ABSTRACT
Introduction: Virus infections have long been considered as a possible cause of type 1 diabetes (T1D). One virus group, enteroviruses (EVs), has been studied extensively, and clinical development of a vaccine against T1D-associated EV types has started.
Areas covered: Epidemiological studies have indicated an association between EVs and T1D. These viruses have a strong tropism for insulin-producing β-cells; the destruction of these cells leads to T1D. The exact mechanisms by which EVs could cause T1D are not known, but direct infection of β-cells and virus-induced inflammation may play a role. Recent studies have narrowed down the epidemiological association to a subset of EVs: group B coxsackieviruses (CVBs). These findings have prompted efforts to develop vaccines against CVBs. Prototype CVB vaccines have prevented both infection and CVB-induced diabetes in mice. This review summarizes recent progress in the field and the specifics of what could constitute the first human vaccine developed for a chronic autoimmune disease.
Expert commentary: Manufacturing of a clinical CVB vaccine as well as preclinical studies are currently in progress in order to enable clinical testing of the first CVB vaccine. Ongoing scientific research projects can significantly facilitate this effort by providing insights into the mechanisms of the CVB-T1D association.
1. Introduction
Type 1 diabetes (T1D) is a multifactorial disease resulting in the autoimmune destruction of the insulin-producing β-cells of the pancreas. It requires lifelong treatment with daily insulin injections and often leads to vascular and neurological complications reducing quality of life and life expectancy and causing considerable societal costs. Therefore, the prevention of T1D has long been one of the main goals in the diabetes research field.
Even though genetic determinants modulate the risk of T1D, with human leukocyte antigen (HLA) genes responsible for most of the genetic susceptibility [Citation1], environmental factors play a clear role in the pathogenesis. The importance of environmental factors is demonstrated by the rapidly increasing incidence of T1D and by studies in identical twins and immigrants [Citation2]. Based on the marked increase in T1D, it has been estimated that the elimination of the harmful effect of these exogenous factors would prevent most of the cases of T1D [Citation3].
The disease process has a clear immunological component reflected by the detection of autoantibodies against β-cell proteins in patients with T1D. Therefore, while multifactorial, T1D is commonly classified as an autoimmune disease. The β-cell damaging process usually progresses slowly over several years, and the diagnosis of clinical-stage T1D (now commonly termed stage 3 T1D) is preceded by a subclinical period of variable duration when autoantibodies against β-cell proteins are detectable in serum (stage 1, with normoglycemia, and stage 2, with dysglycemia) [Citation4].
Since exogenous factors appear to play a key role in the pathogenesis of T1D, several studies have set out to identify such factors. Viral infections are considered one of the most common triggers of autoimmune processes in general, and of T1D in particular. Indeed, viral infections have long been considered as one of the most likely trigger candidates for T1D. First, certain viruses, particularly enteroviruses (EVs), are known to infect the pancreas and cause a T1D-like disease in animals [Citation5,Citation6]. Second, human studies have shown that respiratory infection symptoms (most common clinical presentation of enteroviral infection) precede the initiation of the β-cell damaging process in children who later progress to T1D [Citation7–Citation10]. Third, EVs have been connected to T1D in numerous studies [Citation3,Citation5,Citation6,Citation11,Citation12]. Certain EV types, such as group B coxsackieviruses (CVB), have tropism for pancreatic tissue, leading to infection of pancreatic islets in humans [Citation13].
We have previously reviewed the studies evaluating the association between EVs and T1D [Citation11]. The present update focuses on recent reports on EVs and particularly on CVB. We will present evidence for CVB as a trigger of T1D, evidence which has reached critical mass resulting in a biopharmaceutical effort to develop and commercialize a vaccine to prevent acute infection and T1D, by the company Provention Bio, Inc (Oldwick, New Jersey, USA).
2. Enteroviruses
Human EVs are small RNA viruses comprising four EV species (species A-D) and three rhinovirus species (A-C) including altogether more than 200 different genotypes (www.picornaviridae.com). They are the most common human viruses. Their primary replication takes place in the lymphatic tissues of the oropharynx and intestine, from where the viruses can spread to the blood, causing viremia [Citation14]. Cases with severe clinical disease represent only the tip of the iceberg as most infections are asymptomatic or cause mild common-cold-type symptoms, often remaining unrecognized and/or undiagnosed. The organs which are affected vary depending on the EV type and other factors regulating the tropism of the virus. Typical severe manifestations include myocarditis, pancreatitis, meningitis, paralysis, rash, and severe systemic infections in newborn infants. Polioviruses are the best-characterized example, comprising three serotypes and causing paralysis by infecting the motor neurons of the spinal cord. The mechanisms mediating the tropism of a specific EV type for certain organs or cells are not completely understood. The mechanisms include the expression of viral receptors on the cell surface, factors regulating intracellular viral replication, and the nature of the local innate immune response in the target organ. The tropism of CVB for pancreatic β-cells has been elucidated, and this correlates well with their expression of a specific receptor, as described below.
EVs can cause persistent infection, and chronic cardiomyopathy probably represents such a condition in humans [Citation15]; CVBs are currently the most common cause of viral myocarditis [Citation16]. In mouse models, persistent EV infection causes inflammatory myopathies, cardiac injury, and central nervous system damage [Citation17,Citation18]. Immunocompromised patients, especially those with humoral immunodeficiency, also suffer from chronic EV infections [Citation19]. Recent studies have suggested that viral persistency is associated with deletions in the 5ʹ non-coding regions of the viral genome and reduced replication of viral genome [Citation20]. However, other mechanisms may also be involved in the development of viral persistence.
Poliomyelitis has been extensively studied as a model disease for enteroviral infections. Three EV types have been named as polioviruses (poliovirus types 1, 2, and 3) due to their ability to cause polio paralysis. Less than 1% of poliovirus-infected individuals develop paralysis, and postinfectious autoimmunity has been invoked as a potential cause. However, it is not known why these viruses so selectively destroy one particular cell type (spinal cord motor neurons) [Citation14,Citation21]. The expression of poliovirus receptors (CD 155) on target cells is a prerequisite for this specificity, yet it does not fully explain the tropism. For example, the ability of cells to produce type 1 interferon can vary which may also contribute to the cellular tropism of these viruses [Citation22].
3. Role of EVs in T1D
The strong evidence suggesting a role for EVs in the pathogenesis of T1D has originated from a plethora of studies including human studies (epidemiological studies, case reports, ecological studies, case–control studies, prospective cohorts, autopsy studies) and in vitro and in vivo mechanistic animal studies. Since T1D clearly involves autoimmune phenomena, the main research focus has been on the activation of autoimmune responses against β-cell proteins. Discovery of common antigenic structures in viral and β-cell proteins suggests that immunological cross-reactivity may play a role in T1D [Citation23,Citation24]. Other possible mechanisms include bystander activation of autoreactive T-cell clones driven by virus-induced inflammation, and the secretion of proinflammatory cytokines [Citation25]. Virus-induced apoptosis or necrosis are also possible, since EVs can infect and damage human β-cells in vitro [Citation26,Citation27]. These hypotheses are summarized in detail below.
3.1. Animal studies
It has long been known that viral infections cause diabetes in animals, including cattle [Citation28] and mice [Citation6]. In the latter model, encephalomyocarditis virus, a picornavirus, causes β-cell damage by directly infecting these cells in the pancreas. The diabetogenic nature of certain encephalomyocarditis virus variants is determined by the sequence of the viral VP1 protein [Citation29], although it is not known if similar nucleotide sequences modulate the diabetogenicity of EVs in human T1D. Infection with a lower viral dose leads to slower β-cell damage [Citation30] and attenuated virus vaccine or immunization with recombinant VP1 prevent diabetes in mice [Citation31,Citation32].
The ability of certain EV types (particularly CVBs) to cause pancreatitis in mice has been known for a long time from studies performed to classify EVs based on their pathogenetic features in mouse models. The main tropism of CVBs in mice is for the exocrine pancreatic tissue. However, as most commonly happens in humans, certain EV strains also infect the endocrine pancreatic tissue (the pancreatic islets containing β-cells) and can cause subtle diabetes in mice [Citation6,Citation33,Citation34]. In suppressors of cytokine signaling (SOCS)-transgenic mice, whose β-cells lack interferon responses, CVBs spread to the β-cells causing rapid damage and overt diabetes [Citation35]. In non-obese diabetic (NOD) mice, which develop spontaneously autoimmune-mediated β-cell damage and diabetes, CVB infection can accelerate an already ongoing disease process [Citation36]. Interestingly, if these mice are infected early at the time when the autoimmune process has not yet started, the infection paradoxically delays the development of diabetes. This protective effect is also seen with many other interventions in NOD mice, suggesting that it is not a specific feature of EVs but probably reflects a nonspecific viral effect on the murine immune system. Finally, CVB infection can also cause mild diabetes in primates [Citation37].
