ABSTRACT
Introduction: Neisseria meningitidis serogroup B (MenB) is the most common cause of bacterial meningitis in many industrialized countries and occurs at any age. The highest incidence is in infants aged <1 year, followed by children and adolescents. Four-component MenB vaccine (4CMenB, Bexsero) is the only MenB vaccine authorized for use in all age-groups. Experience with 4CMenB is growing as it is implemented in different countries/age-groups encompassing university students, children, adolescents, and infant mass vaccination programs.
Areas covered: An update of recently available data describing the mechanism of immunogenicity of 4CMenB and real-world evidence of vaccine effectiveness and disease impact. We discuss the appropriate age for vaccination to maximize population impacts.
Expert commentary: Invasive meningococcal disease is uncommon and sufficiently powered efficacy studies were not feasible during 4CMenB development. Additionally, several thousand genetically diverse invasive MenB strains circulate globally, varying widely in surface protein expression. This posed significant challenges in predicting clinical protection with MenB vaccines. Five years of 4CMenB use post-licensure confirm the clinical benefit of vaccination as predicted during development. Preliminary evidence suggests an extended impact on other meningococcal serogroups and Neisseria gonorrhoeae.
1. Introduction
Neisseria meningitidis serogroup B (MenB) is the most common cause of bacterial meningitis in many industrialized countries, and infants less than 1 year of age are most frequently affected. While the burden of illness due to meningococcal serogroups such as serogroup C (MenC) in Europe and Australia, and serogroup A in the meningitis belt has reduced dramatically in countries that employ meningococcal conjugate vaccines in routine childhood vaccination schedules, invasive meningococcal disease (IMD) due to MenB has continued unchecked. In 2012 in Europe, 68% of confirmed IMD cases were caused by MenB, with an overall case fatality of approximately 7%, compared to 14% for MenC and 8% for MenY [Citation1]. In 2016, 35% of all confirmed IMD in the United States (US) was due to MenB [Citation2], and in 2015, 62% of cases in Australia were due to MenB [Citation3].
The incidence of MenB in infants usually vastly exceeds the incidence in other age groups: for example, the incidence was 11-fold higher in <1 year olds (11.5 cases per 100,000) than in 15–24 year-olds in Europe (1.1 cases per 100,000) (2012), and was almost 6-fold higher in <1 year olds (0.67 cases per 100,000) than in 16–23 year-olds (0.12 cases per 100,000) in the US (2016) [Citation1,Citation2].
4CMenB is a four-component vaccine that contains three main Neisseria protein antigens discovered by mining the genome of N. meningitidis with the so-called ‘reverse vaccinology’ approach [Citation4]. They are Neisseria adhesin A (NadA), Neisserial Heparin Binding Antigen (NHBA), and variant 1 of factor H binding protein (fHbp). The three protein antigens were selected on the basis of their ability to induce serum bactericidal antibodies (SBA) to homologous and heterologous MenB strains [Citation5]. fHbp is a lipoprotein expressed on the surface of the bacterium that binds human complement regulator factor H; thus allowing the bacterium to escape complement killing and to grow in human blood. There are three noncross reactive variants of fHbp named variants 1, 2, and 3 that have also been described as subfamilies; subfamily B containing variant 1, and subfamily A containing variants 2 and 3. NadA is a surface exposed protein that binds human epithelial cells, monocytes and macrophages, and is involved in bacterial adhesion and invasion of human epithelial cells. NHBA is a lipoprotein also exposed on the bacterial surface which binds heparin, improving the survival of bacteria in blood, and mediates adhesion to epithelial cells. fHbp and NHBA antigens were fused respectively to two accessory antigens known as Genome-derived Neisseria Antigen (GNA) 2091 and GNA 1030, which were selected for their ability to induce bactericidal antibodies and/or to induce protection in an adult mouse model [Citation4].
The fourth component of 4CMenB is the OMV derived from a MenB outbreak strain (B:4:P1.7–2.4) from New Zealand and included in 4CMenB to provide protection against strains expressing the serosubtype P1.4 of PorA [Citation6,Citation7] (). The inclusion of four components in the final vaccine aimed at inducing high levels of SBA capable of providing broad protection against the majority of IMD caused by MenB, and to induce antibodies able to block important steps contributing to meningococcal virulence.
Table 1. Antigen components of 4CMenB and rLP2086 and rationale for their selection.
4CMenB (Bexsero, GSK), was first authorized in the European Union (EU) in 2013 and in the United States in 2015. 4CMenB is licensed in Europe, and other countries including Canada, Australia, Brazil, Argentina, for use in infants from 2 months of age as a three-dose schedule plus booster and from 3 months of age as a two-dose schedule plus booster [Citation8], and as a two-dose schedule from 2 years and above. In the United States, it is licensed for 10–25 year-olds as a two-dose schedule with a minimum interval of 1 month between doses. 4CMenB is currently recommended in infant universal mass immunization (UMV) programs in the United Kingdom (UK), Ireland, and Italy [Citation9–Citation12].
The second MenB vaccine, rLP2086 (Trumenba, Pfizer), was authorized in the United States in October 2014 and in the EU in 2017. rLP2086 is licensed for use as a two- or three-dose schedule in 10–25 year-olds in the United States and in individuals aged 10 years and older in the EU. rLP2086 contains two fHbp variants (variants 1 and 3 or subfamilies A and B) in a lipidated form ().
Because IMD is uncommon, it was not feasible to conduct sufficiently powered studies to demonstrate vaccine efficacy prior to licensure. Therefore, 4CMenB and rLP2086 were both authorized on the basis of immunogenicity demonstrated in clinical trials in terms of the percentage of subjects who achieved threshold levels considered protective in SBA assay using human serum as complement source (hSBA). An hSBA titer of at least 1:4 is widely accepted as a surrogate marker of protection against meningococcal disease [Citation13]. However, several thousand genetically diverse invasive MenB strains circulate globally, and these vary widely in their surface protein expression. Establishing the breadth of coverage of these new vaccines across the potential range of invasive strains proved difficult, and relied on SBA and novel in vitro assays such as the Meningococcal Antigen Typing System (MATS).
At present, the published data are not sufficient to enable evaluation of the full impact of rLP2086 vaccination. For 4CMenB, the journey to understand MenB vaccine efficacy started some 20 years ago when the genome sequence of MenB was decoded (). Since then, our understanding of MenB vaccine efficacy, in particular of 4CMenB, has advanced extensively. Effectiveness in preventing IMD at a population level has been demonstrated [Citation14], and the wider population impacts of vaccination continue to be explored (). In addition to direct protection of vaccinated persons, additional benefits could include reductions in carriage of MenB or other serogroups that share antigens in common with the vaccine, and potentially cross-species impacts on diseases such as gonorrhea.
