ABSTRACT
Background
Most adults, and disproportionately fewer African-Americans, have not received herpes zoster (HZ) vaccination despite current recommendations. This study (GSK study identifiers: 208677/HO-17-18066) assessed HZ vaccination preferences among adults aged ≥ 50 years.
Research design and methods
In this discrete choice experiment, respondents chose among a ‘no vaccine’ option and two HZ vaccine profiles, characterized by seven attributes, in a series of choice questions. Random-parameters logit results were used to predict likely vaccine uptake. Subgroup and latent class analysis of African-American’s preferences were performed.
Results
The preference weight for choosing HZ vaccines over no vaccine was statistically significant among the 1,454 respondents (71.9% whites; 25.2% African-Americans). Out-of-pocket (OOP) cost and vaccine effectiveness (VE) were the most important attributes. The African-American and the non-African-American subgroups had statistically significant differences in preferences (χ2 = 59.91, p < 0.001), mainly driven by OOP cost and VE. Latent class analysis identified three groups of African-American respondents with systematically different preferences; two comprised likely-vaccinators, with one being more cost sensitive at lower price thresholds, and one likely non-vaccinators.
Conclusions
For all respondents, HZ vaccine choices were most sensitive to total OOP cost, followed by VE.
PLAIN LANGUAGE SUMMARY
What is the context?
Herpes zoster, or shingles, is a viral disease characterized by a painful, localized skin rash. It affects approximately 32% of US citizens at least once in their lifetime.
The risk of contracting shingles increases with age.
Most American adults over 50 years have not received the shingles vaccine, and vaccination rates are especially low for African-Americans. What is new?
This is the first study to evaluate what drives shingles vaccination decisions among US adults ≥ 50 years of age. We also assessed the differences between African-American and non-African-American adults, and inside the African-American group.
In this choice experiment, 1,454 people ≥ 50 years completed a survey of 8 choice questions, as well as questions on their previous experiences with vaccines, socioeconomic, and demographic characteristics. Seven factors were evaluated.
We found that American adults preferred to get vaccinated, and the most influential factors were costs and vaccine effectiveness while location of vaccination was the least important. There were differences in preferences between African-American and non-African-American adults, mainly driven by costs and vaccine effectiveness. 3 different groups of African-American adults with systematically different preferences could be identified; two were likely to vaccinate, with one being more cost sensitive at lower price thresholds, and the third was unlikely to vaccinate.
What is the impact?
Decisions on shingles vaccination appear to be mostly driven by costs, which could be a barrier to those who do not have appropriate insurance, especially among some African-Americans.
However, healthcare professionals should continue to educate patients on other vaccine characteristics, as they also influence vaccination decisions.
Graphical abstract

1. Introduction
One million people in the United States (US) are estimated to develop herpes zoster (HZ) every year, with about 32% of US population developing HZ once in their lifetime [Citation1]. Additionally, some will suffer from HZ more than once, with an estimated cumulative recurrence increasing by 1% each year after incident HZ episode [Citation2]. The risk of HZ increases with age, especially after the age of 50 years [Citation1,Citation3,Citation4]. Autoimmune diseases and other comorbidities are associated with a higher risk of HZ [Citation5]. In the US, estimates of the annual economic burden associated with HZ are between $782 million [Citation6] and $5 billion [Citation7]; it is therefore a significant source of health care resource consumption. This vaccine-preventable disease [Citation8] burden can be reduced with case-avoidance strategies [Citation9] that would advocate for policies encouraging vaccination.
A single dose Zoster Vaccine Live (ZVL; Zostavax, Merck & Co., Inc) was available for use in the US from 2006 [Citation10] to November 2020 [Citation11]. However, only one HZ vaccine is currently available: the two-dose adjuvanted Recombinant Zoster Vaccine (RZV; Shingrix, GSK) licensed in 2017 [Citation11,Citation12]. With licensure of the new vaccine in 2017, the Advisory Committee on Immunization Practices (ACIP) revised its HZ vaccine recommendations for older adults in the US so that all adults aged ≥ 50 years should receive the preferentially recommended RZV vaccine regardless of prior HZ vaccination status [Citation13].
During a decade of availability with the first HZ vaccine (ZVL), vaccination coverage (VC) remains low, increasing from 6.7% in 2008 [Citation14] to 34.9% in 2017 [Citation15] among adults aged ≥ 60 years. In addition, VC among adults aged ≥ 60 years in 2017 was also associated with an ethnicity disparity in coverage (39.3% VC in whites, 17.1% in African-Americans, and 19.9% in Hispanics) [Citation15]. This specific discrepancy in HZ prevention may result in disparities in the growing public health and economic burden of HZ as the population aged ≥ 50 years increases over time [Citation16].
Very little is known about what drives individual HZ vaccination decisions. To date, only one published study has examined HZ vaccine preferences and acceptance among older adults [Citation17]. However, the focus of this Dutch study evaluated the relative importance of HZ vaccination decision-making relative to pneumococcal, pertussis, and influenza vaccines and disease characteristics, and did not consider the relative importance of the characteristics of HZ vaccines themselves.
The objective of this study was to explore the extent to which different HZ vaccine and respondent characteristics influenced personal vaccination decisions; it further evaluated systematic differences in preferences between African-American respondents and non-African-American respondents and preference heterogeneity within African-American respondents. Finally, it explored whether and how these characteristics influenced respondents stated intent to receive the vaccination and likely compliance with the second dose.
