ABSTRACT
Cholera remains endemic in >50 countries, putting millions at risk, especially young children for whom killed vaccines offer limited protection. An oral, live attenuated vaccine – CVD 103-HgR (Vaxchora vaccine) – was licensed by the US FDA in 2016 for adults aged 18–64 years traveling to endemic regions, based on clinical trials in human volunteers showing the vaccine was well tolerated and conferred 90% efficacy within 10 days. The evidence base for Vaxchora vaccine has expanded with additional clinical trial data, in older adults (aged 46–64 years) and children (aged 2–17 years), demonstrating that the vaccine produces a strong vibriocidal antibody response. Over 68,000 doses have been administered in the United States, with no new safety signals. The dose volume has been reduced in children to improve acceptability, and cold chain requirements are less st ringent, at +2°C─+8°C. The vaccine has recently been licensed in the Untied States for children aged 2–17 years, in Europe for individuals aged ≥2 years, and for home administration in Europe. Next steps include a Phase 4 study in infants (6–23 months). Additional information is needed regarding duration of immunity, the need for and timing of revaccination, and efficacy data from lower-middle-income countries.
1. Introduction
1.1. What is cholera?
Cholera is an acute, secretory, diarrheal infection caused by the ingestion of food or water contaminated with toxigenic serogroups of the gram-negative bacterium, Vibrio cholerae (V. cholerae) [Citation1–3]. Severe cholera can affect both adults and children, is characterized by profuse, watery diarrhea – described as ‘rice water stools’ owing to a pale, milky appearance – and is usually accompanied by vomiting [Citation1,Citation3]. Severe disease (cholera gravis) can result in complications such as dehydration, renal failure, and shock, and can be fatal within hours if not promptly treated [Citation2–4].
1.2. Cholera biology
There are more than 200 serogroups of V. cholerae but only two, O1 and O139, secrete cholera toxin and cause disease [Citation1,Citation3]. The serogroup O1 is subdivided into classical and El Tor biotypes, both of which can be further divided into two cross-reacting serotypes, Ogawa and Inaba. El Tor (O1 serogroup) currently accounts for almost all worldwide cases of cholera, whereas serogroup O139 is rarely isolated. The cholera toxin produced during infection consists of one toxin A subunit and five toxin B subunits. The B subunits mediate binding and uptake of the toxin into intestinal epithelial cells [Citation1]. Once endocytosed, the A and B subunits dissociate, and the A subunit activates adenylate cyclase, which increases intracellular levels of cyclic adenosine monophosphate (cAMP). This results in reduced sodium absorption, increased chloride secretion, and a net flow of water, potassium, and bicarbonate into the bowel lumen [Citation1,Citation5].
1.3. Impact of cholera
Since 1817, there have been six cholera pandemics caused by the O1 classical biotype, which have killed millions of people worldwide [Citation3,Citation4,Citation6]. Cholera remains a major public health problem affecting the world’s most vulnerable communities, which are often burdened by conflict, malnutrition, poor sanitation, and inadequate access to health services [Citation2,Citation3]. The ongoing seventh pandemic, caused by the O1 El Tor biotype, began in Indonesia in 1961 and subsequently spread to Africa, before reaching the Americas in the 1990s, Haiti in 2010 following a natural disaster, and most recently resulting in a severe epidemic in Yemen [Citation4,Citation6–8]. Cholera is now endemic in approximately 50–60 countries, where seasonal or sporadic outbreaks occur [Citation9,Citation10].
2. Epidemiology
Approximately 1.3 billion people are at risk of cholera in endemic regions [Citation3], although the true global burden is largely unknown because only a fraction of cases are reported [Citation2,Citation11]. Underreporting occurs mainly due to insufficient surveillance systems, but fear of the economic impact on industries, such as tourism or exports, can also act as a barrier to accurate reporting [Citation11]. Model-based estimates suggest that the true incidence of cholera could range from 1.3 to 4 million cases per year in endemic countries, with an estimated 21,000–143,000 deaths [Citation12]. These estimates are based on spatial regression, the proportions of at-risk populations that have access to sanitation and drinking water, cholera incidence rates from an international surveillance program, and weighted averages of country-specific case fatality rates. During 2019, Yemen experienced the world’s largest cholera outbreak, accounting for 93% of all global cholera cases reported in that year [Citation13,Citation14]. Since 2017, when the outbreak began, a cumulative total of 2.2 million cases and 3750 deaths had been reported as of December 2019 [Citation15].