Figure 1. 4CMenB: the journey from early research to real world experience.
MATS = Meningococcal Antigen Typing System, hSBA = serum bactericidal antibodies using human complement, IMD = invasive meningococcal disease. Figure references: 1 = [Citation26], 2 = [Citation80], 3 = [Citation81], 4 = [Citation14].
![Figure 1. 4CMenB: the journey from early research to real world experience.MATS = Meningococcal Antigen Typing System, hSBA = serum bactericidal antibodies using human complement, IMD = invasive meningococcal disease. Figure references: 1 = [Citation26], 2 = [Citation80], 3 = [Citation81], 4 = [Citation14].](/cms/asset/e0061f97-4081-49a6-865f-38f62f7f92bc/ierv_a_1547637_f0001_oc.jpg)
Since authorization, new data include effectiveness estimates against MenB from the UK infant UMV program, and data suggestive of possible protection against serogroup W (MenW) [Citation14,Citation15] and cross-protection against Neisseria gonorrhoeae infection have become available [Citation16,Citation17]. Additionally, data from in vitro studies with human monoclonal antibodies induced by 4CMenB provide first indications of the mechanism of 4CMenB immunogenicity, and the synergistic effect of the antibodies induced by the vaccine antigens. Here we provide an update of the mechanism of action of 4CMenB that support the antigen make-up of the vaccine, and real-world evidence of vaccine effectiveness and impact. We also discuss the appropriate age for vaccination to maximize population impacts. A summary contextualizing the results and potential clinical relevance and impact is displayed in the Focus on the Patient Section ().
2. Mechanistic insights into 4CMenB action
Meningococcal vaccines work by inducing complement-mediated killing of bacteria through antibodies directed against specific surface structures. However, conservation at the amino acid level and a threshold density of the target antigen is required for efficient complement activation [Citation18]. For the OMV component of 4CMenB, efficacy is linked to bactericidal antibodies directed mainly against the homologous PorA, which is highly abundant on the surface of the bacterium, but varies quite remarkably in terms of primary sequence among different isolates [Citation19]. For strains in which expression of the target antigens is low, or when their sequences are highly diverse compared to the forms present in the vaccine, multiple targets may be needed for antibody binding to reach sufficient antibody density on the bacterial surface to trigger the activation of complement [Citation20].
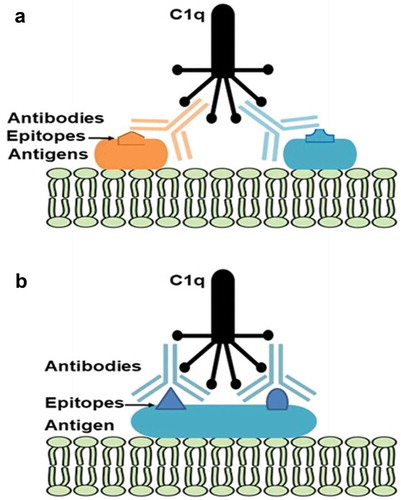
A recent study by Giuliani et al [Citation21], showed that fHbp, NadA, and NHBA in 4CMenB each contain multiple protective epitopes. Antibodies targeting the different epitopes work synergistically, enhancing the bactericidal response to each antigen. For example, monoclonal antibodies to fHbp that individually did not induce SBA with human complement, elicited strong SBA responses when combined with monoclonal antibodies targeting different epitopes on the same protein. In addition, it has been reported that antigens like fHbp and NHBA may work in synergy to elicit bactericidal killing of strains that would be resistant to antibodies raised against each of the two, separately [Citation22]. These findings show that the presence of multiple epitopes and multiple antigen targets in the same vaccine promotes a robust bactericidal response, even when a single target is not sufficient, thereby substantiating the inclusion of multiple antigens in 4CMenB. A model for this phenomenon is proposed in . As the majority of invasive MenB strains contain genes for more than one 4CMenB component, it is likely that the antibodies to each antigen, in addition to working on their own, contribute to a synergistic bactericidal response when combined. This is particularly important when the level of expression of an antigen is not high enough to guarantee an adequate density of the target on the bacterial surface. In the particular case of fHbp, it is well known that the level of antigen expression varies markedly between strains, and can be particularly low in strains expressing some peculiar var2 and var3 subvariants [Citation23–Citation25]. In these cases, the amount of antibodies directed to this target might not reach the threshold for achieving effective complement activation and bacterial killing. However, this gap could be partially filled by the presence of antibodies directed against other antigens (e.g. NHBA), which, although per se not sufficient for complement activation, could help to reach the threshold in cooperation with antibodies against fHbp, thus ultimately contributing to bacterial killing and protection.
Figure 3. Synergy of C1q engagement by (a) monoclonal antibodies binding to two different antigens on the bacterial surface or (b) two monoclonal antibodies binding to the same antigen.
So far, MATS has been used to predict the coverage of IMD-causing meningococcal strains in different countries. MATS measures the phenotypic expression and cross-reactivity of each of the three main antigens, fHbp, NadA, and NHBA on individual MenB strains. Combined with conventional genotyping for PorA, MATS provides an estimation of whether a given isolate would be susceptible to bactericidal killing by antibodies induced by 4CMenB. Since MATS provides an estimation of the contribution of each antigen independently, the cooperation of antibodies recognizing different antigens is not measured, providing a mechanistic explanation for the observed underestimation of 4CMenB protection. The underestimation of protection predicted by MATS was shown in the United Kingdom where the MATS predicted coverage was 73% on circulating clinical strains, while the bactericidal assay showed coverage of 88% of the strains [Citation26]. Similarly, a recent study conducted in Spain reported that strains predicted not to be covered by MATS were killed when tested in hSBA with human sera [Citation27]. Finally, the overall underestimation has been confirmed by real effectiveness of 82.9% from the use of 4CMenB in the UMV program in the United Kingdom [Citation14].
3. Recent evidence of 4CMenB vaccine effectiveness
3.1. Canada
In 2003, an outbreak of a MenB ST269 clone began in Quebec which primarily affected children and adolescents [Citation28]. The outbreak peaked in the Saguenay-Lac-Saint-Jean region where the incidence of MenB IMD reached 6.2/100,000 [Citation29]. A short-term mass vaccination campaign with 4CMenB commenced in 2014, targeting all individuals aged 2 months to 20 years living in the Saguenay-Lac-Saint-Jean region. Eighty-two percent of the target population (49,000 individuals) received at least one vaccine dose [Citation29]. No cases of MenB IMD were reported in vaccinated individuals between 2015 and 2016, from an incidence of MenB IMD in the target population of 11.4 per 100,000 in 2006–2014. No cases occurred in 10,000 unvaccinated individuals in the target population, and there were no cases among 2700 unvaccinated infants born in 2015 after the campaign had finished, which is suggestive of herd protection. During this period, in the entire region there were only two cases of IMD in nonvaccinated adults. The adjusted risk of disease in Saguenay-Lac-Saint-Jean was significantly decreased: relative risk 0.22 (95% confidence interval [CI] 0.05–0.92, p = 0.04). No safety concerns were raised during the campaign [Citation30].