2. Patients and methods
2.1. Survey design
A cross-sectional discrete choice experiment (DCE) survey developed using good practices [Citation18] and administered in January 2019 to adults aged ≥ 50 years in the US (GSK study identifiers: 208677/HO-17-18066).
The survey consisted of: a) DCE questions; b) questions about compliance with a two-dose vaccine; and c) measures of respondents characteristics (Supplementary File 1). Respondents were assigned to one of two survey versions based on their age-specific risk of HZ: a lower risk for those aged 50–64 years and higher risk for those aged ≥ 65 years [Citation1,Citation19,Citation20].
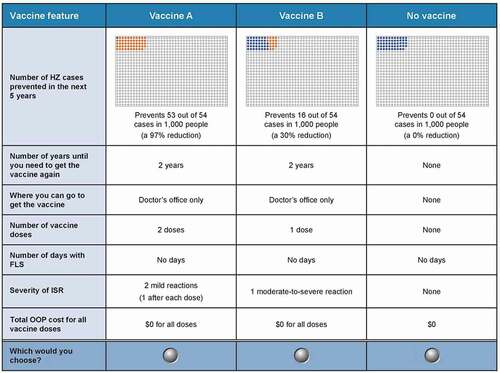
Respondents were presented with a series of choices, each with two hypothetical HZ vaccines (Vaccine A or Vaccine B) and a no-HZ-vaccine option. The selection of seven attributes and attributes levels were informed by the features that could both characterize and differentiate the HZ vaccines: vaccine effectiveness (VE), duration of protection, location of vaccination, number of doses, days with flu-like symptoms (FLS), injection-site reactions (ISR) severity, and total out-of-pocket (OOP) cost (). The ranges for the levels were based on the product labels for the available HZ vaccines and on published clinical data [Citation10,Citation12,Citation13,Citation21,Citation22].
Table 1. Attributes and levels for the DCE
2.1.1. DCE questions
The vaccine profiles in each choice question were determined by an experimental design using the attributes and levels in . DCE applications commonly use a D-optimal experimental design to reduce the number of paired comparisons to the smallest number for efficient estimation of the preference weights [Citation23–26]. The full design included 72 unique choice questions. However, there is a limit to the number of questions each respondent can answer before becoming fatigued. A review of empirical DCE studies in health care showed that the number of questions presented to each respondent is 12 or fewer in most studies [Citation27]. In keeping with this practice, the current study was designed in nine blocks, each consisting of eight DCE questions. Each respondent was randomly assigned to one of the nine blocks and the order of the questions was randomized across respondents to limit the influence of question order or fatigue on choice question’s responses. An example choice question is shown in . Before answering the DCE questions, the vaccine features were defined one-by-one and respondents were shown partial DCE questions to familiarize them with the question content and format. There were also a number of questions to assess respondent comprehension of the survey content. For example, for the vaccine feature ‘number of HZ cases prevented in the next 5 years’, the respondents read a description of the attribute and a description of the risk pictograms. Questions were asked to assess respondent comprehension of the pictograms and upon successfully testing, the survey respondents proceeded (see the survey in the Supplementary File 1 for details).
Figure 1. Sample DCE question from the high-baseline-risk (aged ≥ 65 years, Medicare population) version
2.1.2. Compliance questions
Immediately following the DCE questions, respondents were presented with a shorter series of stated-preference questions to assess compliance with a two-dose vaccine. Each question described a two-dose HZ vaccine and the side effects and OOP cost experienced upon completing the first dose of the vaccine. In particular, respondents were provided with hypothetical vaccine profiles requiring two doses, which were meant to be given 12 months apart, were 97% effective, and would last for 12 years. These levels were similar to current ACIP vaccination recommendations and other published data [Citation13,Citation21,Citation22]. Respondents were then presented with three pairs of questions in which each pair described a specific ‘first-dose’ experience in terms of FLS and ISR, where the levels were determined by a full factorial experimental design, and an OOP cost of $8–$13. The first question in each pair asked respondents whether they would have a second dose if the experience was the same as the first in terms of FLS, ISR, and OOP cost. If the respondent said that they would definitely or probably have the second dose, then the question was repeated with a higher OOP cost ($140–$150 per dose) and constant FLS and ISR levels. If the respondent said that they would definitely or probably not have the second dose based on their ‘first-dose’ experience, the question was repeated with a lower OOP cost ($0 per dose).
2.1.3. Measures of respondent characteristics
The final online survey also contained questions on previous experience with vaccines and vaccination history, socioeconomic and demographic characteristics, as well as questions designed to provide information about respondents’ perceptions of vaccines and awareness of HZ in order to examine whether these factors influenced vaccination preferences. To further explore the potential disparities in vaccine preferences between African-American adults and non-African-American adults, the survey included questions designed to measure respondents’ perceptions of the fairness of policies in general as well as health care services, health, and vaccination recommendations.
2.1.4. Pretests
The draft survey instrument was finalized based on the findings from pretest interviews with 15 adults aged ≥ 50 years. The purpose of the interviews was to assess whether the survey was well-understood, the appropriateness of the descriptive information related to HZ, and the difficulty of the DCE questions (details in Supplementary File 2).
2.2. Study population
Eligible respondents were aged ≥ 50 years, living in the US, and able to read and understand English. Dynata (https://www.dynata.com/), a health-related market research firm, recruited survey respondents from their general consumer panel. As compensation for their time, respondents received loyalty points for completing the survey, which could be accumulated and be exchanged for cash or gifts. All respondents provided informed consent electronically before completing the survey. The survey was granted an exemption from review by RTI International’s institutional review board (Federal Wide Assurance #3331).