In summary, animal studies have provided evidence that EVs and other picornaviruses, and in particular CVBs, can induce diabetes and damage β-cells by direct infection in the pancreas. The diabetogenic effect of the virus is modulated by genetic factors of the host (e.g. mouse strain and sex), the local immune response in the infected islets (e.g. interferon response), and mutations in the viral genome resulting in specific diabetogenic viral strains.
3.2. Cell models
Human pancreatic islets (containing insulin producing β-cells and other endocrine cells) can be isolated from the pancreatic tissue and used for in vitro studies. These islet cell cultures have been widely used to study the interactions between EVs and β-cells. The in vitro studies have shown that human islet cells are permissive for a number of different EV types [Citation26]. The course of infection varies from acute lytic infection to silent replication of the virus without causing any clear cytopathic effect, and different EV strains (even of the same serotype) can differ in this respect [Citation26,Citation38]. Studies have also suggested that CVB serotypes predominantly infect endocrine cells of the pancreas, while exocrine cells were not affected [Citation39]. Viral infection leads to activation of the innate immune system responses in islet cells including β-cells [Citation40], and different viral strains show considerable variation in their ability to induce such responses, a phenomenon which may modulate the course of the infection on the cellular and systemic level [Citation38,Citation41]. In addition, β-cells seem to generate substantially weaker innate immune response that other islet cells possibly contributing to their susceptibility to EV invasion [Citation42]. Recent studies have indicated that CVBs can also establish a chronic infection in pancreatic cells [Citation43]. In summary, cell-based studies have demonstrated that several different EVs infect and damage islet cells in vitro and that CVB group EVs have a clear tropism for insulin producing β-cells.
3.3. Detection of EVs in human pancreatic tissue
Animal studies have shown that EVs, and CVBs in particular, have a strong tropism for pancreas. However, due to the retroperitoneal location of the organ, it has been difficult to obtain samples for viral analyses from living patients with T1D. Autopsy studies in nondiabetic children who died of EV infection have shown that CVB spreads to pancreatic islets causing severe inflammation and damage [Citation13,Citation44]. Interestingly, this islet tropism seems to be characteristic of CVB serotypes, and not of other EV types studied [Citation13].
In addition, autopsy studies in patients affected by T1D have revealed that the majority of T1D patients are positive for EV VP1 protein in the pancreatic islets when analyzed by immunohistochemistry [Citation45]. Most remarkably, EV VP1 is expressed mainly in β-cells while other islet cells are virus negative. Furthermore, EV RNA has been detected in the islets in some of these patients using in situ hybridization [Citation26]. These findings have recently been confirmed in the Network for Pancreatic Organ Donors with Diabetes (nPOD) and Persistent Virus Infection in Diabetes (PEVNET) projects, which analyze pancreatic tissues from cadaver organ donors with T1D or preclinical T1D [Citation46,Citation47]. One of the most important recent discoveries has been made in a study which collected pancreas biopsies from living patients with newly diagnosed T1D [Citation47,Citation48]. In this study, EV was detected in the pancreatic islets in the majority of the cases with T1D using immunohistochemistry and sensitive reverse transcription polymerase chain reaction (RT-PCR) [Citation47]. Importantly, the exocrine part of the organ was EV-negative. It should be noted that an earlier study has also found EV RNA in the pancreatic islets but not in the exocrine part of the pancreas of T1D patients using an in situ hybridization assay [Citation26]. However, another study failed to detect EV RNA using similar in situ technology [Citation49]. Technically very challenging, EVs have been isolated from the pancreas of patients with T1D on only two occasions, and in both cases the virus was identified as the CVB4 serotype [Citation50,Citation51]. Importantly, it is known that pancreatic islets express the main receptor for CVB group EVs (coxsackie and adenovirus receptor, CAR) [Citation26,Citation52]. A recent study showed that the CAR isotype that serves as the receptor for CVBs is strongly expressed only in β-cells, locating in the insulin secretion granules, thus offering one possible mechanistic explanation for the tropism of CVBs to β-cells [Citation53]. Additional viral receptors may also play a role [Citation26]. Altogether, the analysis of pancreatic tissue shows that EVs can be detected in a considerable proportion (60–70%) of patients with T1D. Interestingly, EVs are also detected in the pancreas of patients with T1D at higher rates than in unaffected controls (~10–20%) [Citation45].
The viral signal comes from the pancreatic islets (from β-cells in those experiments which addressed the cellular level) and is associated with the expression of markers of innate immune system activation (expression of the double-stranded RNA-dependent protein kinase and interferon-alpha) and HLA class I hyperexpression on the infected islets [Citation54]. The amount of virus is low – usually only a few cells are infected in each islet, only few islets contain EV-positive cells and only a few copies of viral RNA are detected by RT-PCR in these islets. These facts, together with the frequent detection of the virus in the pancreas of T1D patients analyzed, fits with a persisting low-grade infection in the pancreatic islets in these subjects. The infection leading to viral persistence may have occurred long before the diagnosis of T1D, possibly at the time when the β-cell damaging process has started.
Recent molecular studies have shown that such persisting pancreatic infections can be caused by EV strains which have a specific mutation in their genome leading to reduced replication and defective synthesis of viral proteins [Citation15]. The same mechanism is believed to result in chronic cardiomyopathy after EV infection in the myocardium. Due to the low titers of the virus, it has not been possible to identify the serotypes present in the pancreatic tissue of T1D patients, except in the two cases mentioned, in whom CVB4 was isolated from the pancreas [Citation50,Citation51]. Currently, large international studies are aiming at confirming these results using multiple methods in collaboration between several research groups (e.g. nPOD and PEVNET studies).
3.4. Epidemiological studies
The role of EVs in T1D has been evaluated in numerous case reports, case–control studies, as well as ecological and prospective studies by measuring antibody responses against EVs or by detecting the virus directly in the study subjects. The early serological studies in the 1970s suggested an association between the CVB group and T1D [Citation55,Citation56], and since then a number of studies have been carried out with variable results depending on the methodology used. Meta-analyses of published serological studies have shown no clear association [Citation57], yet meta-analysis based on direct detection of EVs from the pancreas or blood of T1D patients indicate a clear risk association (odds ratios ranging from 4 to 10) [Citation58].
Studies carried out in prospective cohorts of children who have been followed from an early non-diabetic stage (often from birth) have made it possible to study the role of EV infections at different stages of the disease process, including the time before islet autoimmunity has started. Recently, a large prospective study from Finland showed an increased frequency of EV RNA in stool samples long (more than a year) before the onset of islet autoimmunity [Citation59], which is in line with earlier reports about an increased frequency of viral RNA in serum in this same cohort study [Citation60]. On the other hand, smaller prospective studies have indicated no risk association based on the detection of EV RNA in stool samples [Citation61,Citation62], even though the small number of children and/or infrequent sample collection have compromised their statistical power to detect such an association. A recent prospective study from Norway found an increased frequency of EV RNA in blood samples at the onset of islet autoimmunity [Citation63]. Prospective studies using serology have also observed this risk association [Citation64,Citation65]. In addition, EV infections during pregnancy may also be associated with increased risk of T1D in the offspring, but this association may not be as consistent as that seen between postnatal infections and T1D [Citation66,Citation67]. In any case, a recent meta-analysis showed a significant association between EV infection during pregnancy and clinical T1D during childhood in the offspring (odds ratio 2.16, 95% CI; 1.22–3.80; P = 0.008), but no such association was seen with islet autoimmunity (1.45, 0.63–3.31; P = 0.38) [Citation68]. EVs have also been detected in the intestinal mucosa of patients with T1D [Citation69] but this finding was not confirmed in another study [Citation70].
Altogether, the markers of EV infections (presence of virus in blood, stool or pancreas or presence of viral antibodies in serum) have shown a risk association with T1D. Although the magnitude of this association has varied between studies, there is clear consistency between studies carried out in large pediatric cohorts. Several factors including differences in the study design, study populations and their demographics, matching criteria used to select the control children, and assays used to detect virus infections may influence the outcome of the study. Since the frequency of EV infections is high in the healthy background population, a large number of case and control children needs to be analyzed using optimal sample collection and methodology to get reliable information about the association between EVs and T1D. Studies in large prospective cohorts (e.g. international The Environmental Determinants of Diabetes in the Young [TEDDY] and the Finnish Type 1 Diabetes Prediction and Prevention [DIPP] cohorts) are particularly important since they can evaluate this risk association in different stages of the β-cell damaging process. It should also be noted that EVs may play a role in a subgroup of T1D. A recent study suggested that CVB infections could trigger autoantibodies against insulin rather than against GAD65, and play a role in the recently discovered phenotype of T1D characterized by insulin autoantibodies as the first appearing autoantibody [Citation71].