3.2. United Kingdom
Routine meningococcal UMV was pioneered in the United Kingdom with the introduction of the first infant MenC vaccination campaign in 1999 in response to a prolonged clonal MenC outbreak [Citation31]. During the MenC campaign individuals from two months to 18 years of age were vaccinated with the conjugate vaccine. After the initial success of the infant and catch-up campaign, the MenC schedule received several adjustments and now comprises one dose in the second year of life and a second dose given as quadrivalent serogroups A, C, W, Y conjugate vaccine (MenACWY) at 14 years of age.
UMV with 4CMenB was introduced into the infant vaccination schedule in September 2015, and the UK authorities elected to use a reduced 2-dose priming schedule at 2 and 4 months of age, with booster at age 12 months [Citation11]. One year after the introduction of infant UMV, the number of IMD cases in vaccine-eligible infants decreased from an average of 74 in the previous four years, to 37 in 2015–16 [Citation14]. The overall effectiveness of two doses of 4CMenB against MenB IMD was calculated to be 82.9% (95% CI 24.1–95.2). Recent data showed that during the second year the vaccine impact was consistent with estimates reported for first year, suggesting similar ongoing efficacy in year 2 [Citation32]. Effectiveness data from the UK substantiate prelicensure estimates and provide re-assurance that 4CMenB is highly effective in preventing MenB IMD.
3.3. University outbreaks in the United States
4CMenB has been used in six MenB IMD outbreaks in university campuses since 2013 [Citation33]. In outbreaks that occurred in 2014 (University of California, Santa Barbara and Princeton University New Jersey) and in 2016 (Santa Clara University, California) [Citation33–Citation36], no cases of MenB have occurred in vaccinated students following the campaigns [Citation36].
A seroprevalence study conducted in vaccinated students at Princeton University showed that 87% and 100% of vaccinated students had positive hSBA against reference strains for fHbp and NadA, respectively, while 66.1% of vaccinated students had hSBA titers ≥1:4 against the outbreak strain [Citation37]. Nevertheless, no MenB IMD occurred in vaccinated students. This study confirms the findings of Goldschneider et al., published in 1969 [Citation38], who showed that hSBA underestimates protection, and that while a positive result on hSBA assay indicates protection against IMD, a negative hSBA result is not an indication of susceptibility [Citation39]. Indeed, whole blood from persons vaccinated with 4CMenB can kill meningococci even if negative in hSBA [Citation40].
4. Evidence for cross-protection
4.1. Can 4CMenB offer protection against meningococci beyond MenB?
The antigens contained in 4CMenB are common to meningococcal serogroups other than MenB to a varying degree [Citation41]. Thus it is expected that 4CMenB may induce some level of cross-protection against non-B serogroups. For example, the NadA and NHBA antigens are conserved also in the hypervirulent MenW ST11 strain responsible for a growing number of MenW cases in many countries including the United Kingdom, Australia, Chile, and Argentina [Citation15,Citation42,Citation43]. Conversely, most MenW ST11 strains express fHbp as variant 2 or 3, therefore while cross-protection from fHbp variant 1 present in 4CMenB cannot be anticipated, cross-protection might be afforded through NadA and NHBA. Studies using sera from 4CMenB-vaccinated persons support this hypothesis, showing bactericidal activity against MenW clinical isolates from England and Wales, Germany, France, and Brazil, and serogroup X isolates from Africa [Citation15,Citation44,Citation45]. Of note, MATS has not been developed to estimate coverage of non-B strains.
The UK commenced an emergency vaccination program using MenACWY at the same time (2015) when the UMV infant vaccination program with 4CMenB began [Citation46]. The target groups for MenACWY vaccination were adolescents 13–14 years of age, with a catch-up phase for 14–18 year olds, and university entrants. In the first year after the vaccination program only 36.6% coverage of the target population was achieved. Yet the number of MenW IMD cases increased in all age groups except the target age group aged 15–19 years (31% reduction in cases), and interestingly, in infants <1 year of age (35% reduction in cases) [Citation46]. The authors of this study concluded that the observed decrease in MenW cases in infants was consistent with the bactericidal activity elicited by 4CMenB against the MenW strains.
Although these data are suggestive of cross-protection by 4CMenB against other serogroups, large, well-designed studies are needed to provide conclusive evidence of cross-protection. Such studies will be difficult to perform in view of the low numbers of IMD cases caused by ACWY serogroups, and because of the confounding effects of concurrent meningococcal ACWY conjugate vaccination.
4.2. Can 4CMenB prevent N. gonorrhoeae infection?
4.2.1. Background and disease burden
N. gonorrhoeae causes infections of the genital tract with potentially severe long term consequences including infertility and chronic pelvic pain and is transmitted through sexual contact. N. gonorrhoeae also causes conjunctivitis, pharyngitis, pelvic inflammatory disease, and occasionally disseminated disease or meningitis [Citation47]. Antimicrobial resistance among clinical isolates is a growing concern [Citation48]. The World Health Organization estimated that today there are more than 78 million cases of gonorrhea worldwide, affecting almost 1% of the global population [Citation49]. N. gonorrhoeae infections are thus a major public health concern.
N. meningitidis and N. gonorrhoeae share 80–90% sequence homology at the genome level [Citation50]. In a study that examined 111 N. gonorrhoeae isolates from 28 countries, genes for fHbp, nhba, gna1030, and gna2091 were present in all isolates, whereas nadA was not identified in any isolate [Citation51]. NHBA, GNA 1030, and GNA 2091 were highly conserved across N. gonorrhoeae strains, while fHbp was present as variant 3 and shown to be nonlipidated and not surface exposed in gonococci [Citation52]. Compared to a reference MenB strain, amino acid sequence identity was 60.9% for gonococcal fHbp and 90.2% for NHBA. Expression of these proteins was variable [Citation51].
4.2.2 Evidence supporting cross-protection against N. gonorrhoeae
No clinical trials have been conducted to evaluate the effect of 4CMenB vaccination on N. gonorrhoeae infection. A modeling study predicted that assumed effectiveness of 4CMenB against gonorrhea of as low as 20% could prevent more than 83,000 infections per birth cohort in the United States, with substantial cost savings [Citation53]. Population-based surveillance in regions where 4CMenB has been widely used suggests that 4CMenB may show some cross-protection in the real-world setting [Citation16,Citation54].