2.2.1. Stratification and sample size
The study population was stratified such that half of the participants should have received an influenza vaccine at least once in the last two years – a subgroup which was considered a proxy for patients who tend to get vaccines. Approximately 25% of the participants were to be African-American, and approximately 20% of the participants were to be at increased risk for HZ due to condition or treatment for select conditions (i.e. rheumatoid arthritis, ulcerative colitis, Crohn’s disease, chronic obstructive pulmonary disease, type 2 diabetes, lupus, and psoriasis). Participants aged between 50–64 years old were likely to be covered by commercial health plans and those aged ≥ 65 years were likely to be covered by Medicare.
Most published DCE studies have a sample size between 100 and 300 respondents [Citation27]. Therefore, a sample size of 1,450 respondents was expected to be sufficient to estimate preference weights for each attribute level included in the study.
2.3. Statistical methods
The vaccine-choice data from the DCE survey were analyzed using a random-parameters logit (RPL) model (details in Supplementary File 3) including all attribute levels shown in .
The regression parameters from an RPL model are preference weights, or utilities of attribute levels, indicating the relative strength of preference for each attribute level. More-preferred outcomes have higher preference weights. Conditional relative importance of an attribute was calculated on the difference between the preference weight for the attribute level with the highest preference weight and the preference weight for the level of the same attribute with the lowest preference weight. This difference represents the maximum change in utility achievable with any attribute, conditional upon the levels chosen for the attributes in the study. The RPL parameters were also used to predict likelihood of choosing a specific HZ vaccine profile. Finally, the RPL results were used to estimate the minimum acceptable benefits (MAB) for various changes in vaccine-related features (details in Supplementary File 3).
Differences in preferences were assessed for four pairs of mutually exclusive respondent subgroups. This paper describes the results of one of the subgroup analyses – the analysis of the African-American and non-African-American subgroups. The remaining subgroup analyses are available in the supplementary materials. To evaluate differences in preferences, a dummy-coded variable identifying respondents belonging to a subgroup was interacted with each level of the study attributes. The interaction terms can be interpreted as the difference in preferences between a baseline group (not represented by the identifier dummy-coded variable) and a subgroup of interest. Thus, a chi-squared (χ2) test of joint significance of all interaction terms provided the necessary information to determine whether preferences were systematically different between these subgroups. The χ2 tests were performed separately for each set of interaction terms involving a particular dummy-coded interaction term.
To further explore preference heterogeneity among the African-American subgroup, a post-hoc analysis using latent class (LC) models (details in Supplementary File 3) [Citation28] was conducted to identify groups within the African-American respondents with distinct preferences. The preference weights from the LC models were used to estimate the conditional relative attribute importance for each group. The results from the LC model were also applied to HZ vaccine profiles with fixed effectiveness, duration of protection, and doses as well as where patients can get the vaccine, varying levels of cost, ISR, and FLS to predict likely uptake, or the proportion of African-Americans within each LC group that would select a vaccine with certain characteristics over no vaccine.
Finally, responses to the DCE questions were analyzed using logit regression to explore whether and how vaccine and respondent characteristics influenced stated willingness to get vaccinated. For these analyses, the dependent variable was 1 if the respondent chose one of the hypothetical vaccine profiles and 0 if no vaccine was chosen. Descriptive statistics were conducted using SAS version 9.4 (SAS Institute Inc, Cary, NC), while the analysis of the DCE data were performed with NLOGIT (Econometric Software, Plainview, NY).
3. Results
3.1. Sample characteristics
In total, the final survey was accessed by 8,524 individuals of whom 3,353 were eligible and gave consent to participate; 1,454 (43%) of these completed the survey. Only completed surveys were included in the analysis. summarizes respondents’ characteristics. The mean age was 64.4 years (standard deviation 7.8). Half of the respondents had received an influenza vaccination in the past two years and 25.2% were identified as Black or African-American.
Table 2. Respondent characteristics (N = 1,454)
3.2. Preference weights and conditional relative importance for overall sample
As shown in , a value of −3.3 on the alternative specific constant indicates that, in general, adults ≥ 50 years had a strong and statistically significant preference for choosing HZ vaccination rather than opting out of HZ vaccination. The estimated preference weights for all attributes were consistent with the natural ordering of the levels – that is, better outcomes or features were preferred to worse outcomes or features (). Therefore, on average, respondents preferred the following:
Figure 2. Preference weights and conditional relative importance of attributes of HZ vaccines for the full sample (N = 1,454)
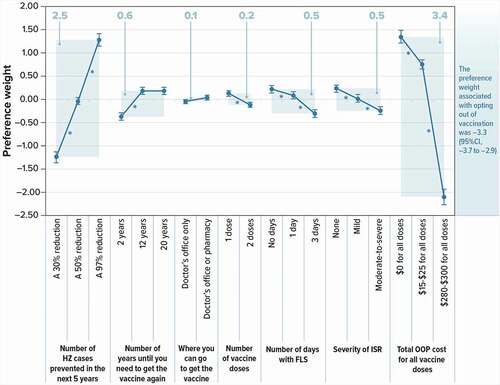
Vaccines that prevented more HZ cases in the next 5 years
Vaccines with one dose (rather than 2)
Vaccines with fewer days of FLS and less-severe ISR
Vaccines with lower total OOP cost
The preference weights of all adjacent levels of the same attribute were statistically significantly different from one another (p < 0.05) with the following exceptions:
Respondents did not differentiate between 12-year and 20-year duration until the vaccine would need to be administered again.