3. Cholera in travelers
Most imported cases of cholera identified in the published literature have been in tourists who had traveled for less than 2 weeks in regions where cholera is endemic or epidemic [Citation16]. While the number of reported cases is relatively small, and travelers are generally considered at low risk of cholera infection if hygienic precautions are taken, the evaluation of travelers’ diarrhea does not typically include testing for cholera, and the true incidence is likely higher than appreciated [Citation3,Citation10,Citation16]. Additionally, cases of severe cholera with acute renal failure and intensive care unit admissions have occurred in returning travelers [Citation17–19]. The risk of infection may be higher for humanitarian/emergency relief workers in cholera-affected areas, especially if they are likely to be exposed directly to cholera patients [Citation3,Citation10]. Based on the 2017 recommendations of the Advisory Committee on Immunization Practices (ACIP), the United States Centers for Disease Control and Prevention (CDC) recommends cholera vaccination for US adults aged 18–64 years who will be traveling to areas with active cholera transmission within a country with endemic or epidemic cholera caused by V. cholerae O1 [Citation9,Citation10]. The World Health Organization (WHO) recommends that oral cholera vaccines (OCV) be considered for travelers at high risk (emergency relief workers), who are likely to be directly exposed to cholera patients or contaminated food or water, particularly those staying in areas with poor access to healthcare facilities [Citation20]. Canada, Australia, and a number of European countries have also formulated recommendations for cholera vaccination that take into account the reason for travel (healthcare professional, aid worker, sea voyage worker, visiting friends/relatives), and underlying medical conditions (such as bowel, heart or kidney disease, diabetes, or low gastric acidity) that might increase the risk of developing severe disease [Citation1].
4. Cholera immunity and correlates of protection
V. cholerae induces long-lasting immunity in most who recover from infection [Citation21–25]. This persistence has been demonstrated in surveillance studies in Bangladesh and in an experimental cholera infection trial, although the latter relied on a small number of subjects [Citation26]. Research on adaptive immunity to cholera has mainly focused on antibody responses directed at the O1-specific polysaccharide component of bacterial lipopolysaccharide (LPS) and cholera toxin, and suggests that a small number of key antigens are the primary triggers of the immune response [Citation27]. Toxin-specific and antibacterial (vibriocidal) antibodies are produced in response to both natural infection and vaccination [Citation3]. However, toxin-specific antibodies confer only short-term immunity, while vibriocidal responses may be associated with long-term immunity [Citation27]. Currently, the best correlate of protection against cholera in controlled clinical studies of cholera-naïve populations is the vaccine-induced increase in serum vibriocidal antibody (SVA) titer – the minimum concentration of serum required for antibody-dependent, complement-mediated killing – which inversely correlates with susceptibility to infection [Citation1,Citation27–30]. However, in scenarios for which the time since exposure is unknown, the relationship between SVA titer and protection from disease is difficult to establish, probably because there is no direct mechanistic connection between levels of circulating SVAs and prevention of V. cholerae colonization at the intestinal mucosal surface [Citation27,Citation28,Citation30]. SVA titers, or fold-increases in titer when pre-exposure levels are available, are useful as correlates of protection soon after vaccination, but decline steadily after their early peaks and may be markers for an intestinal protective immune response [Citation31]. Protective immunity persists even when SVA titers have dropped considerably from these early peaks. Assessment of anamnestic responses involving memory B cells may be a better method for assessing the duration of immunity [Citation31]. Even in the absence of circulating antibodies, protective immunity may be maintained long term by V. cholerae-specific memory B cells, which are stimulated by either vaccination or infection [Citation26,Citation27,Citation30,Citation32]. Upon exposure to antigen, memory B cells can rapidly expand and differentiate into plasmablasts, which are precursors to antibody-secreting cells [Citation27].