The diabetogenic effect of EVs may also be characterized by changes in population dynamics of EV infections. In fact, ecological comparisons between different countries have suggested that the frequency of EV infections in the background population shows an inverse correlation with the incidence of T1D and that the increasing incidence of T1D is associated with decreasing frequency of EV infections in the background population over time [Citation72–Citation74]. These observations have led to the ‘polio hypothesis,’ which claims that the diabetogenic effect of EV infections is strongest in countries where these viruses are relatively rare [Citation72]. A mouse study elegantly demonstrated that maternal EV antibodies prevented the induction of diabetes by EV infection in the offspring [Citation75], and this phenomenon has been recently replicated in the DIPP study, where maternal antibodies against CVB1 were associated with substantially (~50%) lower rate of islet autoantibody seroconversion in the offspring [Citation76]. This important observation suggests that a vaccine against CVB could prevent a substantial subset of T1D cases.
In any case, even if a risk association seems to exist between EV infections and T1D, this association per se does not indicate causality – it can also be secondary to the effect(s) of potential confounding factor(s). However, the biology of EVs, including their tropism to β-cells, strong expression of CAR receptors on β-cells, as well as the increased frequency of EV infections prior to the onset of islet autoimmunity, support the assumption that this association can be causal. However, intervention studies, discussed below, will be needed to prove causality.
3.5. Identification of diabetogenic EV types
The identification of the type of diabetogenic EVs has long been desired as an essential step in the development of vaccines and to better understand the mechanisms of EV-induced diabetes by comparison with nondiabetogenic EVs. The diabetogenic properties of EVs can be linked to specific viral subtypes that have tropism for insulin-producing β-cells and mutations in the viral genome that can further modulate the diabetogenicity in the same way as the neurovirulence of polioviruses is modulated by nucleotide changes in the coding and noncoding regions of the viral genome [Citation14]. Early serological studies in the UK suggested that CVBs, a group with six serotypes, CVB 1–6, and especially the CVB4 serotype, may be linked to T1D [Citation55,Citation56]. CVBs have also been isolated from the pancreas of T1D patients on two occasions (both viruses were of CVB4 serotype) [Citation50,Citation51]. Autopsy studies among children who died of CVB infection have indicated islet cell damage and insulitis in their pancreas, while no such damage was seen in infections caused by other EV serotypes [Citation13]. CVBs can also cause diabetes and pancreatitis in mouse models and in monkeys [Citation6]. In addition to CVBs, some other EVs, particularly those belonging to species B, have been linked to islet autoimmunity and T1D (see below).
In order to achieve sufficient statistical power to identify the precise nature of the EV association, large sero-epidemiological studies have been established. The largest of these studies is the DIPP, established in 1994 in Finland to screen newborn infants for genetic risk of T1D and to follow them for the development of β-cell autoimmunity and T1D [Citation77]. As the time of this writing, more than 220,000 infants have been screened for HLA-conferred risk for T1D and around 10% have been identified as genetically at risk, of whom ~80% have joined the follow-up starting from birth. Of these ~17,000 children, 50% have remained in follow-up to the age of 15 years and ~9% of them have developed at least two T1D-associated autoantibodies and ~6% (approximately 500 children) have progressed to clinical T1D. Importantly, the serological analysis showed that CVBs were the only EV type associated with T1D risk in the DIPP study [Citation76]. Furthermore, in a subgroup of the children who developed T1D, EV RNA was detected in stool and serum samples significantly more frequently prior to autoantibody seroconversion when compared to carefully matched controls [Citation59,Citation60].
The relationship between islet autoantibodies and CVB serotypes has also been observed in other cohorts collected in European countries [Citation78] and subsequently in prospective studies carried out in Europe, North America, and Australia [Citation79,Citation80]. Recently, the large international TEDDY birth cohort study reported an association between detection of species B EVs (particularly CVBs) in stool samples by Illumina mass sequencing and subsequent initiation of islet autoimmunity (data presented in the Immunology of Diabetes Society meeting in London, October 2018). The additional finding of almost 50% reduction in the CVB-infection-associated risk of islet autoantibodies in the offspring of mothers with anti-CVB antibodies supports the idea that a CVB vaccine might be effective in preventing the disease [Citation76].
Two main mechanisms may explain how a CVB infection can induce or accelerate T1D onset. Viral-induced damage of infected β-cells is hypothesized to lead to autoimmunity by (1) enhanced presentation of β-cell peptides by professional antigen-presenting cells and (2) prolonged inflammation due to viral persistence, replication, endoplasmic stress, posttranslational modifications in β-cell autoantigens, and, finally, enhanced antigenic stimulation [Citation81,Citation82].
Altogether, we conclude that CVBs are currently the major candidates for diabetogenic EVs in humans. However, since certain other species B EVs (mainly echoviruses) have also been linked to T1D in case reports and smaller human studies [Citation83–Citation85], additional large-scale studies should be carried out in different populations.
3.6. Virus–gene interactions
Several factors regulate the host’s susceptibility to EV infections. General risk factors for severe EV disease include young age, male gender, humoral immunodeficiency, lack of maternal antibodies, and short duration of breastfeeding, all of which are connected to a poor immune protection against EVs [Citation86]. In addition, the presence of EV proteins in β-cells is associated with activation of the innate immune system in the infected islets [Citation54]. Part of this activation, including hyperexpression of HLA class I molecules, is seen in the infected islets of T1D cases but not in the infected islets of control subjects, suggesting that abnormally strong inflammation response may also play a role. Certain genes, the polymorphisms of which have been linked to T1D, are related to immune responses against EVs. These genes include HLA genes as well as genes regulating innate immune system responses [Citation87–Citation90]. The innate immune system plays an important role in the protection against EVs and in virus-induced inflammation. One such gene is Interferon Induced with Helicase C Domain 1 (IFIH1), which encodes an innate immune system receptor for EVs [Citation88]. T1D-associated IFIH1 alleles seem to be associated with enhanced innate immune activation [Citation91]. In addition, the polymorphisms in other innate immune system genes such as tyrosine kinase 2 (TYK2) [Citation92,Citation93] and those regulating the IRF7-driven innate immune system responses are also associated with increased risk of T1D [Citation42,Citation89]. A recent mouse study showed that one of the most important non-HLA susceptibility genes for T1D, protein tyrosine phosphatase, non-receptor type 22 (PTPN22), modulates also innate immune responses against CVB-like viruses and that the T1D-associated PTPN22 allele is associated with weak innate immune response making the host susceptible to the virus [Citation90]. Altogether, viral interactions with the innate immune system are likely players involved in the mechanisms mediating the diabetogenic effect of EVs.
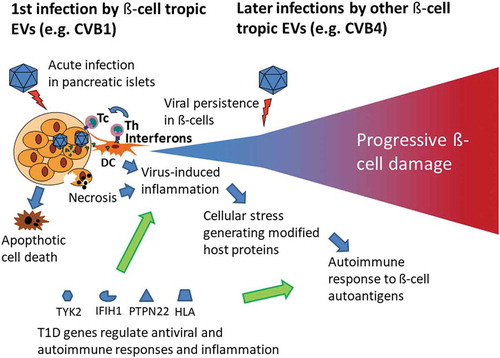
In summary, if EVs and, in particular, CVBs cause T1D, they probably follow similar pathogenetic features as known from other EV diseases. The existing evidence supports the role of direct CVB infection in the pancreatic islets which can damage β-cells by virus-mediated effects and/or by a slower immune-mediated damage. Viral persistence and the activation of the innate immune system likely play an important role in immune-mediated β-cell damage and may cause posttranslational modifications in β-cell autoantigens leading to the activation of autoimmune responses. Persistent infection may also deteriorate β-cell function without killing the cells by shutting off the protein synthesis (including insulin synthesis). These elements and a hypothetical disease model are summarized in .
Figure 1. Model for enterovirus (EV)-induced β-cell damage leading to type 1 diabetes (T1D). The virus infects insulin-producing β-cells in the pancreas leading to viral persistence, cell death and inflammation in infected islets. Host antiviral response is modulated by polymorphisms in genes regulating the immune response against EVs. These polymorphisms are associated with T1D (e.g. IFIH1, PTPN22, TYK2, HLA). The diabetogenic virus-gene interaction is characterized by an inflammation response and subsequent induction of an autoimmune process.