Respondents did not differentiate between the levels of where you can go to get the vaccine (doctor’s office only or doctor’s office or pharmacy).
also shows the conditional relative importance estimate for each attribute. Preferences were strongest for total OOP cost, choosing vaccine alternatives over no vaccine, and VE. As an example, an increase in VE from 30% to 97% had a relative importance of approximately 2.5 while a decrease of total OOP cost from $280–$300 to $0 had a relative importance of 3.4; thus, this change in cost was therefore 1.4 times (= 3.4 ÷ 2.5) more important than the change in VE.
The MAB for various changes in vaccine-related features are presented in Supplementary Table 1.
3.3. Subgroup analyses
The results of the χ2 test for joint significance indicated that there were statistically significant differences in preferences between African-Americans and non-African-Americans (χ2 = 59.91, p < 0.001). The systematic differences in preferences were largely driven by cost, VE, and the utility associated with the non-vaccine alternative.
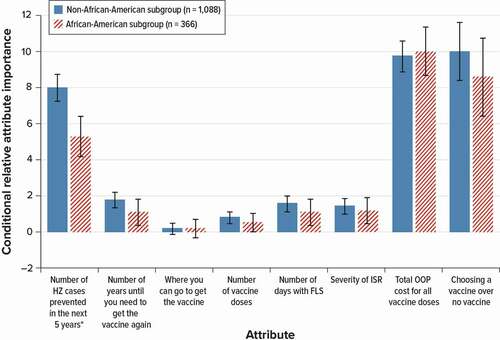
OOP cost and getting a vaccine rather than not getting a vaccine were the most important for the African-American and the non-African-American subgroups, but OOP cost was slightly more important to the African-American subgroup than it was to the non-African-American subgroup, though the different was not statistically significant (). The African-American subgroup placed less importance on VE than the non-African-American subgroup.
Figure 3. Conditional relative importance weights of attributes of HZ vaccines for African-American subgroups
The results of the χ2 test for joint significance indicated that there were statistically significant differences in preferences between all mutually exclusive subgroups, with the exception of respondents who were autoimmune compromised or had other at-risk conditions compared with respondents who were not autoimmune compromised or did not have other at-risk conditions; therefore, no conditional relative importance weights were calculated for this subgroup. The conditional relative importance results for the remaining subgroups are reported in Supplementary Figures 1 and 2.
3.4. Preference heterogeneity among African-Americans
The LC analysis identified three distinct groups (or classes) of African-American respondents with systematically different vaccine preferences. Respondents had a 30% probability of being in Group 1, a 21% probability of being in Group 2, and a 49% probability of being in Group 3. To better illustrate the differences in the disutility associated with the opt-out alternative among the three groups, displays the conditional relative importance weights for changes that result in a net disutility as negative values. shows that the differences in preferences across these groups lie in the importance placed on getting an HZ vaccine over no vaccine, total OOP cost, and the duration of VE:
Figure 4. Conditional relative importance weights of attributes of HZ vaccines for latent groups within the African-American subgroup (N = 366)
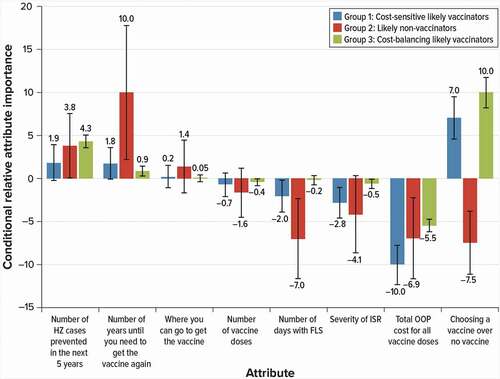
Group 1 respondents can be described as cost-sensitive likely vaccinators. The most important attribute to this group was OOP cost, relative to the other attributes and levels included in the study, and these respondents were more likely to vaccinate rather than opt out of vaccination.
Group 2 respondents were likely non-vaccinators. Their most important attribute was duration of VE relative to the other attributes and levels included in the study; however, group 2 respondents were unlikely to opt for vaccination.
Group 3 respondents were the cost-balancing likely vaccinators. Choosing HZ vaccine over no vaccine was their most important attribute relative to the other attributes and levels included in the study, and respondents in this group were most sensitive to changes in total OOP cost.
illustrates the differences in predicted choice probabilities (likelihood of choosing a vaccine) between the three groups given specific changes in OOP cost (panel A), ISR (panel B) and FLS (panel C). For example, panel A shows how predicted uptake would change for specific changes in OOP cost, holding ISR and FLS constant, while panel B shows how predicted uptake would change for specific changes in ISR, holding OOP and FLS constant. The results in can be interpreted in the following ways:
Figure 5. Predicted choice probabilities or uptake for HZ vaccines with different attributes among latent groups within the African-American subgroup (N = 366)
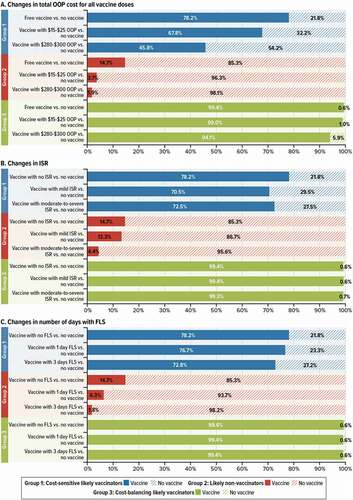
Panel A shows that Group 1 respondents were the most sensitive to changes in total OOP cost, as predicted uptake was 78.2% when the vaccine cost was $0 and 45.8% when the cost was $280–$300. In contrast, Group 3 respondents are not as sensitive to cost, with predicted uptake only going down by approximately 5 percentage points when the cost of the vaccine goes from $0 to $280–$300. Group 2 respondents were unlikely to vaccinate, even when the vaccine had no cost (14.8% of predicted uptake).
