent binding to Mpro (PDB ID: 5R80) through two fundamental points. First, phenoxy and triazole parts interact with Gln189 and Met49 via hydrogen bonding (). These interactions highly stabilise the compound inside the binding pocket. Second, the amide linker of the lipophilic terminal part interacts with Cys145. On the other hand, the phenylpyrazole entity does not exhibit any meaningful prominent accommodation or binding to the active site residuesCitation46. Given the fundamental interactions exhibited by the middle region of this scaffold, we envisioned another round of chemical modification by the replacement of the phenylpyrazole part with chemical isosteric moiety bearing the same heteroatoms; N-aminophthalimide fragmentCitation46. Phthalimide has emerged as a privileged scaffold in the drug discovery process. The promising biological activities (including anti-SARS-CoV-2) of some reported phthalimide derivatives encouraged us to decide this replacementCitation3–5,Citation47–50. In addition, we will try various substituted phenyl groups at the right part connected to the triazole core directly or through the ordinary methyl amide linker. Moreover, a polar fragment of nucleosides will also be investigated and compared to non-polar ones.
Materials and methods
General chemistry
All reagents and solvents used were of the highest quality of analytical reagent grade and were used without further purification. Fine chemicals and solvents were purchased from BDH Chemicals Ltd. and Sigma-Aldrich. Melting points were measured on a Stuart Scientific SMP1 and are uncorrected. TLC was performed on UV fluorescent Silica gel Merck 60 F254 plates, and the spots were visualised using a UV lamp (254 nm). Fourier transform infra-red spectroscopy (FT-IR) was conducted on a Perkin-Elmer 1430 series FT-IR spectrometer. 1H and 13C nuclear magnetic resonance (NMR) spectra were obtained on a Bruker spectrometer (400 MHz) with TMS as an internal reference. Elemental analyses were performed using a GmbH-Vario EL III Elementar AnalyserCitation51.
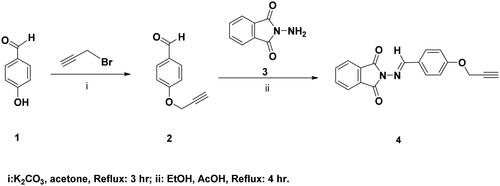
Synthesis and characterisation of 4-(prop-2-yn-1-yloxy) benzaldehyde (2)
Compound 2 was prepared in accordance with our previously published workCitation38.
Synthesis and characterisation of (E)-2-((4-(prop-2-yn-1-yloxy)benzylidene)amino)isoindoline-1,3-dione (4)
A stirring solution of compound 2 (5 mmol), 2-aminoisoindoline-1,3-dione (3) (5 mmol) and few drops of acetic acid in ethanol (40 ml) was heated under reflux for 4 h. After cooling, the resulting precipitate was collected by filtration and recrystallized from ethanol to give the desired Schiff base 4 as colourless crystals in 92% yield, mp: 183–184 °C. IR (υ, cm−1): IR (KBr): 1550 (C = C), 1700 (C = O, C = N), 2140 (C≡C), 3280 (≡CH).1H NMR (400 MHz, DMSO-d6): δH = 3.65 (s, 1H, ≡CH), 4.91 (s, 2H, OCH2), 7.15 (d, 2H, J = 8.0 Hz, Ar-H), 7.85–7.93 (m, 6H, Ar-H), 9.11 (s, 1H, HC = N). 13C NMR (100 MHz, DMSO-d6): δC = 54.09 (OCH2); 79.15 (C≡CH); 79.27 (C≡CH); 115.82, 123.86, 126.86, 130.35, 130.51, 135.34, 160.52, 165.02 (Ar-C, C = N, C = O). Calculated for: C18H12N2O3: C, 71.05; H, 3.97; N, 9.21. Found: C, 71.24; H, 3.90; N, 9.10.
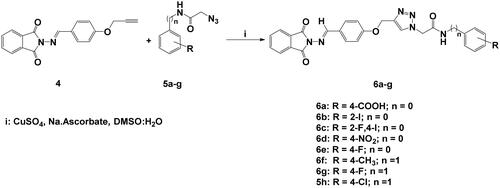
General click procedure for the synthesis of 1,4-disubstituted 1,2,3-triazoles 6a-h, 8a-h and 10a,b
A solution of O-propargylated Schiff Base 4 (1 mmol) in DMSO (10 ml) was added with stirring to a solution of copper sulphate (0.10 g) and sodium ascorbate (0.15 g) in water (10 ml). The reaction mixture was then stirred at 80 °C for 5–10 h with the appropriate phenylacetamide azides 5a-h, aromatic azides 7a-h, and/or glycosyl azides 9a,b (1 mmol). TLC (hexane-ethylacetate) was used to monitor the reaction. After the reaction completed, the reaction mixture was poured onto crushed ice. The resulting precipitate was collected by filtration, washed with a saturated solution of ammonium chloride, then water, and recrystallized from ethanol/DMF to provide the desired 1,2,3-triazoles carrying phthalimide Schiff bases 6a-h, 8a-h, and/or 10a,b.
Characterisation of (E)-4–(2-(4-((4-(((1,3-dioxoisoindolin-2-yl)imino) methyl)phenoxy)methyl)-1H-1,2,3-triazol-1-yl)acetamido)benzoic acid (6a)
This compound was obtained as white pellets in 87% yield, mp: 264–265 °C. IR (υ, cm−1): 1575 (C = C), 1700 (C = N), 1730 (C = O), 2970 (CH-Al), 3045 (CH-Ar). 1H NMR (400 MHz, DMSO-d6): δH = 5.27 (s, 0.3H, OCH2), 5.31 (s, 1.7H, OCH2), 5.35 (s, 2H, NCH2CO), 7.12 (dd, 1H, J = 4 Hz, J = 8 Hz, Ar-H), 7.24–7.32 (m, 4H, Ar-H), 7.39–7.44 (m, 2H, Ar-H), 7.71–7.85 (m, 5H, Ar-H), 8.21 (s, 1H, CH-1,2,3-triazole), 9.08 (s, 0.3H, HC = N), 9.80 (s, 0.7H, HC = N), 10.12 (s, 1H, NH), 10.12 (s, 1H, COOH). 13C NMR (100 MHz, DMSO-d6): δC = 52.62 (NCH2); 61.70 (OCH2); 115.71, 115.80, 121.47, 123.70, 126.52, 130.37, 130.57, 135.24, 135.40, 142.32, 142.52, 159.90, 160.68, 160.78, 161.49, 164.59, 165.00 (Ar-C, C = N, C = O, COOH). Calculated for: C27H20N6O6: C, 61.83; H, 3.84; N, 16.02. Found: C, 61.93; H, 3.89; N, 16.09.
Characterisation of (E)-2–(4-((4-(((1,3-dioxoisoindolin-2-yl)imino) methyl)phenoxy)methyl)-1H-1,2,3-triazol-1-yl)-N-(2-iodophenyl) acetamide (6b)
This compound was obtained as white pellets in 88% yield, mp: 213–214 °C. IR (υ, cm−1): 1560 (C = C), 1690 (C = N), 1720 (C = O), 2930 (CH-Al), 3030 (CH-Ar). 1H NMR (400 MHz, DMSO-d6): δH = 5.23 (s, 0.3H, OCH2), 5.25 (s, 1.7H, OCH2), 5.36 (s, 2H, NCH2CO), 6.98 (dd, 1H, J = 4 Hz, J = 8 Hz, Ar-H), 7.15–7.21 (m, 3H, Ar-H), 7.33–7.40 (m, 3H, Ar-H), 7.79–7.88 (m, 5H, Ar-H), 8.25 (s, 1H, CH-1,2,3-triazole), 9.04 (s, 0.3H, HC = N), 9.82 (s, 0.7H, HC = N), 9.97 (s, 1H, NH). 13C NMR (100 MHz, DMSO-d6): δC = 52.46 (NCH2); 61.82 (OCH2); 96.65, 115.71, 115.78, 123.91, 127.17, 127.77, 128.71, 129.35, 130.33, 130.52, 132.36, 139.12, 139.61, 160.95, 163.47, 165.08, 165.12 (Ar-C, C = N, C = O). Calculated for: C26H19IN6O4: C, 51.50; H, 3.19; N, 13.86. Found: C, 51.69; H, 3.27; N, 13.74.
Characterisation of (E)-2–(4-((4-(((1,3-dioxoisoindolin-2-yl)imino) methyl)phenoxy)methyl)-1H-1,2,3-triazol-1-yl)-N-(2-fluoro-4-iodophenyl)acetamide (6c)
This compound was obtained as white pellets in 88% yield, mp: 228–229 °C. IR (υ, cm−1): 1580 (C = C), 1685 (C = N), 1725 (C = O), 2920 (CH-Al), 3090 (CH-Ar). 1H NMR (400 MHz, DMSO-d6): δH = 5.29, 5.32 (2s, 2H, OCH2), 5.42 (s, 2H, NCH2CO), 7.02 (s, 2H, J = 8 Hz, Ar-H), 7.25–7.45 (m, 5H, Ar-H), 7.85–7.90 (m, 5H, Ar-H), 8.31 (s, 1H, CH-1,2,3-triazole), 9.12 (s, 0.2H, HC = N), 9.88 (s, 0.8H, HC = N), 10.01 (s, 1H, NH). 13C NMR (100 MHz, DMSO-d6): δC = 52.46 (NCH2); 61.88 (OCH2); 96.49, 115.64, 115.76, 120.89, 123.75, 123.98, 126.52, 127.16, 127.66, 129.31,129.37, 130.35, 130.58, 132.19, 132.32, 135.42, 139.15, 139.64, 142.37, 163.64, 165.07 (Ar-C, C = N, C = O). Calculated for: C26H18FIN6O4: C, 50.02; H, 2.91; N, 13.46. Found: C, 50.19; H, 2.98; N, 13.57.