A retrospective cohort study was conducted in the Saguenay-Lac-Saint-Jean region in Canada, where 49,000 individuals 2 months to 20 year of age received 4CMenB [Citation16]. Notifications of gonorrhea to the public health authority were compared for the prevaccination period (Jan 2006–June 2014) and the post-vaccination period (July 2014–June 2017). There were 231 notifications from 2006 until June 2017 in those aged 14 years and older. The number of gonorrhea cases decreased in 14–20 year-olds during the postvaccination period, but increased in those ≥21 years, although this trend was not significant; vaccine effectiveness against gonococcal infection in 14–20 year-old was 59% (95% CI −22–84, p = 0.1) [Citation16].
Other evidence comes from the 2004 to 2008 New Zealand campaign to control a prolonged MenB epidemic [Citation54]. The MenZB OMV vaccine – the same OMV component in 4CMenB – was given to individuals <20 years of age and 81% of the target group (approximately 1 million individuals) received vaccination. A retrospective case control study estimated vaccine effectiveness in preventing gonorrhea among 14,730 vaccinees aged 15–30 years-recruited from sexual health clinics [Citation17]. Cases were gonorrhea positive and controls were chlamydia positive. Vaccine effectiveness among fully vaccinated 15–30 year-olds was 31% (95% CI 21–39, p < 0.0001).
The statistically significant finding of 31% effectiveness of MenB OMV in preventing gonorrhea is very encouraging, particularly considering that additional potentially cross-protective antigens are present in 4CMenB. The evidence to date suggests that the degree of cross-protection could contribute to the overall population impact of 4CMenB vaccination programs.
4.3. Impact on meningococcal carriage
In a study conducted in UK adolescents, 4CMenB vaccination was associated with a significant decrease (26.6%, 95% CI 10.5–39.9) in the carriage of other capsular groups (BCWY, mainly serogroup Y), during the first year after vaccination. A trend in reduction in carriage of MenB strains was also detected, although not statistically significant, possibly because of the low level of carriage during the study [Citation55]. Carriage of MenB and carriage acquisition did not decrease after three doses of rLP2086 in a limited number of adolescents in the United States who were vaccinated as part of a MenB outbreak control program [Citation56]. In a recently published study, neither 4CMenB nor rLP2016 had an impact on carriage, although the study was probably too small to provide meaningful results [Citation57].
A large randomized control trial to assess the impact of 4CMenB on pharyngeal meningococcal carriage in adolescents is currently underway in Australia (www.clinicaltrials.gov NCT03089086) [Citation58].
4.4. What is the appropriate age for MenB vaccination? arguments for 4CMenB in infant UMV programs
While MenB infections occur in all age groups, the highest incidence is overwhelmingly in infants. Of all notified MenB IMD cases in Europe (2012), Australia (2015) and the US (2016), between 31.5% and 72% were in children less than 5 years of age, and 9% to 21% were in children aged less than 1 year [Citation1–Citation3].
Experience with meningococcal conjugate vaccines has shown that rapid disease control is achieved with infant UMV combined with a catch-up program in older age groups to rapidly induce herd protection effects [Citation31,Citation59]. For conjugate vaccines, booster doses during adolescence are required to prevent waning immunity [Citation59].
Adolescents play a pivotal role as carriers of meningococci and are the main source of transmission. Adolescent vaccination against meningococcal disease is considered key to interrupting carriage and transmission, thereby influencing the epidemiology of IMD in all age groups [Citation59]. However, results from a modeling study suggest that whilst adolescent-only vaccination programs against IMD can help to prevent disease in adolescents themselves, such strategies are not immediately effective in reducing disease in infants [Citation60]. In the United States, adolescent-only vaccination was pursued commencing in 2005 using a single dose of MenACWY in 11–18 year olds, with a booster dose added in 2010 due to concerns around waning immunity [Citation61]. Uptake of the vaccine was slow but steady, with coverage of one dose reaching 40% in 2008 and 82% by 2016 [Citation62]. Adolescents are traditionally difficult to access for vaccination because they are infrequent users of healthcare services and so have fewer opportunities to receive vaccination from a healthcare provider [Citation63].
For MenB vaccines, in the absence of solid carriage data for 4CMenB or for rLP2086, it is not known whether an adolescent-only strategy using MenB vaccines would have the desired effect on carriage and transmission to infants. If vaccination does interrupt transmission, the choice of an adolescent-only or combined adolescent plus infant strategy could influence the vaccine impact. A modeling study of 4CMenB vaccination in the United Kingdom has suggested that an adolescent-only vaccination strategy is the most favorable from a cost-effectiveness viewpoint, providing direct protection to the target population in whom a peak in IMD occurs. However, if vaccination interrupts transmission it would take 20 years for this strategy to substantially reduce the number of IMD cases [Citation60] (). In this model, most cases were prevented using a combined infant-adolescent vaccination strategy [Citation60].
Figure 4. Effect of alternate vaccination strategies on annual disease cases.
VEC = vaccine efficacy against carriage, SC = strain coverage, CU = one-off catch up vaccination. Routine vaccination infant at 2, 3, 4, and 12 months of age (blue line) and adolescent vaccination at age 13 years (2 doses) (dashed yellow line). Combined strategy refers to routine vaccination infant at 2, 3, 4, and 12 months (3 + 1 doses) and adolescent at 13 years (2 doses). This could be switched after 10 years to routine infant at 2, 4, and 12 months (2 + 1 doses) and adolescent at 13 years (2 doses) (dashed green line). Reproduced from [Citation60]: "Re-evaluating cost effectiveness of universal meningitis vaccination (Bexsero) in England: modelling study." Hannah Christensen, Caroline L Trotter, Matthew Hickman, W John Edmunds, 2014;349:g5725. With permission from BMJ Publishing Group Ltd.