Panel B shows that Group 1 is somewhat sensitive to changes in ISR, with predicted uptake going up by approximately 8 percentage points when ISR goes from mild-to-severe to none. In contrast, as with OOP cost, Group 3 is not sensitive to changes in ISR with uptake varying very little if at all from 99%, and Group 2 respondents were unlikely to vaccinate even with no ISR (14.7% of predicted uptake).
Panel C shows that, as for changes in ISR, Group 1 is somewhat sensitive to changes in FLS, with predicted uptake going up by approximately 5 percentage points when the days with FLS go from 3 to none. As for OOP cost and ISR, Group 3 was also the least sensitive to changes in FLS, with stable predicted uptake of 99.4%, while Group 2 remains unlikely to vaccinate even when the vaccine has no days with FLS (14.7% of predicted uptake).
3.5. Stated willingness to get an HZ vaccine
The logit regression analysis results indicate that respondents who trust health care providers (HCPs) vaccine recommendations were more likely to choose having HZ vaccination than not having HZ vaccination (Supplementary Figure 3). Respondents who believed that HZ is a serious disease and that they would likely contract HZ in the next five years were also more likely to choose HZ vaccination rather than not vaccinating, as were respondents with recent influenza vaccination experience, experience with a two-dose vaccine, and those who agreed that vaccines were safe. In contrast, the following respondent characteristics were associated with a lower likelihood of choosing HZ vaccination over not vaccinating: had never experienced any ISR, had ever chosen not to get a recommended vaccine, and thinking that it was unlikely or unsure that the HZ vaccine would work well for them.
The logit regression results also indicated (Supplementary Table 2) that increasing VE, or its duration, was associated with a higher likelihood of choosing HZ vaccination. Vaccines with two doses, FLS for three days, moderate-to-severe ISR, and total OOP cost of $15–$25 or $280–$300, were associated with a lower likelihood of choosing HZ vaccination over not vaccinating.
3.6. Stated compliance with a two-dose vaccine
Approximately 75% of the sample would opt for a second dose of a two-dose, 97% effective HZ vaccine that lasts 12 years if the vaccine costs $8–$13 per dose (). Of those, 51.2% would still have a second dose at the higher cost of $140–$150, holding the FLS and ISR constant. The majority (> 80%) of the respondents who would not have a second dose at the fixed cost of $8–$13 would still not have a second dose even if it costs $0.
Table 3. Frequency of responses to the second dose compliance questions for the full sample (N = 1,454)
4. Discussion
This DCE study on preferences for HZ vaccines in the US found that adults aged ≥ 50 years preferred to get an HZ vaccine over no vaccine and that the total OOP cost was the most important vaccine characteristic conditional on the attributes and levels shown in the study; VE was the next most important characteristic. Local and systemic reactions associated with HZ vaccines and number of doses were of lesser importance; VE was approximately five times more important than reducing the number of days with FLS and the severity of ISR, and ten times more important that decreasing the number of doses from two to one. The least important characteristic was the location where the vaccine would be administered. Most respondents would complete a series of vaccination doses at the lowest suggested fixed price for the second dose ($8–$13), but nearly half would not do so if the second dose costs $140–$150.
To our knowledge, this is the first study designed to comprehensively assess US adult preferences for HZ vaccines. Other studies, conducted on either general or specific (other than HZ) vaccine preferences among adults, have also shown that VE and OOP cost are key factors in vaccination decision-making [Citation29–37]. Not all DCE studies have considered the same set of vaccine attributes. Two studies conducted in the Netherlands showed that VE, longer duration of protection, disease risk, and mortality rates were the determining factors in vaccination decision-making [Citation17,Citation30]; cost of the vaccine was not included in the vaccine attributes considered in these studies. A study from South Africa showed that the most favored vaccine profile would have 90% VE [Citation31]. Interestingly, the decision to be vaccinated was positively associated with increasing VC, i.e. respondents were more likely to choose vaccination if more people around them were getting vaccinated [Citation31]. A study on preferences for hepatitis B vaccines in China showed that protection rates and protection duration were important attributes, while location for receiving the vaccination and number of doses were less important [Citation29]. In China, those born before 1995 must pay for hepatitis B vaccination [Citation29], and this study showed that lower OOP cost was preferred; however, respondents were willing to pay more for increased VE. Thus, they were willing to pay a two times higher price for protection lasting 20 instead of 10 years, and a two and a half times higher price for a 10% increase in protection (from 89% to 99%) [Citation29]. In the US, a study using a stated preference binary choice experiment showed that VE was the most important factor influencing vaccination decisions, followed by OOP cost and recommendation from a primary care physician [Citation32]. The risk of side effects and vaccination location were not crucial factors [Citation32]. The study included 989 adolescents and adults, of whom adults were mainly whites (74.6%) and college-educated [Citation32].