Characterisation of (E)-2–(4-((4-(((1,3-dioxoisoindolin-2-yl)imino)methyl)phenoxy)methyl)-1H-1,2,3-triazol-1-yl)-N-(4-nitrophenyl)acetamide (6d)
This compound was obtained as white pellets in 90% yield, mp: 242–243 °C. IR (υ, cm−1): 1590 (C = C), 1675 (C = N), 1725 (C = O), 2920 (CH-Al), 3035 (CH-Ar). 1H NMR (400 MHz, DMSO-d6): δH = 5.30 (s, 2H, OCH2), 5.47 (s, 2H, NCH2CO), 7.24 (d, 2H, J = 8 Hz, Ar-H), 7.85–7.92 (m, 8H, Ar-H), 8.27 (d, 2H, J = 8 Hz, Ar-H), 8.33 (s, 1H, CH-1,2,3-triazole), 9.12 (s, 1H, HC = N), 11.12 (s, 1H, NH). 13C NMR (100 MHz, DMSO-d6): δC = 52.85 (NCH2); 61.71 (OCH2); 115.63, 115.78, 123.71, 124.00, 125.76, 126.53, 130.35, 142.71, 142.52, 143.09, 144.98, 159.90, 160.77, 161.48, 165.01, 165.81 (Ar-C, C = N, C = O). Calculated for: C26H19N7O6: C, 59.43; H, 3.64; N, 18.66. Found: C, 59.59; H, 3.57; N, 18.57.
Characterisation of (E)-2–(4-((4-(((1,3-dioxoisoindolin-2-yl)imino) methyl)phenoxy)methyl)-1H-1,2,3-triazol-1-yl)-N-(4-fluorophenyl) acetamide (6e)
This compound was obtained as white pellets in 89% yield, mp: 219–220 °C. IR (υ, cm−1): 1590 (C = C), 1675 (C = N), 1720 (C = O), 2920 (CH-Al), 3035 (CH-Ar). 1H NMR (400 MHz, DMSO-d6): δH = 5.23 (s, 1.7H, OCH2), 5.26 (s, 0.3H, OCH2), 5.30 (s, 2H, NCH2CO), 7.11–7.21 (m, 4H, Ar-H), 7.52–7.56 (m, 2H, Ar-H), 7.76–7.88 (m, 6H, Ar-H), 8.25 (s, 1H, CH-1,2,3-triazole), 9.04 (s, 0.7H, HC = N), 9.82 (s, 0.3H, HC = N), 10.53 (s, 1H, NH). 13C NMR (100 MHz, DMSO-d6): δC = 52.63 (NCH2); 61.67 (OCH2); 115.71, 115.78, 115.98, 116.16, 121.57, 121.63, 123.38, 123.91, 126.52, 127.08, 130.52, 130.57, 132.37, 134.91, 135.24, 135.40, 157.83, 159.74, 160.94, 161.51, 164.63, 165.09 (Ar-C, C = N, C = O). Calculated for: C26H19FN6O4: C, 62.65; H, 3.84; N, 16.86. Found: C, 62.41; H, 3.91; N, 16.75.
Characterisation of (E)-2–(4-((4-(((1,3-dioxoisoindolin-2-yl)imino) methyl)phenoxy)methyl)-1H-1,2,3-triazol-1-yl)-N-(4-methylbenzyl) acetamide (6f)
This compound was obtained as colourless crystals in 88% yield, mp: 201–202 °C. IR (υ, cm−1): 1560 (C = C), 1670 (C = N), 1715 (C = O), 2930 (CH-Al), 3070 (CH-Ar). 1H NMR (400 MHz, DMSO-d6): δH = 2.28 (s, 3H, CH3), 4.30 (d, 2H, J = 8.0 Hz, NHCH2), 5.23 (s, 2H, OCH2), 5.30 (s, 2H, NCH2CO), 7.20–7.32 (m, 5H, Ar-H), 7.39–7.48 (m, 5H, Ar-H), 7.81 (d, 2H, J = 8.0 Hz, Ar-H), 8.29 (s, 1H, CH-1,2,3-triazole), 9.05 (t, 1H, J = 8.0 Hz, NH), 9.80 (s, 1H, HC = N). 13C NMR (100 MHz, DMSO-d6): δC = 25.63 (CH3); 42.23 (NHCH2); 52.15 (NCH2); 61.70 (OCH2); 115.72, 125.32, 127.18, 128.70, 128.98, 129.45, 130.56, 131.16, 132.45, 137.45, 142.44, 163.23, 166.02 (Ar-C, C = N, C = O). Calculated for: C28H24N6O4: C, 66.13; H, 4.76; N, 16.53. Found: C, 66.31; H, 4.68; N, 16.64.
Characterisation of (E)-2–(4-((4-(((1,3-dioxoisoindolin-2-yl)imino) methyl)phenoxy)methyl)-1H-1,2,3-triazol-1-yl)-N-(4-fluorobenzyl) acetamide (6 g)
This compound was obtained as colourless crystals in 90% yield, mp: 195–196 °C. IR (υ, cm−1): 1585 (C = C), 1690 (C = N), 1715 (C = O), 2920 (CH-Al), 3070 (CH-Ar). 1H NMR (400 MHz, DMSO-d6): δH = 4.33 (d, 2H, J = 8.0 Hz, NHCH2), 5.24 (s, 2H, OCH2), 5.29 (s, 2H, NCH2CO), 7.26–7.36 (m, 6H, Ar-H), 7.45–7.52 (m, 4H, Ar-H), 7.87 (d, 2H, J = 8.0 Hz, Ar-H), 8.32 (s, 1H, CH-1,2,3-triazole), 9.10 (t, 1H, J = 8.0 Hz, NH), 9.81 (s, 1H, HC = N). 13C NMR (100 MHz, DMSO-d6): δC = 42.36 (NHCH2); 52.11 (NCH2); 61.79 (OCH2); 115.69, 115.93, 127.45, 129.31, 130.25, 130.46, 131.84, 132.33, 139.46, 143.52, 157.52, 161.35, 163.56, 166.21 (Ar-C, C = N, C = O). Calculated for: C27H21FN6O4: C, 63.28; H, 4.13; N, 16.40. Found: C, 63.01; H, 4.02; N, 16.57.
Characterisation of (E)-N-(4-chlorobenzyl)-2–(4-((4-(((1,3-dioxoisoindolin-2-yl)imino)methyl)phenoxy)methyl)-1H-1,2,3-triazol-1-yl)acetamide (6h)
This compound was obtained as colourless crystals in 90% yield, mp: 208–209 °C. IR (υ, cm−1): 1570 (C = C), 1680 (C = N), 1720 (C = O), 2940 (CH-Al), 3055 (CH-Ar). 1H NMR (400 MHz, DMSO-d6): δH = 4.31 (d, 2H, J = 8.0 Hz, NHCH2), 5.21 (s, 2H, OCH2), 5.28 (s, 2H, NCH2CO), 7.23–7.38 (m, 10H, Ar-H), 7.89 (d, 2H, J = 8.0 Hz, Ar-H), 8.26 (s, 1H, CH-1,2,3-triazole), 9.03 (t, 1H, J = 8.0 Hz, NH), 9.86 (s, 1H, HC = N). 13C NMR (100 MHz, DMSO-d6): δC = 42.12 (NHCH2); 52.05 (NCH2); 61.75 (OCH2); 115.63, 127.03, 128.75, 129.67, 130.25, 131.99, 132.33, 138.24, 142.23, 163.41, 166.05 (Ar-C, C = N, C = O). Calculated for: C27H21ClN6O4: C, 61.31; H, 4.00; N, 15.89. Found: C, 61.63; H, 4.10; N, 15.70.
Characterisation of (E)-2-((4-((1–(4-fluorophenyl)-1H-1,2,3-triazol-4-yl)methoxy)benzylidene)amino) isoindoline-1,3-dione (8a)
This compound was obtained as colourless crystals in 87% yield, mp: 238–239 °C. IR (υ, cm−1): 1540 (C = C), 1670 (C = N), 1720 (C = O), 2930 (CH-Al), 3075 (CH-Ar). 1H NMR (400 MHz, DMSO-d6) δH = 5.37, 5.39 (2s, 2H, OCH2), 7.24–7.61 (m, 6H, Ar-H), 7.87–7.91 (m, 6H, Ar-H), 8.81 (s, 1H, CH-1,2,3-triazole), 9.12 (s, 0.7H, HC = N), 9.89 (s, 0.3H, HC = N). 13C NMR (100 MHz, DMSO-d6): δC = 61.51 (OCH2); 115.72, 117.51, 117.71, 123.91, 125.10, 126.07, 126.68, 126.87, 130.57, 131.92, 143.41, 153.10, 155.59, 160.68, 163.35, 164.99 (Ar-C, C = N, C = O). Calculated for: C24H16FN5O3: C, 65.30; H, 3.65; N, 15.87. Found: C, 65.50; H, 3.76; N, 15.75.