HLA: human leukocyte antigen; IFIH1: Interferon Induced with Helicase C Domain 1; PTPN22: protein tyrosine phosphatase, non-receptor type 22; TYK2: tyrosine kinase 2.
4. Current status of preventive strategies for T1D
Even though scientific research has generated tremendous amount of information about the pathogenesis of T1D, this has not led to a breakthrough in the prevention of the disease. Several clinical trials have been carried out with immunosuppressive and immunomodulatory agents among subjects with recent onset T1D or preclinical T1D characterized by positivity for multiple T1D associated autoantibodies. These agents prevent or delay the onset of diabetes in the NOD mouse model, but so far, these attempts have not been successful in human T1D [Citation94]. Some of the measures which have been tested in human trials have been successfully used in the treatment of other autoimmune diseases such as rheumatoid arthritis, while others have been based on tolerance induction by delivering β-cell autoantigens orally, intranasally or subcutaneously. None of them has provided a clear long-term beneficial effect on the preservation of β-cell function even though some promising effects have been observed in certain subgroups of patients with anti-CD3, anti-CD20, LFA3-Fc, and CTLA-4Ig [Citation94–Citation96]. Only one large-scale primary prevention trial has been carried out. That trial tested the possible beneficial effect of the elimination of complex dietary proteins in early infancy. The outcome was negative with no difference between the two trial groups in the progression rate to overt T1D up to a median age of 11.5 years [Citation97]. Altogether, currently, there is no treatment available which could be used for the prevention of T1D.
Since immune interventions have not given long-term benefits in clinical prevention trials, the concept of the autoimmunity as the primary force driving the β-cell damaging process has been challenged [Citation95,Citation98]. Recent studies of the pancreas tissues have also suggested that the T-cell-mediated inflammation in the pancreatic islets is often mild with relatively low number of infiltrating cells and low fraction of affected islets [Citation99,Citation100]. Thus, it is possible that the autoimmune component is not as dominant as previously thought based on the NOD mouse model, and the role of other factors may be more essential. From this perspective, the fact that EVs have been detected in the pancreatic islets of the majority of patients with T1D is interesting, since the autoimmune process could well be driven by a persisting EV infection in the islets. Autoimmune phenomena are common in chronic virus infections, where it is difficult to distinguish the autoimmune, antiviral, and inflammation components of the immune response.
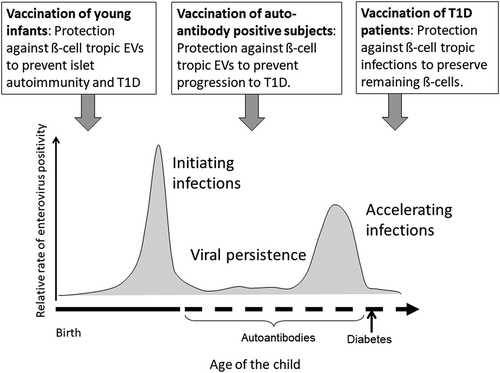
Since the potential of EV vaccines or antiviral drugs in the prevention of T1D has never been tested, they are currently among the most attractive candidates to be studied in clinical prevention trials. Vaccine intervention has the advantage that EV vaccines are known to provide a long-term and effective protection against EVs. They should be given at an early age to prevent the first diabetogenic EV infection and subsequent development of β-cell damage. Theoretically, one could also speculate that such vaccines might have some beneficial effect at later stages of the process by preventing further damage on β-cells by later EV infections in subjects with presymptomatic or newly diagnosed T1D. This would be an advantage in the trial design since substantially smaller number of study subjects would be needed than in vaccine trials aimed at primary prevention of T1D. Polyvalent vaccine including a cocktail of T1D-associated EVs would be ideal in both scenarios. The scenarios for different vaccination options are illustrated in .
Figure 2. Summary of the scientific concept of preventing type 1 diabetes (T1D) by enterovirus (EV) vaccines. The figure shows the relative rate of enterovirus infections in different stages of the β-cell damaging process in children who develop T1D and different scenarios for the prevention of these infections by different vaccination regimens. Early infection by a β-cell tropic enterovirus strain has been implicated to play a role in the initiation of the β-cell damaging process. Later infections by other β-cell tropic virus strains may generate cumulative β-cell damage that eventually progress to T1D. The primary aim is to vaccinate children before the age of 6 months to prevent infections which are associated with the initiation of the β-cell damaging process. Polyvalent vaccine given at this point would protect also from later infections. In addition, the vaccine could also be given to autoantibody-positive but still nondiabetic children to prevent infections that could accelerate the progression of the β-cell damaging process. Theoretically, the vaccine could also give some benefit to subjects with established T1D by protecting their remaining β-cell reserve against additional viral hits. One additional scenario is to vaccinate pregnant women to protect newborn infants by maternal EV antibodies transferred via placenta and breast milk.
On the other hand, the use of antiviral drugs could be indicated in subjects who have a persisting virus infection in the pancreas. In this scenario, antiviral drugs could be tested in subjects with presymptomatic T1D and/or newly diagnosed T1D for their ability to eradicate persisting virus and maintain β-cell function. The first trial with antiviral drugs has recently started among patients with newly diagnosed T1D (Principal Investigator: Prof. Knut Dahl-Jörgensen, University of Oslo, Norway).
5. Rationale for a CVB vaccine to prevent T1D
The ultimate goal of a primary prevention trial with a vaccine directed against diabetogenic CVB types will be to assess whether the observed CVB-T1D association is indeed causal. Even a modest reduction in the incidence rate of T1D by such a vaccine would be not just clinically meaningful, but also scientifically highly valuable to prove the concept of the causal role of EVs/CVBs in the pathogenesis of T1D. Ethical and social arguments also support the development of such a vaccine since safe and potentially effective treatments should be tested to reduce the burden of this disease. A CVB vaccine would also be attractive to reduce the substantial costs attributable to the treatment of T1D and its complications. In addition, such a vaccine would generate huge savings in indirect costs and improve the quality of life in families avoiding the appearance of T1D in their child. The vaccine would also decrease the disease burden caused by acute CVB infections. The required vaccine technologies are in place since the same technologies used in the production of poliovirus vaccines and EV 71 vaccines can be applied to many other EVs [Citation101].
The main risk in the development of such a vaccine is the possibility that the observed association between CVBs and T1D is not causal but an epiphenomenon reflecting unknown confounding factors. While ultimately only a clinical trial will answer this key question, several large-scale studies continue to address the role of EVs and CVBs in T1D (e.g. nPOD, TEDDY, PEVNET, DIPP), and their results will help shape research and development in T1D over the next few years ().
Table 1. Topics for additional research to facilitate the development of enterovirus vaccine for the prevention of T1D.
6. Challenges and our approach in EV vaccine development
In order to advance toward a vaccine against CVBs, the EV types most closely associated with T1D, we conducted a risk analysis evaluation to facilitate the required close collaboration between academic and industrial partners.
Formalin-inactivated poliovirus vaccine has proved to be one of the safest and most effective vaccines ever produced. In addition, formalin-inactivated whole-virus EV vaccines have been developed against EV71. These vaccines have been tested in three phase III clinical trials indicating high safety and immunogenicity [Citation102], further reducing risk in this field. Two of these vaccines have also received regulatory approval in China.
A few years ago, two of the co-authors (HH and MK) together with their collaborators set out to explore the development of an EV vaccine against T1D, and established a consortium which included both academic research groups and vaccine companies. One of the main aims of this work was the identification of the serotype of EVs which are associated with T1D. The other important aim was to produce experimental vaccines against the identified EV types and test their efficacy and safety in animal models.
The identification of the serotype of diabetogenic EVs was a key question since more than 100 different EV types have been identified so far. The maximum number of serotypes which can be included in a single vaccine depends on many factors, but typically should be less than 10 in any formalin-inactivated whole-virus vaccine. Therefore, this consortium started systematic surveys to identify those EV serotypes which show an association with T1D in large epidemiological studies. The main approach was based on the screening of neutralizing antibodies, which are specific for the serotype used in the assay. The other approach was to molecularly type EVs which are detected by RT-PCR in subjects with preclinical or clinical T1D. The initial neutralizing antibody screening study included children who had been followed from birth and who developed T1D or turned positive for multiple autoantibodies as a marker of a subclinical disease process. Antibodies were screened against 41 different EV serotypes in longitudinal follow-up samples in the Finnish DIPP study. This study led to the identification of the CVB1 serotype as one major risk virus [Citation76]. In addition, other CVB serotypes interacted with CVB1 in a manner suggestive of immunological cross-protection.