![Figure 4. Effect of alternate vaccination strategies on annual disease cases.VEC = vaccine efficacy against carriage, SC = strain coverage, CU = one-off catch up vaccination. Routine vaccination infant at 2, 3, 4, and 12 months of age (blue line) and adolescent vaccination at age 13 years (2 doses) (dashed yellow line). Combined strategy refers to routine vaccination infant at 2, 3, 4, and 12 months (3 + 1 doses) and adolescent at 13 years (2 doses). This could be switched after 10 years to routine infant at 2, 4, and 12 months (2 + 1 doses) and adolescent at 13 years (2 doses) (dashed green line). Reproduced from [Citation60]: "Re-evaluating cost effectiveness of universal meningitis vaccination (Bexsero) in England: modelling study." Hannah Christensen, Caroline L Trotter, Matthew Hickman, W John Edmunds, 2014;349:g5725. With permission from BMJ Publishing Group Ltd.](/cms/asset/61f67ad5-5a53-4f74-8b36-618ed7501f2f/ierv_a_1547637_f0004_c.jpg)
4.4.1. The pivotal role of infant vaccination
There are very few vaccines for which the target group for vaccination is not the population most affected by disease. ‘Cocooning’ of newborn infants by administering pertussis vaccines to their contacts, and maternal immunization are examples of such policies, both of which provide almost immediate, short-term benefits to the vulnerable infant. While adolescent-only vaccination is likely to reduce MenB IMD in this age group, benefits to infants, in whom the disease burden is the highest, are unlikely to be realized for decades [Citation60]. Direct protection of infants through infant UMV is the only way to achieve rapid and early prevention of disease in the age group most at risk. The track record of infant UMV vaccination programs in achieving rapid and high coverage of new antigens is excellent, especially when the new antigens can be incorporated to the routine vaccination schedule and coadministered with other vaccines [Citation64]. If an effect of 4CMenB on nasopharyngeal carriage of meningococci can be demonstrated, then infant UMV would ideally be combined with an adolescent dose to maximize potential herd effects, interrupt transmission and reap the potential benefits of cross-protection. While this is the most costly option in the available model, the potential benefits of cross-protection and an extended impact on sexually transmitted disease (namely gonorrhea) have not been accounted for, and could markedly improve the cost-effectiveness of 4CMenB vaccination.
4.4.2. Evidence supporting 4CMenB vaccination in infants
4CMenB is the only MenB vaccine approved for use in individuals starting from 2 months of age (although this indication is not in place worldwide). Immunogenicity and safety data supporting licensure of 4CMenB in infants have been recently reviewed [Citation65]. Prelicensure studies established immunogenicity and a clinically acceptable safety profile in more than 5800 infants using three-dose primary vaccination plus booster, with or without co-administration with other routinely administered vaccines [Citation6,Citation66–Citation70]. 4CMenB was evaluated in commonly used schedules as three-dose priming with booster or reduced two-dose priming with booster. A pivotal study conducted in over 2600 infants in Europe showed that three doses of 4CMenB induced hSBA titers ≥1:4 in 84%–100% of participants against strains expressing antigens contained in the vaccine [Citation69]. One month after a booster dose at 12 months of age the percentage with hSBA titers ≥1:4 increased to between 95% and 100% [Citation69]. More recently, immunogenicity of a two-dose 3.5 and 5 month schedule and a 2.5, 3.5, and 5 month schedule with booster has been demonstrated [Citation71]. One month after two priming doses, 98–100% of infants had hSBA titers ≥1:4 against fHbp, NadA and PorA reference strains, and 49–59% had hSBA titers ≥1:4 against the NHBA reference strain.
4CMenB may be co-administered with diphtheria, tetanus, acellular pertussis, Haemophilus influenzae type b, inactivated poliomyelitis, hepatitis B, heptavalent pneumococcal conjugate, meningococcal C conjugate, rotavirus, measles, mumps, rubella, and varicella vaccines [Citation72]. The available data suggested that the overall responses to 4CMenB and the coadministered vaccines were not impacted by concomitant administration [Citation6,Citation66–Citation69,Citation73].
4CMenB was associated with local and systemic reactions in infants that were short-lived and largely of mild-to-moderate severity. Local and systemic reactions including fever were more frequent when 4CMenB was coadministered with other routinely administered vaccines [Citation67,Citation69], but these symptoms were significantly reduced when paracetamol was administered at the time of vaccination [Citation68]. There was no evidence that the administration of prophylactic paracetamol influenced the immune response to vaccination, and paracetamol administration is recommended by the UK and Australian authorities when 4CMenB is administered to infants.
The United Kingdom implemented the world’s first infant UMV program with 4CMenB and has achieved rapid disease control in the target population with effectiveness already demonstrated during the first year of the program which continued as predicted during the second year [Citation14,Citation32]. For the infant UMV programs in Ireland from 2016 and Italy more time is needed to assess effectiveness or impact [Citation9,Citation10].
5. Expert commentary
Our understanding of the mechanism of protection, impact and effectiveness of 4CMenB is rapidly progressing. Prior to large-scale use, predictions of the likely impact of vaccination were challenging because of the microbial population diversity of clinical MenB isolates and the absence of previous experience with meningococcal vaccine containing new non-capsular polysaccharide antigens. Additionally, the rarity of the disease did not allow efficacy trials to be performed, and tools were not available to predict vaccine coverage. Experience with 4CMenB is growing exponentially as it is implemented in different parts of the globe and in diverse age groups, encompassing university students in the United States, individuals aged 2 months to 20 years in one region in Canada, and infant UMV programs in the United Kingdom, Ireland, and Italy. Reassuringly, predictions of effectiveness based on immunogenicity have been borne out and may be conservative. Estimates of effectiveness from the field clearly suggest that predictive methods like MATS underestimate the real degree of protection afforded by 4CMenB.
The UK infant UMV program using a reduced two-dose schedule with booster has provided rigorous examination of the safety and effectiveness of the vaccine. Accumulating evidence suggests 4CMenB is an effective tool in preventing deaths and disease due to MenB IMD. The incidence of IMD peaks in infants and in adolescents, and based on the available evidence, the most effective way to protect these highest risk age-groups is through direct vaccination. As yet it is unclear whether vaccination of adolescents delivers indirect protection of infants, and even if it does, an adolescent-only program will take a long time to offer adequate protection to this age group. A combined infant-adolescent program currently represents the preferred solution for achieving rapid control of MenB IMD.
While immunization programs are ongoing, new aspects of the mechanism of antibody-mediated protection of 4CMenB have been unraveled. For 4CMenB, the presence of multiple epitopes on each vaccine antigen and the synergistic behavior of the antibodies highlight the potential of this multicomponent formulation. Recent evidence suggesting potential impacts of 4CMenB vaccine on non-B serogroups and on N. gonorrhoeae are very promising, and warrant further exploration.
6. Five-year view
Five years after approval, the available data suggest that population impact of 4CMenB vaccination is set to exceed expectations. In the next five years, we will learn much more about the impacts of 4CMenB on carriage, IMD caused by non-B serogroups, and gonorrhea. Even modest impacts in these areas would represent substantial added benefits to 4CMenB vaccination. These data will be used to inform cost-effectiveness analyses and guide decisions on the optimal strategies to achieve maximum impact, and could change the way the vaccine is used in the longer term.
Key issues
Recent evidence shows that the antibodies induced by 4CMenB can act synergistically to enhance the bactericidal response, suggesting that prelicensure estimates of strain coverage and vaccine effectiveness may be conservative.