Characterisation of (E)-4–(4-((4-(((1,3-dioxoisoindolin-2-yl)imino) methyl)phenoxy)methyl)-1H-1,2,3-triazol-1-yl)benzoic acid (8b)
This compound was obtained as pale yellow solid in 86% yield, mp: 287–288 °C. IR (υ, cm−1): 1570 (C = C), 1660 (C = N), 1710 (C = O), 2900 (CH-Al), 3060 (CH-Ar). 1H NMR (400 MHz, DMSO-d6) δH = 5.30 (s, 1.8H, OCH2), 5.33 (s, 0.2H, OCH2), 7.19 (d, 2H, J = 8 Hz, Ar-H), 7.81–7.86 (m, 9H, Ar-H), 8.23 (d, 1H, J = 4 Hz, Ar-H), 9.01 (s, 1H, CH-1,2,3-triazole), 9.05 (s, 0.7H, HC = N), 9.83 (s, 0.3H, HC = N), 12.15 (s, 1H, COOH). 13C NMR (100 MHz, DMSO-d6): δC = 61.64 (OCH2); 115.77, 115.84, 123.74, 123.91, 126.71, 130.54, 130.58, 132.38, 135.40, 139.94, 143.65, 160.84, 161.37, 165.07, 173.11 (Ar-C, C = N, C = O, COOH). Calculated for: C25H17N5O5: C, 64.24; H, 3.67; N, 14.98. Found: C, 64.51; H, 3.77; N, 14.84.
Characterisation of (E)-2-((4-((1–(4-nitrophenyl)-1H-1,2,3-triazol-4-yl)methoxy)benzylidene)amino)isoindoline-1,3-dione (8c)
This compound was obtained as pale yellow solid in 88% yield, mp: 270–271 °C. IR (υ, cm−1): 1550 (C = C), 1680 (C = N), 1715 (C = O), 2870 (CH-Al), 3030 (CH-Ar). 1H NMR (400 MHz, DMSO-d6) δH = 5.29 (s, 1.8H, OCH2), 5.31 (s, 0.2H, OCH2), 7.24 (dd, 2H, J = 4 Hz, J = 8 Hz, Ar-H), 7.40 (t, 2H, J = 4 Hz, Ar-H), 7.80–7.90 (m, 8H, Ar-H), 8.88 (s, 1H, CH-1,2,3-triazole), 9.04 (s, 0.7H, HC = N), 9.82 (s, 0.3H, HC = N). 13C NMR (100 MHz, DMSO-d6): δC = 61.71 (OCH2); 115.89, 122.45, 123.99, 124.16, 126.72, 130.57, 132.88, 134.80, 135.19, 135.73, 136.49, 144.26, 161.41, 165.00 (Ar-C, C = N, C = O). Calculated for: C24H16N6O5: C, 61.54; H, 3.44; N, 17.94. Found: C, 61.25; H, 3.54; N, 17.78.
Characterisation of (E)-2-((4-((1–(3-fluoro-4-methylphenyl)-1H-1,2,3-triazol-4-yl)methoxy)benzylidene)amino) Isoindoline-1,3-dione (8d)
This compound was obtained as pale yellow solid in 87% yield, mp: 255–256 °C. IR (υ, cm−1): 1560 (C = C), 1660 (C = N), 1710 (C = O), 2850 (CH-Al), 3060 (CH-Ar). 1H NMR (400 MHz, DMSO-d6) δH = 2.28 (s, 3H, CH3), 5.32 (s, 2H, OCH2), 7.20 (d, 2H, J = 8 Hz, Ar-H), 7.49 (t, 2H, J = 4 Hz, Ar-H), 7.72–7.85 (m, 4H, Ar-H), 7.88–7.94 (m, 3H, Ar-H), 8.97 (s, 1H, CH-1,2,3-triazole), 9.10 (s, 1H, HC = N). 13C NMR (100 MHz, DMSO-d6): δC = 28.56 (CH3); 61.34 (OCH2); 115.77, 121.31, 123.57, 125.09, 126.82, 128.46, 130.74, 133.15, 134.64, 135.82, 136.82, 143.61, 160.62, 161.89, 165.37 (Ar-C, C = N, C = O). Calculated for: C25H18FN5O3: C, 65.93; H, 3.98; N, 15.38. Found: C, 65.79; H, 3.91; N, 15.46.
Characterisation of (E)-2-((4-((1–(2-fluorophenyl)-1H-1,2,3-triazol-4-yl)methoxy)benzylidene)amino)isoindoline-1,3-dione (8e)
This compound was obtained as pale yellow solid in 86% yield, mp: 248–249 °C. IR (υ, cm−1): 1565 (C = C), 1690 (C = N), 1725 (C = O), 2915 (CH-Al), 3045 (CH-Ar). 1H NMR (400 MHz, DMSO-d6) δH = 5.37 (s, 2H, OCH2), 7.25 (d, 2H, J = 8 Hz, Ar-H), 7.87–8.00 (m, 9H, Ar-H), 8.30 (s, 1H, Ar-H), 9.10 (s, 1H, CH-1,2,3-triazole), 9.12 (s, 1H, HC = N). 13C NMR (100 MHz, DMSO-d6): δC = 61.63 (OCH2); 115.83, 120.78, 122.46, 123.91, 130.54, 130.58, 132.37, 135.40, 136.54, 160.82, 161.35, 165.07 (Ar-C, C = N, C = O). Calculated for: C24H16FN5O3: C, 65.30; H, 3.65; N, 15.87. Found: C, 65.53; H, 3.53; N, 15.72.
Characterisation of ethyl (E)-4–(4-((4-(((1,3-dioxoisoindolin-2-yl)imino) methyl)phenoxy)methyl)-1H-1,2,3-triazol-1-yl)benzoate (8f)
This compound was obtained as pale yellow solid in 88% yield, mp: 224–225 °C. IR (υ, cm−1): 1540 (C = C), 1690 (C = N), 1725 (C = O), 2940 (CH-Al), 3050 (CH-Ar). 1H NMR (400 MHz, DMSO-d6) δH = 1.17 (t, 3H, J = 4 Hz, J = 8 Hz, CH3), 4.37–4.52 (q, 2H, CH2CH3), 5.34 (s, 2H, OCH2), 7.28 (d, 2H, J = 8 Hz, Ar-H), 7.43 (t, 2H, J = 4 Hz, Ar-H), 7.72–7.79 (m, 4H, Ar-H), 7.88–7.95 (m, 4H, Ar-H), 9.03 (s, 1H, CH-1,2,3-triazole), 9.15 (s, 1H, HC = N). 13C NMR (100 MHz, DMSO-d6): δC = 15.31 (CH3); 56.35 (CH2CH3); 61.56 (OCH2); 115.75, 117.81, 121.36, 123.70, 124.43, 125.06, 129.45, 130.42, 131.71, 133.69, 135.35, 144.65, 160.56, 161.39, 165.77 (Ar-C, C = N, C = O). Calculated for: C27H21N5O5: C, 65.45; H, 4.27; N, 14.13. Found: C, 65.29; H, 4.35; N, 14.27.
Characterisation of (E)-2-((4-((1–(3,4-dichlorophenyl)-1H-1,2,3-triazol-4-yl)methoxy)benzylidene)amino)isoindoline-1,3-dione (8 g)
This compound was obtained as pale yellow solid in 87% yield, mp: 266–267 °C. IR (υ, cm−1): 1555 (C = C), 1680 (C = N), 1720 (C = O), 2935 (CH-Al), 3070 (CH-Ar). 1H NMR (400 MHz, DMSO-d6) δH = 5.31 (s, 1.4H, OCH2), 5.34 (s, 0.6H, OCH2), 7.24 (dd, 2H, J = 4 Hz, J = 8 Hz, Ar-H), 7.42 (dd, 1H, J = 4 Hz, J = 8 Hz, Ar-H), 7.51–7.59 (m, 2H, Ar-H), 7.79–7.88 (m, 6H, Ar-H), 8.74 (s, 1H, CH-1,2,3-triazole), 9.07 (s, 0.7H, HC = N), 9.83 (s, 0.3H, HC = N). 13C NMR (100 MHz, DMSO-d6): δC = 61.51, 61.66 (OCH2); 115.74, 115.80, 117.59, 117.75, 123.89, 126.14, 126.56, 130.50, 130.60, 135.36, 135.73, 143.44, 153.38, 155.37, 160.70, 161.43, 163.38, 165.03, 165.04, 165.06 (Ar-C, C = N, C = O). Calculated for: C24H15Cl2N5O3: C, 58.55; H, 3.07; N, 14.23. Found: C, 58.74; H, 3.15; N, 14.12.