Our analysis showed that, within the African-American subgroup, there were three distinct groups of respondents with systematically different preferences suggesting heterogeneity in vaccine decision-making. More specifically, these three groups expressed different willingness to vaccinate and sensitivity to total OOP cost. Likely non-vaccinators were in one group (group 2) and likely vaccinators were in two groups (groups 1 and 3). The probability of belonging to the likely non-vaccinator group was one out of five and was lower than the probability of belonging in the other two groups. For the two likely vaccinator groups, respondents placed different levels of importance on OOP cost, where respondents in group 3 were less sensitive to changes in cost and had a predicted uptake that remained high even when OOP cost was at the highest levels. In contrast to group 3, predicted uptake for group 1 dropped by approximately 10% and 32% when total OOP cost increased from $0 to $15–$25 and $280–$300 respectively. The likely non-vaccinators had a predicted uptake of about 15%, even when the vaccine costs $0, vaccination had no ISR, and no days with FLS.
The concerns about OOP cost elicited in our findings and the other studies highlight a key issue for payers and policymakers as it appears that VC is sensitive to vaccine price, even when the vaccine reduces the incidence of a disease by nearly 100%. Cost burdens faced by the patients could be limited through minimization or elimination of cost sharing [Citation38,Citation39]. High patient copay was a barrier to vaccinations among Medicare Part D patients, as shown in an analysis of 2012–2014 US administrative claims data [Citation40]. Moreover, retrospective examination of a US pharmacy’s 2014 claims data reported that 38.9% of HZ vaccine prescription fills were not dispensed to the respective individuals because the amount of cost sharing involved was higher than expected [Citation41]. Providing vaccines at low or no copay may improve vaccination rates in these adults The implementation of zero cost sharing provisions of the Affordable Care Act in 2010 increased by one million the number of women aged 19–25 years who initiated three-dose series (≥ $390 total cost) of human papillomavirus vaccination [Citation42]. However, our findings show that although the cost was a concern for the majority of respondents, a small but nevertheless considerable number of respondents would not complete a two-dose vaccination series even if the second dose was offered free of charge. Therefore, for some people, price is not the driving factor when deciding whether to complete a vaccination series; these people may be more sensitive to safety and side effects, or less likely to seek vaccination in general [Citation43]. Other people may believe that a second dose is unnecessary, or they may be more familiar with a single dose regimen. More data are needed to understand such attitudes. For these populations, interventions would need to challenge attitudes toward and beliefs about vaccination by providing education. Such interventions would require significant time and effort from HCPs [Citation43].
The logit regression model of vaccine choice helped to explain some of the potential factors that may support improvement of VC in adults aged ≥ 50 years. The results revealed that respondents who have favorable opinions or attitudes related to vaccines, those who are more likely to be pro-vaccine, those who think HZ is a serious disease, and those who have experience with vaccines with more than one dose were more likely to choose a HZ vaccine over no vaccine. In addition, respondents who had received a recommendation to get the HZ vaccine from a nurse, pharmacist, or doctor, and those who trusted their HCP’s vaccine recommendation were also more likely to choose an HZ vaccine over no vaccine. Therefore, HCPs should continue to talk to their patients about the HZ disease burden and the importance of protecting against it through vaccination, as HCP recommendations appear to have a positive impact on the decision to vaccinate.
Several limitations must be considered when interpreting the results presented. The respondents evaluated hypothetical vaccines and their choices among these vaccines, which may not have the same significance as choices involving actual vaccination decisions. Actual vaccine choices may depend upon a number of contextual factors that were beyond the scope of this study. Similarly, some treatment features selected in this survey may not be relevant to all respondents. Another potential limitation is that the sample might not allow generalization. This study used a web panel to recruit study respondents and their preferences may not be representative of the overall population of adults aged ≥ 50 years in the US. Despite these limitations, this study has a number of strengths derived from the use of good practices [Citation18]. In particular, the survey was pretested using in-depth interviews with adults aged ≥ 50 years, used an experimental design developed using good research practices [Citation44], and followed best practices in statistical analysis [Citation45].
5. Conclusions
Adults aged ≥ 50 years preferred getting an HZ vaccine to opting out of vaccination. However, the preferences for vaccination and stated likelihood of completing the two-dose series were sensitive to the total OOP cost. Furthermore, statistics have shown that VC is lower among racial minorities, including African-Americans. The results from this study revealed that two of the three LC groups within the African-American respondents preferred getting a HZ vaccine over no vaccine; however, the analysis identified a LC group that preferred to opt out of vaccination. In all three groups, respondents placed varying levels of importance on total OOP cost, with groups 1 and 2 being the most sensitive to changes in cost. Therefore, costs associated with the vaccine might present a barrier to some African-American adults. VE was the next most influential factor in HZ vaccination decision-making.
Authors contributions
BJ Patterson, K Myers, A Stewart, EM Hillson, and C Poulos were involved in the design of the study; BJ Patterson, K Myers, A Stewart, B Mange, EM Hillson, and C Poulos collected or generated the data; and BJ Patterson, K Myers, A Stewart, B Mange, and C Poulos analyzed and/or interpreted the data. BJ Patterson, K Myers, and C Poulos developed the manuscript outline. All authors participated in the development of this manuscript and in its critical review with important intellectual contributions. All authors had full access to the data and gave approval before submission. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The work described was carried out in accordance with ICMJE recommendations for conduct, reporting, editing, and publications of scholarly work in medical journals.