4CMenB is the only MenB vaccine authorized for use in infants. High immunogenicity and acceptable safety profile were demonstrated in prelicensure studies. Two years of experience in the United Kingdom in which more than 1.5 million infants have received 4CMenB, attest to the tolerability of the vaccine. Vaccine effectiveness against MenB IMD was 82.9% reinforcing the concept that current estimates of coverage obtained by MATS underestimate real protection.
Exploratory analyses and retrospective population-based studies are suggestive of possible impacts of 4CMenB on MenW IMD and on gonorrhea, although these data are yet to be substantiated.
Five years after approval, the available data suggest that population impact of 4CMenB vaccination is set to exceed expectations.
Authors’ Contribution
All authors participated in the development and the review of the manuscript and approved the final submitted version. The corresponding author had final responsibility to submit for publication. Drafts were developed by a professional publication writer according to the recommendations, documentation, and outline provided by the lead author.
Declaration of interest
All authors are full time employees of the GSK group of companies. All authors hold shares in the GSK group of companies as part of their employee remuneration. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Trademarks
Bexsero is a trademark of the GSK group of companies. Trumenba is a trademark of Pfizer Inc.
Additional information
Funding
References
- European Center for Disease Prevention and Control. Surveillance report. Surveillance of invasive bacterial diseases in Europe. 2012. [Cited 2018 Jun 26]. Available from: http://ecdc.europa.eu/en/publications/Publications/Surveillance%20of%20IBD%20in%20Europe%202012.pdf
- Centers for Disease Control and Prevention. Enhanced meningococcal disease surveillance report. 2016. [Cited 2018 May 07]. Available from: https://www.cdc.gov/meningococcal/downloads/NCIRD-EMS-Report.pdf.
- Lahra MM, Enriquez RP, National Neisseria Network. Australian meningococcal surveillance programme annual report, 2015. Commun Dis Intell Q Rep. 2016;40:E503–E511.
- Giuliani MM, Adu-Bobie J, Comanducci M, et al. A universal vaccine for serogroup B meningococcus. Proc. Natl. Acad. Sci. USA. 2006;103:10834–10839.
- Pizza M, Scarlato V, Masignani V, et al. Identification of vaccine candidates against serogroup B meningococcus by whole-genome sequencing. Science. 2000;287:1816–1820.
- Findlow J, Borrow R, Snape MD, et al. Multicenter, open-label, randomized phase ii controlled trial of an investigational recombinant meningococcal serogroup b vaccine with and without outer membrane vesicles, administered in infancy. Clin Infect Dis. 2010;51:1127–1137.
- Serruto D, Bottomley MJ, Ram S, et al. The new multicomponent vaccine against meningococcal serogroup B, 4CMenB: immunological, functional and structural characterization of the antigens. Vaccine. 2012;30(Suppl 2):B87–97.
- European Medicines Agency. Bexsero, meningococcal group-B vaccine (rDNA, component, adsorbed). Product Information. [Cited 2018 Sep 13]. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002333/human_med_001614.jsp&mid=WC0b01ac058001d124
- MenB and Rotavirus Vaccines. Ireland’s health service. [Cited 2018 Oct 13]. Available from: http://www.hse.ie/eng/health/immunisation/news/menbrotavacc.html
- Signorelli C, Chiesa V, Odone A. Meningococcal serogroup B vaccine in Italy: state-of-art, organizational aspects and perspectives. J Prev Med Hyg. 2015;56:E125–132.
- Ladhani SN, Ramsay M, Borrow R, et al. Enter B and W: two new meningococcal vaccine programmes launched. Arch Dis Child. 2016;101:91–95.
- Ladhani SN, Campbell H, Parikh SR, et al. The introduction of the meningococcal B (MenB) vaccine (Bexsero®) into the national infant immunisation programme–new challenges for public health. J Infect. 2015;71:611–614.
- Borrow R, Carlone GM, Rosenstein N, et al. Neisseria meningitidis group B correlates of protection and assay standardization–international meeting report Emory University, Atlanta, Georgia, United States, 16-17 March 2005. Vaccine. 2006;24:5093–5107.
- Parikh SR, Andrews NJ, Beebeejaun K, et al. Effectiveness and impact of a reduced infant schedule of 4CMenB vaccine against group B meningococcal disease in England: a national observational cohort study. Lancet. 2016;388:2775–2782.
- Ladhani SN, Giuliani MM, Biolchi A, et al. Effectiveness of meningococcal B vaccine against endemic hypervirulent Neisseria meningitidis W Strain, England. Emerg Infect Dis. 2016;22:309–311.
- Longtin J, Dion R, Simard M, et al. Possible impact of wide-scale vaccination against serogroup B Neisseria meningitidis on gonorrhoea incidence rates in one region of Quebec, Canada (Abstract LB-3). ID week. 2017. [Cited 2018 Oct 13]. Available from: https://idsa.confex.com/idsa/2017/webprogram/Paper67400.html.
- Petousis-Harris H, Paynter J, Morgan J, et al. Effectiveness of a group B outer membrane vesicle meningococcal vaccine against gonorrhoea in New Zealand: a retrospective case-control study. Lancet. 2017;390:1603–1610.
- Giuntini S, Reason DC, Granoff DM. Complement-mediated bactericidal activity of anti-factor H binding protein monoclonal antibodies against the meningococcus relies upon blocking factor H binding. Infect Immun. 2011;79:3751–3759.
- Tappero JW, Lagos R, Ballesteros AM, et al. Immunogenicity of 2 serogroup B outer-membrane protein meningococcal vaccines: a randomized controlled trial in Chile. JAMA. 1999;281:1520–1527.
- Weynants VE, Feron CM, Goraj KK, et al. Additive and synergistic bactericidal activity of antibodies directed against minor outer membrane proteins of Neisseria meningitidis. Infect Immun. 2007;75:5434–5442.
- Giuliani M, Bartolini E, Galli B, et al. Human protective response induced by meningococcus B vaccine is mediated by the synergy of multiple bactericidal epitopes. Sci Rep. 2018;8:3700.
- Vu DM, Wong TT, Granoff DM. Cooperative serum bactericidal activity between human antibodies to meningococcal factor H binding protein and Neisserial heparin binding antigen. Vaccine. 2011;29:1968–1973.
- Biagini M, Spinsanti M, De Angelis G, et al. Expression of factor H binding protein in meningococcal strains can vary at least 15-fold and is genetically determined. Proc. Natl. Acad. Sci. USA. 2016;113:2714–2719.
- Pajon R, Beernink PT, Harrison LH, et al. Frequency of factor H-binding protein modular groups and susceptibility to cross-reactive bactericidal activity in invasive meningococcal isolates. Vaccine. 2010;28:2122–2129.