Characterisation of (E)-2-((4-((1–(4-acetylphenyl)-1H-1,2,3-triazol-4-yl)methoxy)benzylidene)amino)isoindoline-1,3-dione (8h)
This compound was obtained as pale yellow solid in 88% yield, mp: 275–276 °C. IR (υ, cm−1): 1540 (C = C), 1690 (C = N), 1730 (C = O), 2930 (CH-Al), 3080 (CH-Ar). 1H NMR (400 MHz, DMSO-d6) δH = 2.24 (s, 3H, CH3), 5.32 (s, 2H, OCH2), 7.24 (d, 2H, J = 8 Hz, Ar-H), 7.40 (t, 2H, J = 4 Hz, Ar-H), 7.70–7.81 (m, 4H, Ar-H), 7.89–7.98 (m, 4H, Ar-H), 9.06 (s, 1H, CH-1,2,3-triazole), 9.17 (s, 1H, HC = N). 13C NMR (100 MHz, DMSO-d6): δC = 24.56 (CH3); 61.73 (OCH2); 115.63, 116.96, 119.13, 121.65, 124.65, 125.34, 128.69, 130.25, 131.45, 134.26, 136.09, 143.36, 160.72, 161.75, 165.23 (Ar-C, C = N, C = O). Calculated for: C26H19N5O4: C, 67.09; H, 4.11; N, 15.05. Found: C, 67.27; H, 4.17; N, 15.14.
Characterisation of (2S,3S,4R,5S,6S)-2-(acetoxymethyl)-6–(4-((4-((E)-((1,3-dioxoisoindolin-2-yl)imino)methyl)phenoxy)methyl)-1H-1,2,3-triazol-1-yl)tetrahydro-2H-pyran-3,4,5-triyl triacetate (10a)
This compound was obtained as white pellets in 91% yield, mp: 166–167 °C. IR (υ, cm−1): 1580 (C = C), 1660 (C = N), 1735 (C = O), 2830 (CH-Al), 3070 (CH-Ar). 1H NMR (400 MHz, DMSO-d6) δH = 1.70, 1.90, 1.94, 1.96 (4s, 12H, 4 x CH3CO), 4.01–4.11 (m, 2H, H-6, H-6′), 4.32 (ddd, 1H, J = 4 Hz, J = 8 Hz, J = 12 Hz, H-5), 5.14 (dd, 1H, J = 4 Hz, J = 8 Hz, H-3), 5.21 (s, 1.30H, OCH2), 5.24 (s, 0.70H, OCH2), 5.49 (t, 1H, J = 8 Hz, H-4), 5.62 (dd, 1H, J = 4 Hz, J = 8 Hz, H-2), 6.32 (d, 1H, J = 8 Hz, H-1), 7.16 (dd, 2H, J = 4 Hz, J = 8 Hz, Ar-H), 7.76–7.86 (m, 6H, J = 8 Hz, Ar-H), 8.53 (s, 1H, CH-1,2,3-triazole), 9.03 (s, 0.65H, HC=N), 9.80 (s, 0.35H, HC=N). 13C NMR (100 MHz, DMSO-d6): δC = 20.34, 20.71, 20.85, 20.99 (4 x CH3CO); 61.55 (OCH2); 62.19 (C-5); 67.95 (C-6); 70.57 (C-4); 72.64 (C-3); 73.81 (C-2); 84.36 (C-1); 115.74, 115.82, 123.90, 124.44, 124.50, 126.62, 130.41, 130.48, 130.52, 132.32, 135.39, 143.35, 143.56, 160.90, 161.29, 163.26, 165.08 (Ar-C, C = N, C = O), 169.06, 169.96, 170.17, 170.66 (CH3C=O). Calculated for: C32H31N5O12: C, 56.72; H, 4.61; N, 10.34. Found: C, 56.53; H, 4.69; N, 10.46.
Characterisation of (2S,3R,4R,5S,6S)-2-(acetoxymethyl)-6–(4-((4-((E)-((1,3-dioxoisoindolin-2-yl)imino)methyl)phenoxy)methyl)-1H-1,2,3-triazol-1-yl)tetrahydro-2H-pyran-3,4,5-triyl triacetate (10b)
This compound was obtained as white pellets in 90% yield, mp: 175–176 °C. IR (υ, cm−1): 1555 (C = C), 1670 (C = N), 1730 (C = O), 2890 (CH-Al), 3050 (CH-Ar). 1H NMR (400 MHz, DMSO-d6) δH = 1.71, 1.88, 1.92, 2.12 (4s, 12H, 4 x CH3CO), 3.98 (dd, 1H, J = 4 Hz, J = 12 Hz, H-6), 4.09 (dd, 1H, J = 4 Hz, J = 12 Hz, H-6′), 4.51 (t, 1H, J = 4 Hz, H-5), 5.24 (s, 2H, OCH2), 5.40 (dd, 2H, J = 4 Hz, J = 8 Hz, H-3, H-4), 5.57 (dd, 1H, J = 4 Hz, J = 8 Hz, H-2), 6.34 (d, 1H, J = 8 Hz, H-1), 7.18 (d, 2H, J = 8 Hz, Ar-H), 7.79–7.85 (m, 6H, J = 8 Hz, Ar-H), 8.48 (s, 1H, CH-1,2,3-triazole), 9.81 (s, 1H, HC=N). 13C NMR (100 MHz, DMSO-d6): δC = 20.40, 20.79, 20.88, 20.97 (4 x CH3CO); 61.55 (OCH2); 62.04 (C-5); 67.77 (C-6); 68.18 (C-4); 70.89 (C-3); 73.48 (C-2); 84.77 (C-4); 115.75, 115.81, 116.37, 124.72, 124.98, 126.78, 130.40, 130.63, 132.33, 134.72, 143.19, 143.62, 160.82, 163.28, 164.92 (Ar-C, C = N, C = O), 169.10, 170.07, 170.56, 170.59 (CH3C=O). Calculated for: C32H31N5O12: C, 56.72; H, 4.61; N, 10.34. Found: C, 56.56; H, 4.55; N, 10.45.
Biological evaluation studies
Antiviral assay
The effect of target chemical treatments on SARS-CoV-2 viral load (SARS-CoV-2 isolate EGY/WAT-2 VACCERA) was assessed by the Real-Time PCR test to detect SARS-CoV-2 viral RNACitation52,Citation53. Total RNA was extracted according to the instructions using the genesig® Coronavirus SARS-CoV-2 Real-Time PCR Assay kit (Primer design TM Ltd, Southampton, United Kingdom). All procedures are depicted in the supplementary section.
Cytotoxicity evaluation using a viability assay
The cytotoxic activity was assessed using the 3–(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) colorimetric assay as reported previously. In brief, the tumour cell lines were suspended in medium at concentration 5 × 104 cell/well in Corning® 96-well tissue culture plates and then incubated for 24 h. The tested compounds with concentrations ranging from 0 to 50 μg/ml were then added into 96-well plates (six replicates) to achieve different conc. for each compound. Six vehicle controls with media or 0.5% DMSO were run for each 96 well plate as a control. After incubating for 24 h, the numbers of viable cells were determined by the MTT testCitation54–56.
SARS-CoV-2-MPro inhibition assay
The COV2-SARS-CoV-2 protease enzyme assayCitation46,Citation57–59 was described in the manufacturing protocol (BPS Bioscience)Citation60. The methodology is described in the supplementary section.
Molecular docking
The interaction of the designated compounds with SARS-CoV‑2 Mpro Enzyme] were performed with the aid of MOE softwareCitation61. Also, we used the graphical user interface program autodock software as a complementary toolCitation62. The protein crystal structure in combination with Bipyridine benzonitrile was extracted from the RCSB PDBCitation63. Preparation of the protein was performed over two steps using Maestro 8.0’s ‘protein preparation wizard. To vert any electronic clash in the protein, the energy of the crystal structure was minimised using OPLS 2005 force field. The binding site was assigned as indicated by the native ligand, and the receptor grid was created using “Grid Generation tool”. Finally, a single best pose was generated as the output for the tested ligands.
Results and discussions
Chemistry
The synthetic route adopted for the synthesis of the targeted phatamide-1,2,3-triazole molecular conjugates was outlined in Schemes 1–4. The precursor phthalimide-based azomethine linkage and alkyne side chain 3 was successfully synthesised in 92% yield through the thermal condensation of 2-aminoisoindoline-1,3-dione (3) and 4-(prop-2-yn-1-yloxy)benzaldehyde (2) in the presence of catalytic amount of acetic acid for 4 h. According to our previously published workCitation46, the alkyne 2 was synthesised by propargylation of 4-hydroxybezaldehyde 1 in refluxing acetone with potassium carbonate as a basic catalyst (Scheme 1).
The chemical structure of the synthesised Schiff base 4 was elucidated by spectroscopic methods. Thus, its IR spectrum was in obvious with the structure. It showed the disappearance of the carbonyl aldehyde and amino groups of the starting materials 2 and 3, respectively, which confirmed their condensation in the formation of the resulted azomethine group (C = N). The 1H NMR spectrum also supported the condensation reaction by the absence of amino (NH2) and aldehyde (CHO) protons, and presence of the distinct singlet at δH 9.11 ppm attributed to the imine proton (HC = N). The spectrum also revealed two characteristic singlets at δH 3.65 and δH 4.91 ppm assigned to the ≡CH and OCH2 protons, respectively. Extra aromatic protons were observed in the aromatic area. Moreover, its 13C NMR spectrum showed the appearance of signals at δC 160.52–165.02 ppm assigned to the imine (C = N) and phthalimide carbonyl amide (C = O) carbons, respectively. The diagnostic acetylenic carbons (-C≡C-) were observed at δC 79.15 and 79.27 ppm. All remaining carbons were recorded at their expected chemical shift (See experimental section).