In the next step, a validation study was carried out among newly diagnosed T1D patients and control subjects recruited in different European populations. This study confirmed the risk association of CVB1 [Citation78]. Independent results from a validation study – a prospective series of children with preclinical T1D– also support the risk association of CVB1 [Citation79]. Furthermore, these studies were expanded by measuring antibodies against 12 different CVB1 strains, and the results confirmed the risk association of CVB1 (unpublished data).
The second approach was based on sequencing the genome of EVs detected by RT-PCR in the prospective birth cohort study in Finland (the DIPP study). Stool and serum samples were analyzed for the presence of EV RNA, and this provided an opportunity to sequence the VP1 region of the virus to identify the serotype. This work showed a trend for higher relative proportion of the CVB group EVs in the serum of children who progressed to T1D compared to that in control children (unpublished data).
Altogether, these results supported previous studies suggesting that, among all EV types, the CVBs are most strongly associated with T1D. These viruses are common and CVB1 antibodies were frequent also in the nondiabetic control subjects. This observation fits with the low attack rate seen in EV infections in general. Most CVB infections are subclinical and lead to complications only in a small fraction of infected individuals. There are six serotypes in the CVB group, which are responsible for a wide spectrum of associated diseases in humans [Citation103–Citation105]. We have estimated that the proportion of children developing T1D after CVB1 infection is less than 5%. Based on these findings, we proposed that a polyvalent vaccine including CVB1 together with other selected CVB serotypes would be an optimal choice for clinical trials. Based on our results from the prospective studies, we have estimated that a CVB vaccine could prevent up to 60% of new cases with T1D. These calculations are based on a scenario comparable to that seen for poliovirus vaccines where the vaccine protects against practically all virus-related disease cases.
A vaccine against CVB would also be of public health interest to prevent acute infection by CVB. Acute CVB infection generally leads to a nonspecific febrile illness, common cold-like symptoms, otitis media, myositis, herpangina, rash, or mild gastrointestinal distress, but may also lead to more severe manifestations, including pericarditis, myocarditis, meningitis, and pancreatitis. CVB group viruses have generally been among the 15 most frequently reported EV serotypes in the national surveillance system in the US [Citation106] indicating that they lead to a significant amount of contacts with the health-care system.
7. Preclinical development of a CVB vaccine against T1D
In the next phase, experimental vaccines against CVBs were produced and their efficacy and safety were tested in mouse models. Both traditional formalin-inactivated vaccine technology and virus-like particle (VLPs) vaccines were selected for these studies. Inactivated CVB1 vaccine prevented CVB1 infection in BALB/c mice, and the spreading of CVB1 to the pancreas [Citation107], without any appreciable side effects. Importantly, the CVB vaccine did not accelerate the development of diabetes in the diabetes-prone NOD mice, even if the natural CVB1 infection did so. VLP-based CVB3 vaccine induced high neutralizing antibody responses in mice as well [Citation108].
Inactivated CVB-based vaccines induced also a strong immune response in mice in the form of neutralizing antibodies. Indeed, inactivated CVB1 generated a strong anti-CVB1 neutralizing antibody response when administered to mice over two to three cycles [Citation107,Citation109]. Recently, further collaboration studies were set out to test whether the inactivated CVB vaccine preventing CVB infection could also prevent virus-induced diabetes in mice. These studies first confirmed that CVB infection can accelerate diabetes onset in NOD and SOCS-1-Tg mice and then proved that the CVB vaccine protects not only from CVB infection but also against CVB-induced diabetes development in SOCS-1-Tg mice [Citation110].
These experimental vaccine studies have demonstrated the feasibility of vaccine production against CVBs: the traditional inactivated vaccines are highly immunogenic and prevent acute CVB infection and T1D. In addition, VLP-based CVB vaccine was as well highly immunogenic. Previous studies have also shown that other modalities of CVB vaccines, such as recombinant subunit vaccines, DNA vaccines, and live attenuated vaccines, are effective in mouse models [Citation111–Citation115].
In addition to efficacy, the safety of a candidate vaccine is of paramount importance, particularly since the target vaccination group includes healthy infants. The extensive experience with poliovirus vaccines suggests that inactivated EV vaccines are generally very safe. However, in addition to the general safety aspects, possible T1D-specific safety issues should be carefully considered. Theoretically, the vaccine could cause β-cell damage if it shares common antigenic structures with β-cell proteins (molecular mimicry). Previous studies have suggested that such epitopes may exist, but their role in the pathogenesis of T1D remains elusive [Citation116]. A clinical vaccine candidate should ideally avoid these epitopes, even if the inactivated CVB vaccine does not exaggerate the development of diabetes in NOD mouse [Citation107], and there are no indications that polio vaccines could cause diabetes, even when children with increased genetic susceptibility to T1D were vaccinated [Citation117]. In addition, maternal antibodies against CVBs in cord blood do not increase the risk of T1D in the child arguing against the role of antibody mediated cross-reactivity [Citation76]. It is thus unlikely that the vaccine would cause T1D, but this safety aspect will be carefully monitored in all stages of the CVB vaccine development program.
8. Current clinical development of a CVB vaccine against T1D
Building on the success of other licensed and clinically advanced EV vaccines (e.g. poliovirus and EV 71), the biopharmaceutical company Provention Bio has initiated the development of a formalin-inactivated CVB whole-virus vaccine (PRV-101). Formalin-inactivated whole-virus vaccine technology was chosen due to the favorable experience with the polio vaccine which has proved to be one of the safest and most effective vaccines ever developed. This fact together with the well-characterized production technology offer an important advantage for the development of PRV-101.
PRV-101 is intended as a polyvalent vaccine encompassing several serotypes of CVB. Based on cross-neutralization studies with hyperimmune sera, immune cross-reactivity between the different involved serotypes is not sufficient to afford appropriate protection if the desired serotype is not included in the vaccine, hence the polyvalency. The specific serotypes will be determined by a combination of epidemiological and experimental approaches.
Potential for molecular mimicry is negligible as PRV-101 does not contain the proven mimicry epitope related to human GAD65 and since the vaccine will not be administered with an adjuvant, which is reported to be an important factor why adjuvanted vaccines might be prone to mimicry [Citation118].
In conclusion, the work by this consortium has provided crucial novel information about the type of EVs associated with T1D and shown that experimental CVB vaccines work well in mouse models. The body of work has recently enabled the start of a clinical vaccine development program with a polyvalent-inactivated CVB vaccine produced in the same way as the current inactivated poliovirus vaccine. The target population of the vaccine would be pediatric (prevention of complications and hospitalizations due to CVB infections) and/or subjects at risk for T1D. The authors are not aware of any other past or present CVB vaccine in clinical development.
9. Expert commentary
Scientific evidence linking EVs, and in particular CVBs, to the pathogenesis of T1D has been strengthened considerably during the past years. Epidemiological, biological, and animal studies have resulted in a compelling rationale to move to the next stage of development, a clinical program to test the hypothesis. The first such clinical development program is now in manufacturing phase for a CVB vaccine to prevent acute infection and potentially T1D. In addition to the potential prevention of T1D, this vaccine can have other important beneficial health effects generated by protection against acute CVB infections. These effects can be substantial since CVB infections are frequent and cause significant morbidity particularly in young children (e.g. upper and lower respiratory infections, otitis media, meningitis, myocarditis, herpangina and hand, foot and mouth disease). This is also illustrated by the fact that CVBs have constantly been among the top 15 EV types reported in the annual surveillance of laboratory confirmed EV infections in the US [Citation106]. The technology is in place to produce the CVB vaccine as demonstrated by the widely used poliovirus vaccine. In addition to the formalin-inactivated whole-virus vaccine, other vaccine technologies could be used once the proof-of-concept has been obtained from the current development program. Ongoing scientific research projects can also significantly facilitate this effort by providing insights into the mechanisms of the CVB-T1D association in different populations. Altogether, these ongoing research efforts have created an international scientific ecosystem that is addressing the fundamental question whether the association between EV infections and T1D is causal. The next few years will be critical in this field and will show the direction into which these efforts will develop.
10. Five-year view
The risk association between CVB and T1D will continue to be validated in further epidemiological and biological studies.
Interactions between T1D-associated genes and diabetogenic EVs will be identified (e.g. the genes regulating innate immune responses).
Mechanisms of persisting EV infection and its role in T1D will be elucidated.
Antiviral drugs will be tested for their ability to preserve β-cell function or prevent T1D in clinical trials.
Clinical development of an EV vaccine against T1D will progress to clinical vaccine trials.
Key issues
T1D is a severe disease, and its incidence is increasing worldwide.
Viral infections cause diabetes in animals.