- Seib KL, Brunelli B, Brogioni B, et al. Characterization of diverse subvariants of the meningococcal factor H (fH) binding protein for their ability to bind fH, to mediate serum resistance, and to induce bactericidal antibodies. Infect Immun. 2011;79:970–981.
- Frosi G, Biolchi A, Lo Sapio M, et al. Bactericidal antibody against a representative epidemiological meningococcal serogroup B panel confirms that MATS underestimates 4CMenB vaccine strain coverage. Vaccine. 2013;31:4968–4974.
- Abad R, Biolchi A, Moschioni M, et al. A large portion of meningococcal antigen typing system-negative meningococcal strains from Spain is killed by sera from adolescents and infants immunized with 4CMenB. Clin Vaccine Immunol. 2015;22:357–360.
- Law DK, Lorange M, Ringuette L, et al. Invasive meningococcal disease in Quebec, Canada, due to an emerging clone of ST-269 serogroup B meningococci with serotype antigen 17 and serosubtype antigen P1.19 (B:17: P1.19). J Clin Microbiol. 2006;44:2743–2749.
- De Wals P, Deceuninck G, Lefebvre B, et al. Impact of an immunization campaign to control an increased incidence of serogroup b meningococcal disease in one region of Quebec, Canada. Clin Infect Dis. 2017;64:1263–1267.
- De Serres G, Gariepy MC, Billard MN, et al. Initial dose of a multicomponent serogroup b meningococcal vaccine in the Saguenay– lac-Saint-Jean region, Québec, Canada: an interim safety surveillance report. 2014. [Cited 2018 Aug 02]. Available from: https://www.inspq.qc.ca/pdf/publications/1902_SerogroupB_Meningococcal_Vaccine.pdf
- Miller E, Salisbury D, Ramsay M. Planning, registration, and implementation of an immunisation campaign against meningococcal serogroup C disease in the UK: a success story. Vaccine. 2001;20(Suppl 1):S58–67.
- Ladhani S. Safety and efficacy of MenB vaccination in the UK. MRF Conference, 2017 November 14–15; London. [Cited 2018 June 26]. Available from https://www.meningitis.org/mrf-conference-2017.
- Grogan J, Roos K. Serogroup B meningococcus outbreaks, prevalence, and the case for standard vaccination. Curr Infect Dis Rep. 2017;19:30.
- McNamara LA, Shumate AM, Johnsen P, et al. First use of a serogroup b meningococcal vaccine in the US in response to a University outbreak. Pediatrics. 2015;135:798–804.
- Patel M, Briere E, Duffy J, et al. Use of a novel serogroup B meningococcal vaccine in response to two university outbreaks in the US (Abstract O22). XIXth International Pathogenic Neisseria Conference (IPNC), 2014. [Cited 2017 Oct 02]. Available from http://neisseria.org/ipnc/2014/IPNC_2014_abstracts.pdf.
- Biswas HH, Han GS, Wendorf K, et al. Notes from the field: outbreak of serogroup B meningococcal disease at a University — California, 2016. MMWR. 2016;65:520–521.
- Basta NE, Mahmoud AAF, Wolfson J, et al. Immunogenicity of a meningococcal b vaccine during a University outbreak. New Eng. J. Med. 2016;375:220–228.
- Goldschneider I, Gotschlich EC, Artenstein MS. Human immunity to the meningococcus. I. The role of humoral antibodies. J. Exp. Med. 1969;129:1307–1326.
- Rappuoli R. Meningococcal B vaccine during a University outbreak. N Engl J Med. 2016;375:1594–1595.
- Granoff DM. Meningococcal B vaccine during a University outbreak. N Engl J Med. 2016;375:1594.
- Wang X, Cohn A, Comanducci M, et al. Prevalence and genetic diversity of candidate vaccine antigens among invasive Neisseria meningitidis isolates in the United States. Vaccine. 2011;29:4739–4744.
- Australian Government Department of health. Invasive meningococcal disease national surveillance report, with a focus on MenW, 31 March 2018. [Cited 2018 Oct 13]. Available from: https://www.health.gov.au/internet/main/publishing.nsf/Content/5FEABC4B495BDEC1CA25807D001327FA/$File/31-Mar18-IMD-Surveillance-report.pdf
- Borrow R, Alarcon P, Carlos J, et al. The global meningococcal initiative: global epidemiology, the impact of vaccines on meningococcal disease and the importance of herd protection. Expert Rev Vaccines. 2017;16:313–328.
- Hong E, Giuliani MM, Deghmane A-E, et al. Could the multicomponent meningococcal serogroup B vaccine (4CMenB) control Neisseria meningitidis capsular group X outbreaks in Africa? Vaccine. 2013;31:1113–1116.
- Tomei S, Biolchi A, Brunelli B, et al. Potential coverage of the BEXSERO® MenB vaccine on non-B meningococci (Abstract P30). XIXth International Pathogenic Neisseria Conference (IPNC), 2014. [Cited 2017 Oct 02]. Available from http://neisseria.org/ipnc/2014/IPNC_2014_abstracts.pdf.
- Campbell H, Edelstein M, Andrews N, et al. Emergency meningococcal acwy vaccination program for teenagers to control Group w meningococcal disease, England, 2015–2016. Emerg Infect Dis. 2017;23:1184–1187.
- Chan PA, Robinette A, Montgomery M, et al. Extragenital infections caused by chlamydia trachomatis and Neisseria gonorrhoeae: A review of the literature. Infect Dis Obstet Gynecol. 2016;2016:5758387.
- The Lancet. Editorial. Curbing the rise in gonococcal AMR. Lancet. 2017;390:204.
- WHO. Antibiotic-resistant gonorrhoea on the rise, new drugs needed. News Release. 2017. [Cited 2018 Jan 23]. Available from: http://www.who.int/mediacentre/news/releases/2017/Antibiotic-resistant-gonorrhoea/en/
- Tinsley CR, Nassif X. Analysis of the genetic differences between Neisseria meningitidis and Neisseria gonorrhoeae: two closely related bacteria expressing two different pathogenicities. Proc. Natl. Acad. Sci. USA. 1996;93:11109–11114.
- Hadad R, Jacobsson S, Pizza M, et al. Novel meningococcal 4CMenB vaccine antigens - prevalence and polymorphisms of the encoding genes in Neisseria gonorrhoeae. APMIS. 2012;120:750–760.
- Jongerius I, Lavender H, Tan L, et al. Distinct binding and immunogenic properties of the gonococcal homologue of meningococcal factor h binding protein. PLoS Pathog. 2013;9:e1003528.