Using the previously reported approachCitation34, focused substituted anilines were effectively converted to the desired phenylacetamide azides 5a-h through their alkylation with chloroacetyl chloride followed by azidolysis reaction. Within the scope on the molecular hybridisation, the desired molecular hybrids 6a-h were obtained with good to excellent yields (87–90%); by the construction of the 1,2,3-triazole via the copper-catalyzed azide-alkyne cycloaddition (CuAAC) between the phthalimide Schiff base bearing alkyne side chain 4 and the synthesised azides 5a-h in the presence of copper sulphate and sodium ascorbate as catalysts in a mixture of DMSO:H2O as solvent at 80 °C for 6–8 h (Schemes 2 and Citation3).
The success of the cycloaddition reaction was confirmed by the spectral data of the resulting click adducts 6a-g. Their IR spectra showed clearly the disappearance of the acetylenic groups (C≡C and ≡C-H) of their precursor 4, which confirmed its involvement on the formation of 1,2,3-triazole ring. The investigation of their 1H NMR spectra revealed the presence of a diagnostic singlet at around δH 8.21–8.33 ppm attributed to the characteristic H-5-triazolyl ring. The amidic protons (CONH) were recorded as singlets at δH 9.03–11.12 ppm, where the methylene protons (OCH2, NCH2 and NHCH2 were observed at δH 4.30–5.47 ppm. The remaining protons were resonated at their respected area and listed in the experimental section. The analysis of the 13C NMR data showed clearly disappearance Sp-carbons and the appearance of the new aliphatic carbon signals (OCH2, NCH2 and NHCH2) between δC 42.12–61.88 ppm. Additional aromatic carbons resonating in their respected chemical shifts (See experimental section).
Furthermore, we have expanded our efforts to synthesise several new phthalimide-1,2,3-triazole hybrids encapsulating lipophilic aromatic rings 8a-h (87–90% yield) utilising the optimised click synthesis outlined above (CuSO4, Na-ascorbate; H2O:DMSO), as shown in Scheme 3. The synthetic strategy started with the synthesis of aromatic azide building blocks 7a-h by diazotisation reactions of their corresponding aniline derivatives as previously reported in our workCitation37.
The structures of compounds 8a-h were in obvious of their IR spectra, which confirmed the participation of the acetylenic groups of the alkyne 4 on the formation of the triazole core. The formation of such ring in compounds 8a-h was also supported by their 1H NMR spectra which displayed the resonance of the distinct H-5-triazolyl proton as a singlet around δH 8.74–9.10 ppm. The spectra also recorded signals attributed to the methylene protons (OCH2) at δH 5.29–5.37 ppm. Additional aromatic protons of the phenyl rings were observed in the aromatic area and are detailed in the experimental section. No Sp-carbons were recorded in their 13C NMR data which evidenced the success of the cycloaddition reaction. All remaining carbons were listed in the experimental section. Owing to the fact that the molecular hybridisation strategy has attracted continuing interest for drug developments, it was interested to vary the azide structure to increase the synergetic effect of the resulted click candidates. Thus, click synthesis of a focused 1,2,3-triazole-phthalimide harbouring glycosyl moieties 10a-b was also carried out in this study. The click ligation of the same precursor alkyne 4 with glycosyl azides 9a-b under the previously adopted copper 1,3-dipolar cycloaddition reaction conditions gave selectively the targeted 1,4-disubstituted-1,2,3-triazoles based phthalimide-N-glycoside molecular hybrids in 90–91% yield (Scheme 4).
The structure elucidation of the resulted click adducts 10a-b were established FT-IR, 1H NMR, 13C NMR spectroscopic methods, which evidenced the success of the click synthesis by the appearance of a distinct doublet at δH 6.32 and 6.34 ppm with J1′,2′ value equal to 8 Hz attributed to the glycosyl anomeric proton (H-1) and confirming the β-configuration. The recorded anomeric protons were correlated with their respected carbons C-1 resonating at δC 84.36 and 84.77 ppm supporting the β-anomers. New singlets were observed in the aliphatic regions in their 1H NMR spectra (δH 1.70–2.12 ppm) and correlated with their carbons (δC 20.34–20.99 ppm) in the13C NMR spectra, these signals were assigned to the diagnostic acetoxy groups (OAc). Furthermore, the triazolyl H-5 were observed at δH 8.48–8.53 ppm confirming the ring closure and cycloaddition reaction. All remaining protons and carbons were recorded at their respect chemical shifts (See experimental section).
Evaluation of antiviral activity against SARS-CoV-2
At the outset of our antiviral investigations, we tested the ability of the synthesised molecules to inhibit SARS CoV-2 in infected Vero E6 cells in terms of (% of inhibition) compared to reference drug. We utilised two concentrations to perform our assay, 1 and 10 uM. In addition, we tested the cytotoxic effect of our molecules in Vero E6 cells to correlate antiviral activity and cytotoxicity and ensure their low or no cytotoxic effects on the cells. The data from these assays are summarised in . At 1 uM concentration, molecules 6a and 8e with 4-carboxyphenyl and 2-fluoroophenyl side chain, respectively, showed comparable activity to Remdesivir (87.82 and 83.58 vs. 92.72%). On the other hand, the other derivatives showed moderate to weak % of inhibition as indicated by their API values.
Table 1. Antiviral activity of novel phthalimide-based triazole inhibitors by propagation in Vero E6 cells.
At 10 uM, the majority of our derivatives started to show exceptional inhibition activities in comparison with Remdesivir. API parameter showed values ranging from 0.9 to 0.98 (). Compound 6a, with 4-propionamidobenzoic acid side chain, showed the highest activity among the tested compounds and the better API value in comparison with Remdesivir (94.96% and 0.98). In order to ensure that any given activity of our compounds is not due to a potential toxicity to the tested cells, we introduced Vero E6 cells cytotoxicity as an additional parameter in our evaluation. To distinguish the performance of our molecules in both sides (antiviral activity and toxicity), we generated a parameter called CT/A parameter which compare the IC50 (in uM) of the tested molecule (include Remdesivir) with the antiviral activity maximum concentration (10 uM). Higher CT/A value implies that the tested molecule was safe to the cells during the assay, and it is applicable to increase the drug concentration to achieve 100% eradication of the virus. Compound 8a bearing 4-fluorophenyl achieved the best CT/A parameter which represents (12.7). This value means that the safety margin of this compound represents 12.7-fold of its antiviral activity. Additionally, Remdesivir came in the 5th place among the molecules that showed the lowest CT/A parameter (4.31). Among the tested compounds, 6a, 6e, 6 g, and 8a displayed the best combination between the antiviral activity and tolerability at 10 uM. Since derivatives 6e and 8a share the same side chain (4-fluorophenyl), we concluded that fluorene atom at that position may be essential for the antiviral activity of this scaffold.
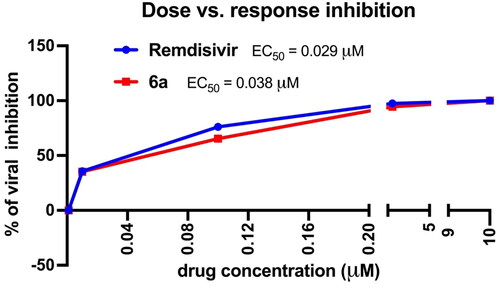
Then, we tested the effect of compound 6a as a representative of the most active compounds in our series, at five different concentrations. We compared the effect of our compound with Remdesivir, the drug of choice for the treatment of COVID-19. The data are summarised in .
The tested molecules (Remdesivir and 6a) showed a dose–response inhibition of the virus with comparable EC50 values (0.029 and 0.039 uM). At a very low dose (0.01 uM) our compound showed ∼ 40% virus inhibition. This value represents 995 times the CT/A value of this compound. This data collectively indicated that this scaffold is a promising starting point to generate an effective treatment for SARS-CoV-2.
SARS-CoV-2 Mpro inhibition assay
In our effort to detect the mechanism of action of our compounds, we tested the inhibition effect of SARS-CoV-2 Mpro by a designated derivative of our designed compounds. In several studies, the SARS-CoV-2 Mpro is the key protease of CoV-2 and was proved as one of the fundamental targets to impact this virusCitation64,Citation65. Many SARS-CoV-2 Mpro inhibitors have been reported that can covalently or non-covalently have been reportedCitation66. Some of the reported compounds carries the same triazol scaffoldCitation67. Such reports encouraged us to envision the impact of our molecule on SARS-CoV-2 Mpro. We tested the effect of the in vitro Mpro inhibition effect of the selected compounds using MTT assay, and compared it with lopinavir, the non-specific Mpro inhibitorCitation68. Results from this assay is summarised in .
Table 2. Inhibitory data of selected derivatives against SARS-CoV-2 Mpro.
Compounds 6a and 6 g showed IC50 values higher than Lopinavir, the non-specific Mpro inhibitor. Compound 6a was significantly more active than the other tested molecules and of the reference molecule. However, none of the tested compounds showed an IC50 concentration comparable to its viral inhibitory effect (10 uM, ). The results suggest that while the virus growth significantly inhibited by some of our compounds, the Mpro function did not highly altered. This observation suggesting that our molecules may have a mechanism of action independent of the Mpro target.