One virus group, EVs, has been associated with human T1D in numerous studies.
EVs have a natural tropism for the pancreas and they infect insulin producing β-cells which are destroyed in T1D.
The majority of patients with T1D carry low amounts of EV proteins and RNA in their pancreatic islets suggesting a low-grade EV infection.
In T1D patients, EV proteins locate in β-cells and their presence correlates with the activation of the innate immune system and with inflammation in the infected islets.
The association between EVs and T1D has been linked to certain specific EV serotypes, including the CVBs.
Experimental vaccines have successfully been produced against CVBs and shown to be effective and safe in mice.
Manufacturing of a polyvalent CVB vaccine has commenced for clinical trials which will test its efficacy for the prevention of acute CVB infections and T1D.
Declaration of interest
H Hyöty and M Knip are shareholders and chairman (HH) of the Board of Vactech Ltd and members of the Scientific Advisory Board of Provention Bio, Inc., which develop vaccines against picornaviruses and CVB. F Leon is an employee and shareholder in Provention Bio. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
We wish to thank Sami Oikarinen, PhD, Hanna Honkanen, PhD, Maarit Oikarinen, PhD, Anita Kondrashova, MD, PhD, Amir-Babak Sioofy-Khojine, PhD, Jutta Laiho, MSc, Olli Laitinen, PhD, Minna Hankaniemi, PhD, and Vesa Hytönen, PhD, who have contributed to the vaccine studies at the University ofTampere, Tampere, Finland (O.L. and M.H. also in Vactech Ltd). In addition, we wish to thank Malin Hodström-Tullberg, PhD, who has been our collaborator in preclinical vaccine trials at Karolinska Institutet, Stockholm, Sweden, as well as the Steering Committee of the Finnish Diabetes Prediction and Prevention (DIPP) study, the members of the international PEVNET and nPOD consortia and several other collaborators contributing to the findings and discussions summarized in this review.
Additional information
Funding
References
- Pociot F, Akolkar B, Concannon P, et al. Genetics of type 1 diabetes: what’s next? Diabetes. 2010;59:1561–1571.
- Knip M, Veijola R, Virtanen SM, et al. Environmental triggers and determinants of type 1 diabetes. Diabetes. 2005;54(Suppl 2):S125–S136.
- Hyöty H. Viruses in type 1 diabetes. Pediatr Diabetes. 2016;17(Suppl 22):56–64.
- Insel RA, Dunne JL, Atkinson MA, et al. Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the endocrine society, and the American Diabetes Association. Diabetes Care. 2015;38(10):1964–1974.
- Hyöty H, Taylor KW. The role of viruses in human diabetes. Diabetologia. 2002;45(10):1353–1361.
- Yoon JW, Jun HS. Viruses cause type 1 diabetes in animals. Ann NY Acad Sci. 2006;1079:138–146.
- Rasmussen T, Witsø E, Tapia G, et al. Self-reported lower respiratory tract infections and development of islet autoimmunity in children with the type 1 diabetes high-risk HLA genotype: the MIDIA study. Diabetes Metab Res Rev. 2011;7:834–837.
- Beyerlein A, Wehweck F, Ziegler AG, et al. Respiratory infections in early life and the development of islet autoimmunity in children at increased type 1 diabetes risk: evidence from the BABYDIET study. JAMA Pediatr. 2013;167:800–807.
- Lönnrot M, Lynch KF, Elding Larsson H, et al. Respiratory infections are temporally associated with initiation of type 1 diabetes autoimmunity: the TEDDY study. Diabetologia. 2017;60(10):1931–1940.
- Mustonen N, Siljander H, Peet A, et al. Early childhood infections precede development of beta-cell autoimmunity and type 1 diabetes in children with HLA-conferred disease risk. Pediatr Diabetes. 2018;19:293–299.
- Hyöty H, Knip M. Developing a vaccine for type 1 diabetes through targeting enteroviral infections. Expert Rev Vaccines. 2014;13(8):989–999.
- Hober D, Alidjinou EK. Enteroviral pathogenesis of type 1 diabetes: queries and answers. Curr Opin Infect Dis. 2013;26(3):263–269.
- Jenson AB, Rosenberg HS, Notkins AL. Pancreatic islet-cell damage in children with fatal viral infections. Lancet. 1980;2:354–358.
- Racaniello VR. One hundred years of poliovirus pathogenesis. Virology. 2006;344(1):9–16.
- Chapman NM, Kim KS. Persistent coxsackievirus infection: enterovirus persistence in chronic myocarditis and dilated cardiomyopathy. Curr Top Microbiol Immunol. 2008;323:275–292.
- Yajima T. Viral myocarditis: potential defense mechanisms within the cardiomyocyte against virus infection. Future Microbiol. 2011;6(5):551–566.
- Tam PE, Schmidt AM, Ytterberg SR, et al. Duration of virus persistence and its relationship to inflammation in the chronic phase of coxsackievirus B1-induced murine polymyositis. J Lab Clin Med. 1994;123:346–356.
- Feuer R, Ruller CM, An N, et al. Viral persistence and chronic immunopathology in the adult central nervous system following coxsackievirus infection during the neonatal period. J Virol. 2009;83:9356–9369.
- MacLennan C, Dunn G, Huissoon AP, et al. Failure to clear persistent vaccine-derived neurovirulent poliovirus infection in an immunodeficient man. Lancet. 2004;363(9420):1509–1513.
- Kim KS, Tracy S, Tapprich W, et al. 5ʹ-Terminal deletions occur in coxsackievirus B3 during replication in murine hearts and cardiac myocyte cultures and correlate with encapsidation of negative-strand viral RNA. J Virol. 2005;79(11):7024–7041.
- Mueller S, Wimmer E, Cello J. Poliovirus and poliomyelitis: a tale of guts, brains, and an accidental event. Virus Res. 2005;111:175–193.
- Ida-Hosonuma M1, Iwasaki T, Yoshikawa T, et al. The alpha/beta interferon response controls tissue tropism and pathogenicity of poliovirus. J Virol. 2005;79:4460–4469.
- Kaufman DL, Erlander MG, Clare-Salzler M, et al. Autoimmunity to two forms of glutamate decarboxylase in insulin-dependent diabetes mellitus. J Clin Invest. 1992;89(1):283–292.
- Härkönen T, Paananen A, Lankinen H, et al. Enterovirus infection may induce humoral immune response reacting with islet cell autoantigens in humans. J Med Virol. 2003;69(3):426–440.
- Horwitz MS, Bradley LM, Harbertson J, et al. Diabetes induced by coxsackie virus: initiation by bystander damage and not molecular mimicry. Nat Med. 1998;4(7):781–785.
- Ylipaasto P, Klingel K, Lindberg AM, et al. Enterovirus infection in human pancreatic islet cells, islet tropism in vivo and receptor involvement in cultured islet beta cells. Diabetologia. 2004;47:225–239.
- Croft SN, Walker EJ, Ghildyal R. Picornaviruses and apoptosis: subversion of cell death. MBio. 2017;8(5):e01009–172.
- Barboni E, Manocchio J. Alterazioni pancreatiche in bovini con diabete mellito post-aftoso. Arch Vet Ital. 1962;13:477–489.
- Jun HS, Kang Y, Notkins AL, et al. Gain or loss of diabetogenicity resulting from a single point mutation in recombinant encephalomyocarditis virus. J Virol. 1997;71(12):9782–9785.
- Jun HS, Yoon JW. The role of viruses in type I diabetes: two distinct cellular and molecular pathogenic mechanisms of virus-induced diabetes in animals. Diabetologia. 2001;44(3):271–285.
- Jun HS, Yoon SW, Kang Y, et al. Cloning and expression of the VP1 major capsid protein of diabetogenic encephalomyocarditis (EMC) virus and prevention of EMC virus-induced diabetes by immunization with the recombinant VP1 protein. J Gen Virol. 1995;76:2557–2566.
- Notkins AL, Yoon JW. Virus-induced diabetes in mice prevented by a live attenuated vaccine. N Engl J Med. 1982;306(8):486.
- Toniolo A, Onodera T, Jordan G, et al. Virus-induced diabetes mellitus. Glucose abnormalities produced in mice by the six members of the coxsackie B virus group. Diabetes. 1982;31(6):496–499.
- Jaidane H, Sane F, Gharbi J, et al. Coxsackievirus B4 and type 1 diabetes pathogenesis: contribution of animal models. Diabetes Metab Res Rev. 2009;25:591–603.
- Flodström M, Maday A, Balakrishna D, et al. Target cell defense prevents the development of diabetes after viral infection. Nat Immunol. 2002;3:373–382.