- Regnier SA, Huels J. Potential impact of vaccination against Neisseria meningitidis on Neisseria gonorrhoeae in the United States: results from a decision-analysis model. Hum Vaccin Immunother. 2014;10:3737–3745.
- Arnold R, Galloway Y, McNicholas A, et al. Effectiveness of a vaccination programme for an epidemic of meningococcal B in New Zealand. Vaccine. 2011;29:7100–7106.
- Read RC, Baxter D, Chadwick DR, et al. Effect of a quadrivalent meningococcal ACWY glycoconjugate or a serogroup B meningococcal vaccine on meningococcal carriage: an observer-blind, phase 3 randomised clinical trial. Lancet. 2014;384:2123–2131.
- Soeters HM, Whaley M, Alexander-Scott N, et al. Meningococcal carriage evaluation in response to a serogroup b meningococcal disease outbreak and mass vaccination campaign at a College-Rhode Island, 2015–2016. Clin Infect Dis. 2017;64:1115–1122.
- McNamara LA, Thomas JD, MacNeil J, et al. Meningococcal carriage following a vaccination campaign with MenB-4C and MenB-FHbp in response to a University Serogroup B meningococcal disease outbreak-Oregon, 2015–2016. J Infect Dis. 2017;216:1130–1140.
- Marshall HS, McMillan M, Koehler A, et al. B Part of it protocol: a cluster randomised controlled trial to assess the impact of 4CMenB vaccine on pharyngeal carriage of Neisseria meningitidis in adolescents. BMJ Open. 2018;8:e020988.
- Vetter V, Baxter R, Denizer G, et al. Routinely vaccinating adolescents against meningococcus: targeting transmission & disease. Expert Rev Vaccines. 2016;15:641–658.
- Christensen H, Trotter CL, Hickman M, et al. Re-evaluating cost effectiveness of universal meningitis vaccination (Bexsero) in England: modelling study. BMJ. 2014;349:g5725.
- Capua T, Katz JA, Bocchini JA. Update on adolescent immunizations: selected review of US recommendations and literature. Curr Opin Pediatr. 2013;25:397–406.
- Walker TY, Elam-Evans LD, Singleton JA, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years - United States, 2016. MMWR. 2017;66:874–882.
- National Vaccine Advisory Committee. The promise and challenge of adolescent immunization. Am J Prev Med. 2008;35:152–157.
- WHO Ad-hoc Working Group. Impact of new vaccines introduction on immunization & health systems. [Cited 2018 Jul 25]. Available from: http://www.who.int/immunization/sage/NUVI_IS_HS_final_Refs_12_April_2010.pdf
- Toneatto D, Pizza M, Masignani V, et al. Emerging experience with meningococcal serogroup B protein vaccines. Expert Rev Vaccines. 2017;16:433–451.
- Esposito S, Prymula R, Zuccotti GV, et al. A phase 2 randomized controlled trial of a multicomponent meningococcal serogroup B vaccine, 4CMenB, in infants (II). Hum Vaccin Immunother. 2014;10:2005–2014.
- Gossger N, Snape MD, Yu L-M, et al. Immunogenicity and tolerability of recombinant serogroup B meningococcal vaccine administered with or without routine infant vaccinations according to different immunization schedules: a randomized controlled trial. JAMA. 2012;307:573–582.
- Prymula R, Esposito S, Zuccotti GV, et al. A phase 2 randomized controlled trial of a multicomponent meningococcal serogroup B vaccine (I). Hum Vaccin Immunother. 2014;10:1993–2004.
- Vesikari T, Esposito S, Prymula R, et al. Immunogenicity and safety of an investigational multicomponent, recombinant, meningococcal serogroup B vaccine (4CMenB) administered concomitantly with routine infant and child vaccinations: results of two randomised trials. Lancet. 2013;381:825–835.
- Torres FM, Safadi MAP, Martinez AC, et al. Immunogenicity and safety of a 2 or 3 dose primary series of meningococcal serogroup b vaccine in infants, and a 2-dose catch-up series in children (Abstract ESP16-0464). 34th meeting of the European Sociey for Pediatric Infectious Diseases (ESPID), 2016. [Cited 2018 Oct 29]. Available from https://espid2016.kenes.com/Documents/ESPID16_Abstracts.pdf.
- Martinon-Torres F, Safadi MAP, Martinez AC, et al. Reduced schedules of 4CMenB vaccine in infants and catch-up series in children: immunogenicity and safety results from a randomised open-label phase 3b trial. Vaccine. 2017;35:3548–3557.
- Bexsero® (Meningococcal Group B Vaccine). Prescribing information. [Cited 2018 Aug 18]. Available from: http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM431447.pdf
- Safadi MAP, Martinon-Torres F, Weckx LY, et al. Immunogenicity and safety of concomitant administration of meningococcal serogroup B (4CMenB) and serogroup C (MenC-CRM) vaccines in infants: A phase 3b, randomized controlled trial. Vaccine. 2017;35:2052–2059.
- McNeil LK, Zagursky RJ, Lin SL, et al. Role of factor H binding protein in Neisseria meningitidis virulence and its potential as a vaccine candidate to broadly protect against meningococcal disease. Microbiol Mol Biol Rev. 2013;77:234–252.
- Serruto D, Spadafina T, Ciucchi L, et al. Neisseria meningitidis GNA2132, a heparin-binding protein that induces protective immunity in humans. Proc. Natl. Acad. Sci. USA. 2010;107:3770–3775.
- Welsch JA, Moe GR, Rossi R, et al. Antibody to genome-derived neisserial antigen 2132, a Neisseria meningitidis candidate vaccine, confers protection against bacteremia in the absence of complement-mediated bactericidal activity. J Infect Dis. 2003;188:1730–1740.
- Bambini S, De Chiara M, Muzzi A, et al. Neisseria adhesin A variation and revised nomenclature scheme. Clin Vaccine Immunol. 2014;21:966–971.
- Comanducci M, Bambini S, Caugant DA, et al. NadA diversity and carriage in Neisseria meningitidis. Infect Immun. 2004;72:4217–4223.
- Fagnocchi L, Biolchi A, Ferlicca F, et al. Transcriptional regulation of the nadA gene in Neisseria meningitidis impacts the prediction of coverage of a multicomponent meningococcal serogroup B vaccine. Infect Immun. 2013;81:560–569.
- Medini D, Stella M, Wassil J. MATS: global coverage estimates for 4CMenB, a novel multicomponent meningococcal B vaccine. Vaccine. 2015;33:2629–2636.
- Vogel U, Taha M-K, Vazquez JA, et al. Predicted strain coverage of a meningococcal multicomponent vaccine (4CMenB) in Europe: a qualitative and quantitative assessment. Lancet Infect Dis. 2013;13:416–425.