Computational studies
Molecular docking analysis
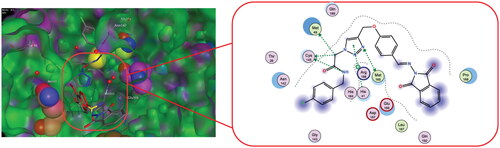
The Mpro active site is located between two-barrel domains, (1st amino acids 10–99, and 2nd amino acids 100–182). The 3rd domain, which consists of residues 198 to 306, is responsible for protein dimerisation and the formation of an alpha helical bundleCitation50. In addition, two important conserved residues, His41 and Cys145, create the catalytic dyad and dimerisation that complete the active site by bringing Ser161 of the second dimer protomer into proximity with Glu166 and promoting creation of the substrate specificity pocket and the oxyanion hole Citation51. In order to stabilise the molecule within the binding pocket, the phenoxy and triazole fragments interacted with the essential residues Gln189 and Met165, His41, and Arg188 via hydrogen bonds and the aromatic stacking effect. The catalytic Cys145 provided a stable hydrogen bonding with the middle amide moiety which might help in the stabilisation of these compounds within S1’ subsite. Reports state that the S2 subsite showed more flexibility than the others, preferring leucine or other hydrophobic residues while accepting smaller substituents in peptide-based inhibitorsCitation57. The majority of specific interaction of active analog occurs centrally through dual hydrogen bonding with Cys145-NH linker of amide, and Met165-n triazole, and a pattern of an aromatic ring formation - stacking interactions with Met165, which drives the terminal phthalimide component to near to S1’ site. Analysing the interaction of compound 6 g (the most prominent derivative in our series) showed no covalent interactions within the binding pocket. The majority of chemical moieties in this molecule contribute to the activity and tolerate the pocket with all catalytic sites, as shown in .
ADMET analysis
Then we used OSIRIS Property Explorer tool to estimate the adverse effects risk of designated analogues 6a, 6 g, and 10a. These effects include tumorigenic effects and reproductive consequences. In addition, we investigated some drug-relevant characters, such as cLogP, LogS as an indication of the compounds solubility, drug-likeness, and overall drug-scoreCitation69,Citation70. We compared the results from our molecules with two drugs, GC-376 and Lopinavir. The results from these calculations are shown in . All the compounds showed an acceptable “predicted” solubility and cLogP values. On the other hand, none of our molecule showed any potential toxicity risks as indicated by the green colour code. In addition, the potential drug-likeness scores of the compound 6a were superior to 6 g and GC-376 and comparable to lopinavir. Chemically, our data showed that that tethering COOH or F as substituents to the peripheral phenyl group sustain a minimal danger of carcinogenic and mutagenic toxicity while appropriately boosting lipophilicity. Generally, the values of drug-score of the representative 6a derivative (0.28) were less than compared to GC-376 (0.37) and better than Lopinavir (0.17).
Table 3. Calculation of certain properties of compounds 6f, 6i, and 10a in comparison with reported drugs as predicted by OSIRIS Property Explorer and Molinspiration tools.
Next, we performed a computational study to predict ADME properties of the synthesised molecules. We calculated several properties such as lipophilicity, absorption (% ABS), topological polar surface area (TPSA), and simple molecular descriptors represented by the Lipinski rule of fiveCitation55,Citation71–73. We used the Molinspiration online property calculation toolkit to perform our calculationsCitation74. Data from these calculations is summarised in . It is worth noting that the software used the Zhao equation (% ABS = 109−(0.345 × TPSA)) to calculate the percentage of absorption (% ABS)Citation75. The predicted percentages of absorption for our compounds were ranging between 68 and 38%. In addition, the TPSA values located within the acceptable range as indicated in . Collectively, data from both calculations revealed that our molecules are most likely drug-like candidatesCitation13,Citation45,Citation46.
Molecular descriptors–based SAR analysis
Next, we used the MOPAC quantum engine module implemented in the MOE software to perform quantum mechanical calculations for the SAR analysis of representative molecules from our series (6a; high activity, 6 g; low activity, and 10a; inactive). The energies of frontier orbitals typically have a significant impact on various chemical and pharmacological process properties. These characteristics provide details about the drugs’ acceptors and donators, and as a result, they provide details about how a charge transfer complex (CTC) forms Citation21,Citation72. The summarises our findings from these calculations. Electron cloud formed by the COOH group to compound 6a is likely to contribute positively to the biological activity of this derivative. On the other hand, the analysis is different in the case of the other two molecules. The presence of another phenyl substitution (F) in case of 6 g or polar nucleoside in the case of 10a altered the calculations. Compounds 6 g and 10a showed slight or major difference in SCF energy and dipole effect, respectively. This data further confirms the impact of certain substitution on the activity of our compounds.
Table 4. Molecular mechanics parameters and molecular orbital spatial distribution and localisation for the HOMO-LUMO of representative compounds, 6a, 6 g, and 10a.
Molecular parameters are calculated by default from MOE program showing number of atoms, orbitals, electrons, SCF energy, dipole moment, and heat of formation. These values are reported for representative derivatives of high (6a), medium (6 g), and low (10a) activity.
Conclusions
This current study details the synthesis of novel molecular conjugates incorporating phthalimide-1,2,3-triazole and assesses their effectiveness in combating SARS-CoV infection. We conducted initial investigations into the mechanism of action to elucidate the process of virus inhibition. The results revealed two highly potent analogues, 6a and 6 g, within the novel scaffold, demonstrating an impressive 94% growth inhibition against the SARS-CoV virus. In vitro inhibition data for Mpro suggested that our compounds might not exclusively target Mpro, implying that the notable virus inhibition observed may be attributed to a different target. The successful development of a stable chemical scaffold with favourable pharmacokinetic properties holds significant promise for advancing antiviral drug discovery. Further studies are imperative to unravel the specific target of these molecules.
Author contributions
Conceptualisation: M.R.A., N.R., H.E.A.A.; Methodology: M.R.A., A.A., M.A., M.A.S., H.E.A.A.; Software: M.A.S., H.E.A.A.; Validation: M.R.A., N.R., S.Y.A., M.A.S., H.E.A.A.; Formal analysis: N.R., A.A., M.A., M.A.S., H.E.A.A.; Investigation: M.R.A., N.R., M.A., H.E.A.A.; Resources: A.A., M.A., S.Y.A.; Data curation: M.R.A., A.A., S.Y.A., M.A.S.; Visualisation: M.R.A., A.A.; Supervision: M.R.A., N.R., H.E.A.A.; Project administration: N.R., A.A., M.A., S.Y.A.; Funding acquisition: N.R., A.A., M.A., S.Y.A.; Writing—original draft preparation: M.R.A., N.R., A.A., M.A.S., H.E.A.A.; Writing—review and editing: M.R.A., N.R., H.E.A.A.; All authors have read and agreed to the published version of the manuscript.
Sample availability
No Samples are available.
Supplemental Material
Download PDF (1.5 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and/or its supplementary materials.
Additional information
Funding
References
- Wu F, Zhao S, Yu B, Chen Y-M, Wang W, Song Z-G, Hu Y, Tao Z-W, Tian J-H, Pei Y-Y, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):1–15.
- Lengerli D, Ibis K, Nural Y, Banoglu E. The 1, 2, 3-triazole ‘all-in-one’ring system in drug discovery: A good bioisostere, a good pharmacophore, a good linker, and a versatile synthetic tool. Expert Opin Drug Discov. 2022;17(11):1209–1236.
- Hashimoto Y. Thalidomide as a multi-template for development of biologically active compounds. Arch Pharm (Weinheim)). 2008;341(9):536–547.
- Shinji C, Nakamura T, Maeda S, Yoshida M, Hashimoto Y, Miyachi H. Design and synthesis of phthalimide-type histone deacetylase inhibitors. Bioorg Med Chem Lett. 2005;15(20):4427–4431.
- Wang F, Yin H, Yue C, Cheng S, Hong M. Syntheses, structural characterization, in vitro cytotoxicities and DNA-binding properties of triphenylantimony di (N-oxy phthalimide) and di (N-oxy succinimide) complexes. J Organomet Chem. 2013;738:35–40.
- Mahmudov I, Demir Y, Sert Y, Abdullayev Y, Sujayev A, Alwasel SH, Gulcin I. Synthesis and inhibition profiles of N-benzyl- and N-allyl aniline derivatives against carbonic anhydrase and acetylcholinesterase – a molecular docking study. Arabian J Chem. 2022;15(3):103645.
- Gümüş M, Babacan ŞN, Demir Y, Sert Y, Koca İ, Gülçin İ. Discovery of sulfadrug–pyrrole conjugates as carbonic anhydrase and acetylcholinesterase inhibitors. Arch Pharm (Weinheim)). 2022;355(1):e2100242.
- Albo Hay Allah MA, Balakit AA, Salman HI, Abdulridha AA, Sert Y. New Heterocyclic Compound as Carbon Steel Corrosion Inhibitor in 1 M H2SO4, High Efficiency at Low Concentration: Experimental and Theoretical Studies. J Adhes Sci Technol. 2023;37(3):525–547.
- Jan JT, Cheng TR, Juang YP, Ma HH, Wu YT, Yang WB, Cheng CW, Chen X, Chou TH, Shie JJ, et al. Identification of existing pharmaceuticals and herbal medicines as inhibitors of SARS-CoV-2 infection. Proc Natl Acad Sci U S A. 2021;118(5):e2021579118.
- Jayk Bernal A, Gomes da Silva MM, Musungaie DB, Kovalchuk E, Gonzalez A, Delos Reyes V, Martín-Quirós A, Caraco Y, Williams-Diaz A, Brown ML, et al. Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients. N Engl J Med. 2022;386(6):509–520.