- Tracy S, Drescher KM. Coxsackievirus infections and NOD mice: relevant models of protection from, and induction of, type 1 diabetes. Ann NY Acad Sci. 2007;1103:143–151.
- Yoon JW, London WT, Curfman BL, et al. Coxsackie virus B4 produces transient diabetes in nonhuman primates. Diabetes. 1986;35:712–716.
- Anagandula M, Richardson SJ, Oberste MS, et al. Infection of human islets of langerhans with two strains of coxsackie B virus serotype 1: assessment of virus replication, degree of cell death and induction of genes involved in the innate immunity pathway. J Med Virol J Med Virol. 2014;86(8):1402–1411.
- Hodik M, Lukinius A, Korsgren O, et al. Tropism analysis of two coxsackie B5 strains reveals irus growth in human primary pancreatic islets but not in exocrine cell clusters in vitro. Open Virol J. 2013;7:49–56.
- Izumi K, Mine K, Inoue Y, et al. Reduced Tyk2 gene expression in β-cells due to natural mutation determines susceptibility to virus-induced diabetes. Nat Commun. 2015;6:6748.
- Hämäläinen S, Nurminen N, Ahlfors H, et al. Coxsackievirus B1 reveals strain specific differences in plasmacytoid dendritic cell mediated immunogenicity. J Med Virol. 2014;86:1412–1420.
- Op de Beeck A, Dl E. Viral infections in type 1 diabetes mellitus – why the β cells? Nat Rev Endocrinol. 2016;12:263–273.
- Sane F, Caloone D, Gmyr V, et al. Coxsackievirus B4 can infect human pancreas ductal cells and persist in ductal-like cell cultures which results in inhibition of Pdx1 expression and disturbed formation of islet-like cell aggregates. Cell Mol Life Sci. 2013;70:4169–4180.
- Bissel SJ, Winkler CC, Deltondo J, et al. Coxsackievirus B4 myocarditis and meningoencephalitis in newborn twins. Neuropathology. 2014;34:429–437.
- Richardson SJ, Willcox A, Bone AJ, et al. The prevalence of enteroviral capsid protein vp1 immunostaining in pancreatic islets in human type 1 diabetes. Diabetologia. 2009;52:1143–1151.
- Richardson SJ, Leete P, Dhayal S, et al. Evaluation of the fidelity of immunolabelling obtained with clone 5D8/1, a monoclonal antibody directed against the enteroviral capsid protein, VP1, in human pancreas. Diabetologia. 2014;57(2):392–401.
- Krogvold L, Edwin B, Buanes T, et al. Detection of a low-grade enteroviral infection in the islets of langerhans of living patients newly diagnosed with type 1 diabetes. Diabetes. 2015;64(5):1682–1687.
- Krogvold L, Edwin B, Buanes T, et al. Pancreatic biopsy by minimal tail resection in live adult patients at the onset of type 1 diabetes: experiences from the DiViD study. Diabetologia. 2014;57(4):841–843.
- Foulis AK, McGill M, Farquharson MA, et al. A search for evidence of viral infection in pancreases of newly diagnosed patients with IDDM. Diabetologia. 1997;40(1):53–61.
- Yoon JW, Austin M, Onodera T, et al. Isolation of a virus from the pancreas of a child with diabetic ketoacidosis. N Engl J Med. 1979;300:1173–1179.
- Dotta F, Censini S, van Halteren AG, et al. Coxsackie B4 virus infection of beta cells and natural killer cell insulitis in recent-onset type 1 diabetic patients. Proc. Natl. Acad. Sci. USA. 2007;104:5115–5120.
- Oikarinen M, Tauriainen S, Honkanen T, et al. Analysis of pancreas tissue in a child positive for islet cell antibodies. Diabetologia. 2008;51:1796–1802.
- Ifie E, Russell MA, Dhayal S, et al. Unexpected subcellular distribution of a specific isoform of the coxsackie and adenovirus receptor, CAR-SIV, in human pancreatic beta cells. Diabetologia. 2018;61(11):2344–2355.
- Richardson SJ, Leete P, Bone AJ, et al. Expression of the enteroviral capsid protein VP1 in the islet cells of patients with type 1 diabetes is associated with induction of protein kinase R and downregulation of mcl-1. Diabetologia. 2013;56(1):185–193.
- Gamble DR, Taylor KW, Cumming H. Coxsackie viruses and diabetes mellitus. Br Med J. 1973;4:260–262.
- Gamble DR, Kinsley ML, FitzGerald MG, et al. Viral antibodies in diabetes mellitus. Br Med J. 1969;3:627–630.
- Green J, Casabonne D, Newton R Coxsackie B virus serology and type 1 diabetes mellitus: a systematic review of published case-control studies. Diabet Med. 2004;21, 507–514.
- Yeung WC, Rawlinson WD, Craig ME. Enterovirus infection and type 1 diabetes mellitus: systematic review and meta-analysis of observational molecular studies. Br Med J. 2011;342:d35.
- Honkanen H, Oikarinen S, Nurminen N, et al. Detection of enteroviruses in stools precedes islet autoimmunity by several months: possible evidence for slowly operating mechanisms in virus-induced autoimmunity. Diabetologia. 2017;60(3):424–431.
- Oikarinen S, Martiskainen M, Tauriainen S, et al. Enterovirus RNA in blood is linked to the development of type 1 diabetes. Diabetes. 2011;60(1):276–279.
- Tapia G, Cinek O, Rasmussen T, et al. Human enterovirus RNA in monthly fecal samples and islet autoimmunity in Norwegian children with high genetic risk for type 1 diabetes: the MIDIA study. Diabetes Care. 2011;34:151–155.
- Simonen-Tikka ML, Pflueger M, Klemola P, et al. Human enterovirus infections in children at increased risk for type 1 diabetes: the babydiet study. Diabetologia. 2011;54(12):2995–3002.
- Cinek O, Stene LC, Kramna L, et al. Enterovirus RNA in longitudinal blood samples and risk of islet autoimmunity in children with a high genetic risk of type 1 diabetes: the MIDIA study. Diabetologia. 2014;57(10):2193–2200.
- Hyöty H, Hiltunen M, Knip M, et al. A prospective study of the role of coxsackie B and other enterovirus infections in the pathogenesis of IDDM. Childhood diabetes in Finland (DiMe) study group. Diabetes. 1995;44(6):652–657.
- Hiltunen M, Hyöty H, Knip M, et al. Islet cell antibody seroconversion in children is temporally associated with enterovirus infections. Childhood diabetes in Finland (DiMe) study group. J Infect Dis. 1997;175(3):554–560.
- Viskari HR, Roivainen M, Reunanen A, et al. Maternal first-trimester enterovirus infection and future risk of type 1 diabetes in the exposed fetus. Diabetes. 2002;51(8):2568–2571.
- Viskari H, Knip M, Tauriainen S, et al. Maternal enterovirus infection as a risk factor for type 1 diabetes in the exposed offspring. Diabetes Care. 2012;35(6):1328–1332.
- Allen DW, Kim KW, Rawlinson WD, et al. Maternal virus infections in pregnancy and type 1 diabetes in their offspring: systematic review and meta-analysis of observational studies. Rev Med Virol. 2018;28:e1974.
- Oikarinen M, Tauriainen S, Oikarinen S, et al. Type 1 diabetes is associated with enterovirus infection in gut mucosa. Diabetes. 2012;61:687–691.
- Mercalli A, Lampasona V, Klingel K, et al. No evidence of enteroviruses in the intestine of patients with type 1 diabetes. Diabetologia. 2012;55(9):2479–2488.
- Sioofy-Khojine AB, Lehtonen J, Nurminen N, et al. Coxsackievirus B1 infections are associated with the initiation of insulin-driven autoimmunity that progresses to type 1 diabetes. Diabetologia. 2018;61(5):1193–1202.
- Viskari HR, Koskela P, Lonnrot M, et al. Can enterovirus infections explain the increasing incidence of type 1 diabetes? Diabetes Care. 2000;23:414–416.
- Viskari H, Ludvigsson J, Uibo R, et al. Relationship between the incidence of type 1 diabetes and enterovirus infections in different European populations: results from the EPIVIR project. J Med Virol. 2004;72:610–617.
- Viskari H, Ludvigsson J, Uibo R, et al. Relationship between the incidence of type 1 diabetes and maternal enterovirus antibodies: time trends and geographical variation. Diabetologia. 2005;48:1280–1287.
- Larsson PG, Lakshmikanth T, Svedin E, et al. Previous maternal infection protects offspring from enterovirus infection and prevents experimental diabetes development in mice. Diabetologia. 2013;56:867–874.