Declaration of interest
BJ Patterson was an employee of the GSK group of companies and held shares in the GSK group of companies at the time of the study conduct and of the manuscript development. K Myers and C Poulos are employees of RTI Health Solutions, and B Mange was an employee of RTI Health Solutions at the time of the study conduct. RTI Health Solutions was contracted by the GSK group of companies to design and implement the present study. A Stewart reports personal fees from the George Washington University, from the Immunization Action Coalition, Take a Stand Project, and from the Advisory Commission on Childhood Vaccines, Health Resources and Services Administration, U.S. Department of Health & Human Services, outside of the present work. EM Hillson was an employee of the GSK group of companies and held shares in the GSK group of companies at the time of the study conduct. The authors declare no other financial and non-financial relationships and activities.
Reviewer disclosures
A reviewer on this manuscript has disclosed that outside this manuscript, they have the following potential conflicts of interest to report: grants from Sanofi Pasteur MSD, the GSK group of companies, Pfizer, Sanofi Pasteur, MSD Italy, Emergent BioSolutions and Seqirus for taking part to advisory boards, expert meetings, for acting as speaker and/or organizer of meetings/congresses and as principal investigator and chief of O.U. in RCTs. This reviewer does not have any competing interest directly related to this paper. Peer reviewers on this manuscript have no other relevant financial or other relationships to disclose.
Previous presentations
IDWeek 2019; 2–6 October 2019, Washington, DC, United States
Trademarks
Shingrix is a trademark owned by or licensed to the GSK group of companies.
Zostavax is a trademark of Merck & Co., Inc.
Supplemental Material
Download Zip (1.7 MB)Acknowledgments
The authors would like to thank Business & Decision Life Sciences platform for editorial assistance and manuscript coordination, on behalf of GSK. Grégory Leroux coordinated manuscript development and editorial support. Athanasia Benekou (Business & Decision Life Sciences, on behalf of GSK) provided medical writing support. The authors also thank Kimberly Moon (RTI Health Solutions) for project management support and Lecia Brown (GSK) for topical discussion about the manuscript content.
Supplemental data
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Harpaz R, Ortega-Sanchez IR, Seward JF. Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2008;57(RR–5):1–30. quiz CE2-4.
- Tseng HF, Bruxvoort K, Ackerson B, et al. The epidemiology of herpes zoster in immunocompetent, unvaccinated adults ≥50 years old: incidence, complications, hospitalization, mortality, and recurrence. J Infect Dis. 2020;222(5):798–806.
- John AR, Canaday DH. Herpes zoster in the older adult. Infect Dis Clin North Am. 2017;31(4):811–826.
- Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014;4(6):e004833.
- Kawai K, Yawn BP. Risk factors for herpes zoster: a systematic review and meta-analysis. Mayo Clin Proc. 2017;92(12):1806–1821.
- Ozawa S, Portnoy A, Getaneh H, et al. Modeling the economic burden of adult vaccine-preventable diseases in the United States. Health Aff (Millwood). 2016;35(11):2124–2132.
- McLaughlin JM, McGinnis JJ, Tan L, et al. Estimated human and economic burden of four major adult vaccine-preventable diseases in the United States, 2013. J Prim Prev. 2015;36(4):259–273.
- World Health Organization. Varicella and herpes zoster vaccines: WHO position paper, June 2014. Wkly Epidemiol Rec. 2014;89(25):265–287.
- Meyers JL, Madhwani S, Rausch D, et al. Analysis of real-world health care costs among immunocompetent patients aged 50 years or older with herpes zoster in the United States. Hum Vaccin Immunother. 2017;13(8):1861–1872.
- Merck [Internet]. Kenilworth (NJ): Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.; c2009–2020. Zostavax [package insert]. Zoster Vaccine Live. Full Prescribing Information; 2019 Sep [cited 2019 Aug 1]. Available from: https://www.merck.com/product/usa/pi_circulars/z/zostavax/zostavax_pi2.pdf.
- Centers for Disease Control and Prevention [Internet]. Atlanta (GA): Centers for Disease Control and Prevention. Shingles (Herpes Zoster). Vaccination; 2019 Jul 1 [cited 2021 Jan 25]. Available from: https://www.cdc.gov/shingles/vaccination.html.
- U.S. Food & Drug Administration [Internet]. White Oak (MD): U.S. Food & Drug Administration. Shingrix [package insert]. Zoster Vaccine Recombinant, Adjuvanted. Full Prescribing Information; 2019 May 17 [cited 2019 Aug 1]. Available from: https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM581605.pdf.
- Dooling KL, Guo A, Patel M, et al. Recommendations of the Advisory Committee on Immunization Practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep. 2018;67(3): 103–108.
- Lu PJ, Euler GL, Harpaz R. Herpes zoster vaccination among adults aged 60 years and older, in the U.S., 2008. Am J Prev Med. 2011;40(2):e1–e6.
- Centers for Disease Control and Prevention [Internet]. Atlanta (GA): Centers for Disease Control and Prevention. Vaccination Coverage among Adults in the United States, National Health Interview Survey, 2017; 2018 Feb 8 [cited 2019 Aug 1]. Available from: https://www.cdc.gov/vaccines/imz-managers/coverage/adultvaxview/pubs-resources/NHIS-2017.html.
- Wingate LT, Stubbs K, Ahmed I, et al. The economic impact of herpes zoster vaccine disparities in elderly United States blacks. Int J Environ Res Public Health. 2018;15(10): 2128.
- Eilers R, De Melker HE, Veldwijk J, et al. Vaccine preferences and acceptance of older adults. Vaccine. 2017;35(21):2823–2830.
- Bridges JF, Hauber AB, Marshall D, et al. Conjoint analysis applications in health--a checklist: a report of the ISPOR Good Research Practices for Conjoint Analysis Task Force. Value Health. 2011;14(4): 403–413.