- Mahase E. Covid-19: Pfizer’s paxlovid is 89% effective in patients at risk of serious illness, company reports. BMJ. 2021;375:n2713.
- Juang Y-P, Chou Y-T, Lin R-X, Ma H-H, Chao T-L, Jan J-T, Chang S-Y, Liang P-H. Design, synthesis and biological evaluations of niclosamide analogues against SARS-CoV-2. Eur J Med Chem. 2022;235:114295.
- Malebari AM, Ahmed H EA, Ihmaid SK, Omar AM, Muhammad YA, Althagfan SS, Aljuhani N, Sayed AAE, Halawa A-A, El-Tahir Hm AH, Turkistani SA, et al. Exploring the dual effect of novel 1,4-diarylpyranopyrazoles as antiviral and anti-inflammatory for the management of SARS-CoV-2 and associated inflammatory symptoms. Bioorg Chem. 2023;130:106255.
- Shyr ZA, Gorshkov K, Chen CZ, Zheng W. Drug discovery strategies for SARS-CoV-2. J Pharmacol Exp Ther. 2020;375(1):127–138.
- Ouyang G, Chen Z, Cai XJ, Song BA, Bhadury PS, Yang S, Jin LH, Xue W, Hu DY, Zeng S. Synthesis and antiviral activity of novel pyrazole derivatives containing oxime esters group. Bioorg Med Chem. 2008;16(22):9699–9707.
- Alsehli M, Aljuhani A, Ihmaid SK, El-Messery SM, Othman DIA, El-Sayed A-A, Ahmed HEA, Rezki N, Aouad MR. Design and synthesis of benzene homologues tethered with 1,2,4-triazole and 1,3,4-thiadiazole motifs revealing dual MCF-7/HepG2 cytotoxic activity with prominent selectivity via histone demethylase LSD1 inhibitory effect. Int J Mol Sci. 2022;23(15):8796.
- Ihmaid SK, Aljuhani A, Alsehli M, Rezki N, Alawi A, Aldhafiri AJ, Salama SA, Ahmed HEA, Aouad MR. Discovery of triaromatic flexible agents bearing 1,2,3-triazole with selective and potent anti-breast cancer activity and CDK9 inhibition supported by molecular dynamics. J Mol Struct. 2022;1249:131568.
- Ihmaid SK, Alraqa SY, Aouad MR, Aljuhani A, Elbadawy HM, Salama SA, Rezki N, Ahmed HEA. Design of molecular hybrids of phthalimide-triazole agents with potent selective MCF-7/HepG2 cytotoxicity: Synthesis, EGFR inhibitory effect, and metabolic stability. Bioorg Chem. 2021;111:104835.
- Al-Masoudi NA, Abood E, Al-Maliki ZT, Al-Masoudi WA, Pannecouque C. Amino acid derivatives. Part 6. Synthesis, in vitro antiviral activity and molecular docking study of new N-α-amino acid derivatives conjugated spacer phthalimide backbone. Med Chem Res. 2016;25(11):2578–2588.
- Mandić L, Benčić P, Mlinarić-Majerski K, Liekens S, Snoeck R, Andrei G, Kralj M, Basarić N. Substituted adamantylphthalimides: Synthesis, antiviral and antiproliferative activity. Arch Pharm (Weinheim)). 2020;353(6):e2000024.
- Dege N, Gökce H, Doğan OE, Alpaslan G, Ağar T, Muthu S, Sert Y. Quantum computational, spectroscopic investigations on N-(2-((2-chloro-4, 5-dicyanophenyl) amino) ethyl)-4-methylbenzenesulfonamide by DFT/TD-DFT with different solvents, molecular docking and drug-likeness researches. Colloids Surf, A. 2022;638:128311.
- Menati S, Azadbakht R, Rudbari HA, Bruno G. Synthesis and characterization of four new azo-Schiff base and their nickel (II) complexes. Polyhedron. 2021;205:115296.
- Assis SP, da Silva MT, de Oliveira RN, Lima VL. Synthesis and anti-inflammatory activity of new alkyl-substituted phthalimide 1H-1,2,3-triazole derivatives. ScientificWorldJournal. 2012;2012:925925–925927.
- Assis SPO, Silva MTD, Silva FTD, Sant’Anna MP, Tenório C, Santos C, Fonseca C, Seabra G, Lima VLM, Oliveira RN. Design and synthesis of triazole-phthalimide hybrids with anti-inflammatory activity. Chem Pharm Bull (Tokyo)). 2019;67(2):96–105.
- Zhang W, Zhao Y, Zhang F, Wang Q, Li T, Liu Z, Wang J, Qin Y, Zhang X, Yan X, et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): The Perspectives of clinical immunologists from China. Clin Immunol. 2020;214:108393.
- Holanda VN, Lima EMA, Silva WVD, Maia RT, Medeiros RL, Ghosh A, Lima VLM, Figueiredo R. Identification of 1,2,3-triazole-phthalimide derivatives as potential drugs against COVID-19: a virtual screening, docking and molecular dynamic study. J Biomol Struct Dyn. 2022;40(12):5462–5480.
- Abutaleb NS, Elhassanny AE, Nocentini A, Hewitt CS, Elkashif A, Cooper BR, Supuran CT, Seleem MN, Flaherty DP. Repurposing FDA-approved sulphonamide carbonic anhydrase inhibitors for treatment of Neisseria gonorrhoeae. J Enzyme Inhib Med Chem. 2022;37(1):51–61.
- Paulsson-Habegger L, Snabaitis AK, Wren SP. Enzyme inhibition as a potential therapeutic strategy to treat COVID-19 infection. Bioorg Med Chem. 2021;48:116389.
- Khan SA, Akhtar MJ, Gogoi U, Meenakshi DU, Das A. An overview of 1,2,3-triazole-containing hybrids and their potential anticholinesterase activities. Pharmaceuticals. 2023;16(2):179.
- Rezki N, Almehmadi MA, Ihmaid S, Shehata AM, Omar AM, Ahmed HEA, Aouad MR. Novel scaffold hopping of potent benzothiazole and isatin analogues linked to 1,2,3-triazole fragment that mimic quinazoline epidermal growth factor receptor inhibitors: Synthesis, antitumor and mechanistic analyses. Bioorg Chem. 2020;103:104133.
- Bozorov K, Zhao J, Aisa HA. 1,2,3-Triazole-containing hybrids as leads in medicinal chemistry: A recent overview. Bioorg Med Chem. 2019;27(16):3511–3531.
- Al-Blewi F, Shaikh SA, Naqvi A, Aljohani F, Aouad MR, Ihmaid S, Rezki N. Design and synthesis of novel imidazole derivatives possessing triazole pharmacophore with potent anticancer activity, and in silico ADMET with GSK-3β molecular docking investigations. Int J Mol Sci. 2021;22(3):1162.
- Alraqa SY, Alharbi K, Aljuhani A, Rezki N, Aouad MR, Ali I. Design, click conventional and microwave syntheses, DNA binding, docking and anticancer studies of benzotriazole-1,2,3-triazole molecular hybrids with different pharmacophores. J Mol Struct. 2021;1225:129192.
- Almehmadi MA, Aljuhani A, Alraqa SY, Ali I, Rezki N, Aouad MR, Hagar M. Design, synthesis, DNA binding, modeling, anticancer studies and DFT calculations of Schiff bases tethering benzothiazole-1,2,3-triazole conjugates. J Mol Struct. 2021;1225:129148.
- Yahya Alraqa S, Alsayed Soliman M, Aljuhani A, Rezki N, Reda Aouad M, Ali I. Synthesis, characterization, DNA binding, docking, and anticancer studies of novel bis-1,2,3-triazoles phthalonitrile. ChemistrySelect. 2020;5(36):11347–11353.
- Aljuhani A, Almehmadi MA, Barnawi IO, Rezki N, Ali I, Messali M, Reda Aouad M. Microwave versus conventional synthesis, anticancer, DNA binding and docking studies of some 1,2,3-triazoles carrying benzothiazole. Arabian J Chem. 2021;14(3):102997.
- Upadhyay HC. Coumarin-1,2,3-triazole hybrid molecules: an emerging scaffold for combating drug resistance. Curr Top Med Chem. 2021;21(8):737–752.
- Belay Y, Muller A, Leballo P, Kolawole OA, Adeyinka AS, Fonkui TY, Motadi LR. Molecular hybrid of 1,2,3-triazole and Schiff base as potential antibacterial agents: DFT, molecular docking and ADME studies. J Mol Struct. 2023;1286:135617.
- Zhao S, Liu J, Lv Z, Zhang G, Xu Z. Recent updates on 1,2,3-triazole-containing hybrids with in vivo therapeutic potential against cancers: A mini-review. Eur J Med Chem. 2023; 251:115254.
- Reda Aouad M, Almehmadi MA, Faleh Albelwi F, Teleb M, Tageldin GN, Abu-Serie MM, Hagar M, Rezki N. Targeting the interplay between MMP-2, CA II and VEGFR-2 via new sulfonamide-tethered isomeric triazole hybrids; Microwave-assisted synthesis, computational studies and evaluation. Bioorg Chem. 2022;124:105816.