- Laitinen OH, Honkanen H. Pakkanen O et al. Coxsackievirus B1 is associated with the induction of beta-cell autoimmunity that portends type 1 diabetes. Diabetes. 2014;63:446–455.
- Haller MJ, Schatz DA. The DIPP project: 20 years of discovery in type 1 diabetes. Pediatr Diabetes. 2016;17(Suppl 22):5–7.
- Oikarinen S, Tauriainen S, Hober D, et al. Virus antibody survey in different European populations indicates risk association between coxsackievirus B1 and type 1 diabetes. Diabetes. 2014;63:655–662.
- Sioofy-Khojine A, Lehtonen J, Cuthbertson D, et al. Coxsackievirus B infections are associated with the initiation of islet autoimmunity – results from the TRIGR study. Annual meeting of the Network for Pancreatic Organ Donors (nPOD); 2017; Fort Lauderdale, Florida, US.
- Ponsonby AL, Pezic A, Cameron FJ, et al. Parental occupational social contact is associated with a reduced risk of incident pediatric type 1 diabetes: mediation through molecular enteroviral indices. PLoS One. 2018;13:e0193992.
- Afonso G, Mallone R. Infectious triggers in type 1 diabetes: is there a case for epitope mimicry? Diabetes Obes Metab. 2013;15(Suppl 3):82–88.
- Petzold A, Solimena M, Knoch KP. Mechanisms of beta cell dysfunction associated with viral infection. Curr Diab Rep. 2015;15(10):73.
- Cabrera-Rode E, Sarmiento L, Tiberti C, et al. Type 1 diabetes islet associated antibodies in subjects infected by echovirus 16. Diabetologia. 2003;46:1348–1353.
- Paananen A, Ylipaasto P, Rieder E, et al. Molecular and biological analysis of echovirus 9 strain isolated from a diabetic child. J Med Virol. 2003;69(4):529–537.
- Williams CH, Oikarinen S, Tauriainen S, et al. Molecular analysis of an echovirus 3 strain isolated from an individual concurrently with appearance of islet cell and IA-2 autoantibodies. J Clin Microbiol. 2006;44(2):441–448.
- Moore M, Kaplan MH, McPhee J, et al. Epidemiologic, clinical, and laboratory features of coxsackie B1-B5 infections in the United States, 1970-79. Public Health Rep. 1984;99(5):515–522.
- Sadeharju K, Knip M, Hiltunen M, et al. The HLA-DR phenotype modulates the humoral immune response to enterovirus antigens. Diabetologia. 2003;46(8):1100–1105.
- Smyth DJ, Cooper JD, Bailey R, et al. A genome-wide association study of nonsynonymous SNPs identifies a type 1 diabetes locus in the interferon-induced helicase (IFIH1) region. Nat Genet. 2006;38:617–619.
- Heinig M, Petretto E, Wallace C, et al. A trans-acting locus regulates an anti-viral expression network and type 1 diabetes risk. Nature. 2010;467(7314):460–464.
- Wang Y, Shaked I, Stanford SM, et al. The autoimmunity-associated gene PTPN22 potentiates toll-like receptor-driven, type 1 interferon-dependent immunity. Immunity. 2013;39(1):111–122.
- Gorman JA, Hundhausen C, Errett JS, et al. The A946T variant of the RNA sensor IFIH1 mediates an interferon program that limits viral infection but increases the risk for autoimmunity. Nat Immunol. 2017;18:744–752.
- Onengut-Gumuscu S, Chen WM, Burren O, et al. Fine mapping of type 1 diabetes susceptibility loci and evidence for colocalization of causal variants with lymphoid gene enhancers. Nat Genet. 2015;47(4):381–386.
- Nagafuchi S, Kamada-Hibio Y, Hirakawa K, et al. TYK2 promoter variant and diabetes mellitus in the Japanese. EBioMedicine. 2015;2(7):744–749.
- Staeva TP, Chatenoud L, Insel R, et al. Recent lessons learned from prevention and recent-onset type 1 diabetes immunotherapy trials. Diabetes. 2013;62(1):9–17.
- Donath MY, Hess C, Palmer E. What is the role of autoimmunity in type 1 diabetes? A clinical perspective. Diabetologia. 2014;57(4):653–655.
- Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, et al. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med. 2009;361(22):2143–2152.
- Knip M, Åkerblom HK, Al Taji E, et al. Effect of hydrolyzed infant formula vs conventional formula on risk of type 1 diabetes: the TRIGR randomized clinical trial. JAMA. 2018;319:38–48.
- Skog O, Korsgren S, Melhus A, et al. Revisiting the notion of type 1 diabetes being a T-cell-mediated autoimmune disease. Curr Opin Endocrinol Diabetes Obes. 2013;20(2):118–123.
- Campbell-Thompson ML, Atkinson MA, Butler AE, et al. The diagnosis of insulitis in human type 1 diabetes. Diabetologia. 2013;56(11):2541–2543.
- In’t Veld P. Insulitis in human type 1 diabetes: the quest for an elusive lesion. Islets. 2011;3(4):131–138.
- Thomassen YE. van ‘t Oever AG, van Oijen MG et al. Next generation inactivated polio vaccine manufacturing to support post polio-eradication biosafety goals. PLoS One. 2013;8(12):e83374.
- Zhou Y, Li JX, Jin PF, et al. Enterovirus 71: a whole virion inactivated enterovirus 71 vaccine. Expert Rev Vaccines. 2016;15(7):803–813.
- Modlin JF, Rotbart HA. Group B coxsackie disease in children. Curr Top Microbiol Immunol. 1997;223:53–80.
- Tam PE. Coxsackievirus myocarditis: interplay between virus and host in the pathogenesis of heart disease. Viral Immunol. 2006;19(2):133–146.
- Huang HI, Shih SR. Neurotropic enterovirus infections in the central nervous system. Viruses. 2015;7(11):6051–6066.
- Abedi GR, Watson JT, Pham H, et al. Enterovirus and human parechovirus surveillance – United States, 2009-2013. MMWR Morb Mortal Wkly Rep. 2015;64:940–943.
- Larsson PG, Lakshmikanth T, Laitinen OH, et al. A preclinical study on the efficacy and safety of a new vaccine against coxsackievirus B1 reveals no risk for accelerated diabetes development in mouse models. Diabetologia. 2015;58(2):346–354.
- Koho T, Koivunen MLR, Oikarinen S, et al. Coxsackievirus B3 VLPs purified by ion exchange chromatography elicit strong immune responses in mice. Antiviral Res. 2014;104:93–101.
- Hankaniemi MM, Laitinen OH, Stone VM, et al. Optimized production and purification of coxsackievirus B1 vaccine and its preclinical evaluation in a mouse model. Vaccine. 2017;35(30):3718–3725.
- Stone VM, Hankaniemi MM, Svedin E, et al. A coxsackievirus B vaccine protects against virus-induced diabetes in an experimental mouse model of type 1 diabetes. Diabetologia. 2018;61(2):476–481.
- Fohlman J, Ilbäck NG, Friman G, et al. Vaccination of Balb/c mice against enteroviral mediated myocarditis. Vaccine. 1990;8(4):381–384.
- Toniolo A, Falcone V, Bernasconi C, et al. DNA immunization of mice against the VP1 capsid protein of coxsackievirus B4. Scand J Immunol. 2002;56(5):448–455.
- Dan M, Chantler JK. A genetically engineered attenuated coxsackievirus B3 strain protects mice against lethal infection. J Virol. 2005;79(14):9285–9295.
- Kim JY, Jeon ES, Lim BK, et al. Immunogenicity of a DNA vaccine for coxsackievirus B3 in mice: protective effects of capsid proteins against viral challenge. Vaccine. 2005;23(14):1672–1679.
- Lan J, Gao Z, Xiong H, et al. Generation of protective immune responses against coxsackievirus B3 challenge by DNA prime-protein boost vaccination. Vaccine. 2011;29(40):6894–6902.
- Coppieters KT1, Wiberg A, von Herrath MG. Viral infections and molecular mimicry in type 1 diabetes. APMIS. 2012;120(12):941–949.
- Viskari H, Oikarinen S, Hoppu S, et al. Live attenuated enterovirus vaccine (OPV) is not associated with islet autoimmunity in children with genetic susceptibility to type 1 diabetes: prospective cohort study. Diabetologia. 2018;61(1):203–2092.
- Ahmed SS, Schur PH, MacDonald NE, et al. 2009 A(H1N1) pandemic influenza, and pandemic influenza vaccinations: what is known and unknown about the neurological disorder, the role for autoimmunity, and vaccine adjuvants. J Autoimmun. 2014;50:1–11.