- Centers for Disease Control and Prevention [Internet]. Atlanta (GA): Centers for Disease Control and Prevention. Vaccination Coverage Among Adults in the United States, National Health Interview Survey, 2016; 2018 Feb 8 [cited 2019 Aug 1]. Available from: https://www.cdc.gov/vaccines/imz-managers/coverage/adultvaxview/pubs-resources/NHIS-2016.html.
- Gabutti G, Valente N, Sulcaj N, et al. Evaluation of efficacy and effectiveness of live attenuated zoster vaccine. J Prev Med Hyg. 2014;55(4):130–136.
- Lal H, Cunningham AL, Godeaux O, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med. 2015;372(22):2087–2096.
- Cunningham AL, Lal H, Kovac M, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016;375(11):1019–1032.
- Dey A. Orthogonal fractional factorial designs. New York (NY): Halstead Press; 1985.
- Huber J, Zwerina K. The importance of utility balance in efficient choice designs. J Mark Res. 1996;33(3):307–317.
- Kanninen BJ. Optimal design for multinomial choice experiments. J Market Res. 2002;39(2):214–227.
- Kuhfeld WF, Tobias RD, Garratt M. Efficient experimental design with marketing research applications. J Mark Res. 1994;31(4):545–557.
- Marshall D, Bridges JF, Hauber B, et al. Conjoint analysis applications in health - how are studies being designed and reported?: an update on current practice in the published literature between 2005 and 2008. Patient. 2010;3(4):249–256.
- Greene WH, Hensher DA. A latent class model for discrete choice analysis: contrasts with mixed logit. Transp Res Part B. 2003;37(8):681–698.
- Guo N, Zhang G, Zhu D, et al. The effects of convenience and quality on the demand for vaccination: results from a discrete choice experiment. Vaccine. 2017;35(21): 2848–2854.
- De Bekker-grob EW, Veldwijk J, Jonker M, et al. The impact of vaccination and patient characteristics on influenza vaccination uptake of elderly people: a discrete choice experiment. Vaccine. 2018;36(11):1467–1476.
- Verelst F, Kessels R, Delva W, et al. Drivers of vaccine decision-making in South Africa: a discrete choice experiment. Vaccine. 2019;37(15):2079–2089.
- Lavelle TA, Messonnier M, Stokley S, et al. Use of a choice survey to identify adult, adolescent and parent preferences for vaccination in the United States. J Patient Rep Outcomes. 2019;3(1):51.
- Marshall HS, Chen G, Clarke M, et al. Adolescent, parent and societal preferences and willingness to pay for meningococcal B vaccine: a discrete choice experiment. Vaccine. 2016;34(5):671–677.
- Poulos C, Curran D, Anastassopoulou A, et al. German travelers’ preferences for travel vaccines assessed by a discrete choice experiment. Vaccine. 2018;36(7): 969–978.
- Poulos C, Standaert B, Sloesen B, et al. Preferences for vaccines against children’s diarrheal illness among mothers in Poland and Hungary. Vaccine. 2018;36(40): 6022–6029.
- Poulos C, Yang JC, Levin C, et al. Mothers’ preferences and willingness to pay for HPV vaccines in Vinh long province, Vietnam. Soc Sci Med. 2011;73(2):226–234.
- Brown DS, Johnson FR, Poulos C, et al. Mothers’ preferences and willingness to pay for vaccinating daughters against human papillomavirus. Vaccine. 2010;28(7):1702–1708.
- American College of Physicians [Internet]. Philadelphia (PA): American College of Physicians; c2019. Addressing the Increasing Burden of Health Insurance Cost Sharing. A Position Paper; 2016 [cited 2019 Aug 1]. Available from: https://www.acponline.org/acp_policy/policies/insurance_cost_sharing_2016.pdf.
- Adult Vaccine Access Coalition [Internet]. Washington (DC): Adult Vaccine Access Coalition; c2015–2020. Medicare Financial Barriers Fact Sheet. Financial Barriers to Adult Immunization. 2017 Mar [cited 2019 Aug 1]. Available from: https://www.adultvaccinesnow.org/wp-content/uploads/2017/03/avac_financial_barriers_FINAL_.pdf.
- Yan S, DerSarkissian M, Bhak RH, et al. Relationship between patient copayments in Medicare Part D and vaccination claim status for herpes zoster and tetanus-diphtheria-acellular pertussis. Curr Med Res Opin. 2018;34(7):1261–1269.
- Akinbosoye OE, Taitel MS, Grana J, et al. Factors associated with Zostavax abandonment. Am J Pharm Benefits. 2016;8(4):84–89.
- Lipton BJ, Decker SL. ACA provisions associated with increase in percentage of young adult women initiating and completing the HPV vaccine. Health Aff (Millwood). 2015;34(5):757–764.
- Ventola CL. Immunization in the United States: recommendations, barriers, and measures to improve compliance: part 2: adult vaccinations. P T. 2016;41(8):492–506.
- Reed Johnson F, Lancsar E, Marshall D, et al. Constructing experimental designs for discrete-choice experiments: report of the ISPOR Conjoint Analysis Experimental Design Good Research Practices Task Force. Value Health. 2013;16(1):3–13.
- Hauber AB, González JM, Groothuis-Oudshoorn CG, et al. Statistical methods for the analysis of discrete choice experiments: a report of the ISPOR Conjoint Analysis Good Research Practices Task Force. Value Health. 2016;19(4):300–315.
