1. Could you briefly summarize your background in the field, your role within B Medical Systems, and the company’s role in the current vaccine roll-out program?
I am Luc Provost and I am the CEO of B Medical Systems. I have been with the company for the last 20 years.
B Medical Systems has been the global market leader in the vaccine cold chain for the past 40 years, and a leading innovator in the medical refrigeration industry. Our company creates safe and reliable medical-grade refrigeration solutions for the storage and transport of vaccines, medicines, blood, blood components, and samples. This way, we have products such as laboratory/vaccine/blood/pharmaceutical refrigerators, plasma/laboratory/vaccine freezers, ultra-low freezers, contact-shock freezers, and transport boxes, to cater to the needs of the national immunization programs, research institutes, hospitals, blood banks, etc. We provide end-to-end solutions for the needs of the medical industry worldwide.
As for immunization campaigns, we have been a trusted partner of global procurement bodies like UNICEF, WHO, etc., for the last 40 years. Across the world, we have worked closely with Central Governments, ministries of health, EPI (Expanded Program on Immunization), etc., to ensure a safe and reliable roll-out of immunization programs. Specific to COVID-19 immunization campaigns, we have been working with governments across the world to roll out successful COVID-19 immunization campaigns.
2. What logistical measures need to be considered to ensure that the roll out of the COVID vaccine is a success, and how can manufacturers, distributors and governments worldwide work together to strengthen the vaccine cold-chain infrastructure?
It is important that nobody is left behind when it comes to COVID-19 vaccines. Equitable distribution of vaccines is important to stop the pandemic and return to normality, but access to the vaccines alone will not end the pandemic. Many of the currently available coronavirus vaccines are highly thermosensitive and it is imperative that a reliable vaccine cold chain is in place before the vaccines arrive from the manufacturer. Governments, with the help of cold-chain suppliers such as us, need to create an immunization plan which considers different storage temperature requirements for the various vaccines and therefore need to make sure that, at every storage center and vaccination center, there will not only be enough cold-chain equipment but also reliable ones. Moreover, the transport of the vaccines from the central storage location to anywhere else in the country needs to be organized, and the use of reliable transport boxes is critical to guarantee safe movement of the vaccines until the last mile. Just as how the global co-operation between governments, industry, and public bodies has resulted in the rapid development of vaccines, the same level of collaboration is required across the globe to ensure that the entire world has access to reliable cold-chain equipment.
3. For more fragile COVID vaccines, such as the Pfizer vaccine, what measures need to be taken to preserve its quality throughout the supply chain?
The core logistical challenge surrounding the COVID-19 vaccines is that they need different types of refrigerators and freezers because of the varying temperature requirements. Vaccines developed by Pfizer-BioNTech and Moderna require very low temperature to be safely stored: −70°C and −20°C respectively. The new mRNA technology which has enabled such a rapid vaccine development has resulted in extremely thermosensitive products which cannot be subject to temperature deviations, especially for long periods of time. Today many countries are not equipped with these types of freezers that can ensure the safe storage of these vaccines. Regular refrigerators and freezers cannot ensure ultra-low temperatures as low as −70°C. Also, there was a recent announcement from the FDA, allowing undiluted frozen vials of the Pfizer-BioNTech vaccine to be transported and stored at conventional temperatures commonly found in pharmaceutical freezers (−25°C to −15°C) for a period of up to 2 weeks. This shouldn’t be seen as a total relaxation of the cold-chain requirement around Pfizer-BioNTech vaccine. This, in fact, is an alternative storage and transportation option, that too for a short duration of time (up to 2 weeks).
We produce reliable ultra-low freezers that can offer storage temperature in the range of −86°C to −20°C, thereby providing a versatile solution for the safe and reliable storage of not just Pfizer’s vaccine, but also Moderna’s vaccines.
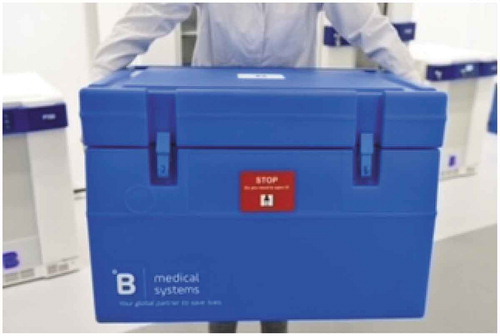
In addition, it’s not just the storage, but vaccine transport can be tricky, and it is important to ensure that vaccines are not exposed to temperature deviations during the transportation. Medical-grade transport boxes () are perfectly suited for safely transporting vaccines and at the right temperatures as they can be used to store vaccines at −70°C, −20°C, or 4°C.