- Aljohani FS, Rezki N, Aouad MR, Elwakil BH, Hagar M, Sheta E, Hussein Mogahed NMF, Bardaweel SK, Hagras NA. Synthesis, characterization and nanoformulation of novel sulfonamide-1,2,3-triazole molecular conjugates as potent antiparasitic agents. Int J Mol Sci. 2022;23(8):4241.
- Çot A, Çeşme M, Onur S, Aksakal E, Şahin İ, Tümer F. Rational design of 1,2,3-triazole hybrid structures as novel anticancer agents: synthesis, biological evaluation and molecular docking studies. J Biomol Struct Dyn. 2023;41(14):6857–6865.
- Alzahrani SAS, Nazreen S, Elhenawy AA, Neamatallah T, Mahboob M. Synthesis, biological evaluation, and molecular docking of new benzimidazole-1,2,3-triazole hybrids as antibacterial and antitumor agents. Polycyclic Aromat Compd. 2023; 43(4):3380–3391.
- Şahin İ, Çeşme M, Güngör Ö, Özgeriş FB, Köse M, Tümer F. New sulfonamide derivatives based on 1,2,3-triazoles: synthesis, in vitro biological activities and in silico studies. J Biomol Struct Dyn. 2023;15:1–18.
- Musa A, Abulkhair HS, Aljuhani A, Rezki N, Abdelgawad MA, Shalaby K, El-Ghorab AH, Aouad MR. Phenylpyrazolone-1,2,3-triazole hybrids as potent antiviral agents with promising SARS-CoV-2 main protease inhibition potential. Pharmaceuticals (Basel). 2023;16(3):463.
- Gupta A, Singh P, Kamble B, Kulkarni A, Chandrasekar MJN. Synthesis, docking and biological evaluation of some novel 5-bromo-2- (5-aryl-1,3,4-thiadiazol-2-yl)isoindoline-1,3-dione derivatives targeting ATP-binding site of topoisomerase II. LDDD. 2012;9(7):668–675.
- Lamie P F, Phillopes J N, El-Gendy A O, Rarova L, Gruz J. Design, synthesis and evaluation of novel phthalimide derivatives as in vitro anti-microbial, anti-oxidant and anti-inflammatory agents. Molecules. 2015;20(9):16620–16642.
- El-Gohary NS, Shaaban MI. Synthesis, antimicrobial, antiquorum-sensing, and cytotoxic activities of new series of isoindoline-1, 3-dione, pyrazolo [5, 1-a] isoindole, and pyridine derivatives. Arch Pharm (Weinheim)). 2015;348(9):666–680.
- Ahmed HEA, Abdel-Salam HA, Shaker MA. Synthesis, characterization, molecular modeling, and potential antimicrobial and anticancer activities of novel 2-aminoisoindoline-1, 3-dione derivatives. Bioorg Chem. 2016;66:1–11.
- Abdulridha AA, Albo Hay Allah MA, Makki SQ, Sert Y, Salman HE, Balakit AA. Corrosion inhibition of carbon steel in 1 M H2SO4 using new Azo Schiff compound: Electrochemical, gravimetric, adsorption, surface and DFT studies. J Mol Liq. 2020;315:113690.
- Genesig CC-gR-TPa, https://www.genesig.com/assets/files/Path_COVID_19_CE_STED_IFU_Issue_500.pdf,. (accessed 12 March 2022.
- Santana PA, Álvarez CA, Valenzuela S, Manchego A, Guzmán F, Tirapegui C, Ahumada M. Stability of ACE2 peptide mimetics and their implications on the application for SARS-CoV2 detection. Biosensors (Basel). 2023;13(4):473–485.
- Ahmed HEA, El-Nassag MAA, Hassan AH, Mohamed HM, Halawa AH, Okasha RM, Ihmaid S, Abd El-Gilil SM, Khattab ESAEH, Fouda AM, et al. Developing lipophilic aromatic halogenated fused systems with specific ring orientations, leading to potent anticancer analogs and targeting the c-Src Kinase enzyme. J Mol Struct. 2019;1186:212–223.
- Ahmed HE, El-Nassag MA, Hassan AH, Okasha RM, Ihmaid S, Fouda AM, Afifi TH, Aljuhani A, El-Agrody A, chemistry m. Introducing novel potent anticancer agents of 1H-benzo [f] chromene scaffolds, targeting c-Src kinase enzyme with MDA-MB-231 cell line anti-invasion effect. J Enzyme Inhib Med Chem. 2018;33(1):1074–1088.
- Alswah M, Bayoumi A, Elgamal K, Elmorsy A, Ihmaid S, Ahmed H. Design, synthesis and cytotoxic evaluation of novel chalcone derivatives bearing triazolo[4,3-a]-quinoxaline moieties as potent anticancer agents with dual EGFR kinase and tubulin polymerization inhibitory effects. Molecules. 2018;23(1):48.
- Morse JS, Lalonde T, Xu S, Liu WR. Learning from the past: possible urgent prevention and treatment options for severe acute respiratory infections caused by 2019-nCoV. Chembiochem. 2020;21(5):730–738.
- Zhang L, Lin D, Kusov Y, Nian Y, Ma Q, Wang J, von Brunn A, Leyssen P, Lanko K, Neyts J, et al. α-Ketoamides as broad-spectrum inhibitors of coronavirus and enterovirus replication: structure-based design, synthesis, and activity assessment. J Med Chem. 2020;63(9):4562–4578.
- Zhu W, Xu M, Chen CZ, Guo H, Shen M, Hu X, Shinn P, Klumpp-Thomas C, Michael SG, Zheng W. Zheng W. Identification of SARS-CoV-2 3CL protease inhibitors by a quantitative high-throughput screening. ACS Pharmacol Transl Sci. 2020;3(5):1008–1016.
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733.
- Molecular Operating Environment (MOE) Chemical Computing Group M, Quebec, Canada. 2012; http://www.chemcomp.com. Accessed on 30/02/2013.
- Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ. Autodock4 and AutoDockTools4: automated docking with selective receptor flexiblity. J Comput Chem. 2009;30(16):2785–2791.
- Douangamath A, Fearon D, Gehrtz P, Krojer T, Lukacik P, Owen CD, Resnick E, Strain-Damerell C, Aimon A, Ábrányi-Balogh P, et al. Crystallographic and electrophilic fragment screening of the SARS-CoV-2 main protease. Nat Commun. 2020;11(1):5047.
- Sabbah DA, Hajjo R, Bardaweel SK, Zhong HA. An updated review on SARS-CoV-2 main Proteinase (MPro): protein structure and small-molecule inhibitors. Curr Top Med Chem. 2021;21(6):442–460.
- Sabbah DA, Hajjo R, Bardaweel SK, Zhong HA. An updated review on betacoronavirus viral entry inhibitors: learning from past discoveries to advance COVID-19 drug discovery. Curr Top Med Chem. 2021;21(7):571–596.
- Pang X, Xu W, Liu Y, Li H, Chen L. The research progress of SARS-CoV-2 main protease inhibitors from 2020 to 2022. Eur J Med Chem. 2023;257:115491.
- Chatterjee S, Kumar N, Sehrawat H, Yadav N, Mishra V. Click triazole as a linker for drug repurposing against SARs-CoV-2: A greener approach in race to find COVID-19 therapeutic. Current Research in Green and Sustainable Chemistry. 2021;4:100064.
- Nukoolkarn V, Lee VS, Malaisree M, Aruksakulwong O, Hannongbua S. Molecular dynamic simulations analysis of ritronavir and lopinavir as SARS-CoV 3CLpro inhibitors. J Theor Biol. 2008;254(4):861–867.
- Bouamrane S, Khaldan A, Maghat H, Ajana MA, Bouachrine M, Lakhlifi T. Quantitative Structure-Activity Relationships and Molecular Docking studies of 1.2.4 triazole derivatives as antifungal activity. Green and Applied Chemistry. 2020;10:33–48.
- Rateb HS, Ahmed HE, Ahmed S, Ihmaid S, Afifi T. Discovery of novel phthalimide analogs: synthesis, antimicrobial and antitubercular screening with molecular docking studies. Excli J. 2016;15:781–796.
- Remko M. Theoretical study of molecular structure, pKa, lipophilicity, solubility, absorption, and polar surface area of some hypoglycemic agents. J Mol Struct Theochem. 2009;897(1-3):73–82.
- Wang S, Jin G, Wang W, Zhu L, Zhang Y, Dong G, Liu Y, Zhuang C, Miao Z, Yao J, et al. Design, synthesis and structure–activity relationships of new triazole derivatives containing N-substituted phenoxypropylamino side chains. Eur J Med Chem. 2012;53:292–299.
- El-Azab AS, Abdel-Aziz AA-M, AlSaif NA, Alkahtani HM, Alanazi MM, Obaidullah AJ, Eskandrani RO, Alharbi A. Antitumor activity, multitarget mechanisms, and molecular docking studies of quinazoline derivatives based on a benzenesulfonamide scaffold: Cell cycle analysis. Bioorg Chem. 2020;104:104345.
- Srivastava R. Theoretical studies on the molecular properties, toxicity, and biological efficacy of 21 new chemical entities. ACS Omega. 2021;6(38):24891–24901.
- Zhao YH, Abraham MH, Le J, Hersey A, Luscombe CN, Beck G, Sherborne B, Cooper I. Rate-limited steps of human oral absorption and QSAR studies. Pharm Res. 2002;19(10):1446–1457.