4. Which leg of the supply chain is most vulnerable to collapse? What measures can we put in place to prevent this?
The last mile. Typically, the preliminary stage of vaccine supply is taken care of by the manufacturer itself. As a manufacturer, these players understand the criticality of the intended temperature ranges. But the last mile – from the country hubs till the point of administration, there are several stakeholders included. We should also not forget that transportation is also included. Some countries can manage this well, but not all of them. Ensuring a reliable cold chain until the last mile is not an easy task!
5. The last mile distribution challenge to the patient’s arm is fundamental to a successful vaccine roll out. What are some unique challenges around this during the current pandemic?
Today, there are several COVID-19 vaccines authorized for use with different temperature requirements (available all over the world). But this creates challenges from a cold chain point of view as not all the equipment offer the same storage temperature. For example, if we were to store Pfizer’s vaccine, then you are looking at ultra-low freezers (as depicted in ) offering storage temperatures in the range of −70°C, while if we were to store the Moderna vaccine, the ideal storage temperature required is −20°C. AstraZeneca vaccines require storage temperatures in the range of 2°C to 8°C. As the doses available are still restricted, the countries need to optimize their vaccine cold-chain capacity in the best possible way, so they are capable of storing any COVID-19 vaccine that they receive, in the most reliable manner.
Then, of course, comes the next challenge. Transportation until the last mile which is a challenge in its own right: being able to ensure that vaccines arrive safely and at the right temperatures to the patient for administration is extremely challenging, especially in places where the vaccines need to travel long distances to reach the people they are intended for. Therefore, having reliable transport boxes which can hold vaccines at different temperatures is extremely important.
6. Could the demand for vaccine cold-chain equipment outstrips supply capabilities? How are manufacturers and distributors adapting to the increase demand?
There has been a great rise in demand for vaccine cold-chain equipment in the past months, and we are expanding to be able to meet it and avoid leaving governments and organizations without a proper cold chain for when they are supplied with vaccines. We are currently expanding our Luxembourg production facility and building a new facility in India to be able to meet the worldwide demand in the future.
7. How can we deliver vaccines successfully in countries with limited cold-chain infrastructure, for example, in low- and middle-income countries?
It is a challenge to ensure that vaccines can be stored in remote areas which lack basic infrastructures, but it is not impossible. At B Medical Systems, we have devised multiple ways to provide for reliable cold chain in these places. In fact, some of our products use the one-power supply that is a constant: the sun. Our Solar Direct Drive Refrigerators and Freezers use our SDD technology and ensure reliable cooling performance even in places lacking reliable electricity in the most remote corners of the world. For example, our Ultra 16 SDD offers an autonomy of almost 30 days, thereby ensuring vaccines can be stored for long durations even in bad weather.
Other solutions, such as our Ice-Lined Refrigerators, are designed with integrated voltage stabilizing solutions that allow for reliable usage of the products even during unstable electric supply. One of the products TCW3000AC can function as a vaccine refrigerator, a vaccine freezer, or even an ice-pack freezer, empowering the countries in terms of cold-chain capability.
Ensuring traceability is also important. The ability to identify a breach in the cold chain in real-time can help life-saving vaccines from getting wasted. We provide Remote Temperature Monitoring Devices (RTMD) () that track the performance of these refrigerators and freezers installed in any part of the world in real time and alert the registered users in case of any deviations.
8. What will be different for B Medical systems after the pandemic, will anything change for your company in a post COVID world?
Although the vaccine cold chain might not be the focus on everyone’s minds in a post-COVID-19 world, there will still be aneed for them: immunization campaigns against other diseases. We have a legacy of working on this supply chain challenge for the last 40 years, and we will continue our work to bring in more innovative cold-chain solutions that can cater to the needs of the world.
On the other hand, I also believe that we will see an increase again in our Blood Management and Medical Refrigeration equipment. Blood banks and hospitals, which due to the pandemic have witnessed dramatic drops in blood donations, will require cold storage for what will hopefully be a renewed increased supply of biological samples such as blood or plasma. Meanwhile, research laboratories will continue to require cooling solutions for the safe storage of important samples for scientific research, and we are excited at the prospect of continuing to have an impact in that field as well.
Disclaimer
The opinions expressed in this interview are those of the interviewee and do not necessarily reflect the views of Taylor & Francis. This interview was not peer reviewed
Declaration of interest
L Provost is the CEO of B Medical Systems. The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.