5. Overview of cholera vaccines
5.1. Killed oral cholera vaccines
The WHO has prequalified four oral cholera vaccines [Citation28,Citation33]: Dukoral vaccine, a monovalent, killed, whole-cell vaccine containing O1 serogroup and recombinant cholera toxin B subunit; Shanchol vaccine, a bivalent, killed, whole-cell vaccine with O1 and O139 serogroups; and Euvichol vaccine/Euvichol-Plus vaccine, which are similar to Shanchol vaccine. In 2010, the WHO recommended use of OCVs in cholera endemic areas, with consideration given for preemptive vaccination in areas at risk for outbreaks as well as reactive vaccination for those experiencing outbreaks [Citation34]. In 2013, the WHO set up an OCV stockpile to help efficient and strategic deployment of these vaccines as part of an outbreak response [Citation35]. Shanchol vaccine and Euvichol vaccine are used in the global OCV stockpile headquartered at the WHO and funded by Gavi, the Vaccine Alliance. Use of killed OCVs is a key component of the strategy of the Global Task Force on Cholera Control (GTFCC) to control endemic cholera in hotspots under nonemergency conditions. During 2013–2018, over 83,500,000 OCV doses were requested by 24 countries, resulting in 104 vaccination campaigns in 22 countries [Citation36].
Two other killed, whole-cell cholera vaccines – mORC-Vax vaccine and Ora Vacs vaccine – are locally licensed in Vietnam and China, respectively (), and another two are currently under development – Cholvax vaccine (bivalent, whole-cell, licensed in Bangladesh) and HillChol vaccine (monovalent, whole cell) [Citation28,Citation33] ().
Table 1. Currently licensed cholera vaccines [Citation1,Citation28,Citation33,Citation47,Citation49,Citation50,Citation51,Citation73,Citation74]
Table 2. Cholera vaccines under development [Citation28]
5.2. Limitations of killed oral cholera vaccines
Killed oral cholera vaccines have demonstrated protection against cholera in clinical trials as well as in endemic, epidemic, and outbreak settings [Citation27,Citation37]. Trials in Bangladesh and Peru demonstrated that the WC/rBS and killed whole-cell formulations provided protective efficacy of 85–90% initially, which declined to about 50% after 6 months [Citation38]. In a randomized, placebo-controlled trial in India, Shanchol vaccine provided protective efficacy against cholera of 67% at 2 years, 66% at 3 years, and 65% at 5 years of follow-up [Citation39–41]. Limitations of these vaccines include the requirement for multiple doses in which repeated vaccination increases the cost of delivery and the time taken to elicit immunity and, in the case of Dukoral vaccine, the need for simultaneous administration of buffer [Citation21,Citation28,Citation37]. However, studies of single-dose killed OCVs have demonstrated protective efficacy ranging from 33% to 87%, although the one prospective randomized trial in Bangladesh noted protection decreasing to 39% at 2 years of follow-up [Citation42–44]. A major limitation of a single-dose killed OCV approach is a decreased level of protection noted in children younger than 5 years of age, a group most vulnerable to cholera infection in endemic areas, which is presumably due to the lower preexisting natural immunity in this age group [Citation21,Citation28,Citation37,Citation38,Citation42,Citation43].
5.3. Live attenuated oral vaccines
5.3.1. Rationale for live attenuated oral vaccines
Antibacterial immunity, rather than antitoxin response, is the dominant mechanism that mediates protection against natural cholera infection [Citation23]. Live bacterial vaccines mimic natural cholera infection more closely than killed strains, with the potential to prime responses to antigens that are expressed in vivo during the course of infection but are not present in a killed vaccine [Citation45]. Live strains are more readily taken up by microfold cells (M cells), the major antigen-sampling cells in the gut, generating a mucosal gut immune response [Citation1,Citation27,Citation28]. Thus, a single oral dose of a live vaccine could result in intestinal colonization and a rapid immune response, removing the need for repeated dosing [Citation28]. An oral cholera vaccine that rapidly confers protection after a single dose is particularly advantageous for individuals from nonendemic areas who must travel at short notice to areas of high cholera transmission, and might also be practical for reactive mass vaccination in so-called ‘virgin soil’ epidemics [Citation46].
5.3.2. CVD 103-HgR
CVD 103-HgR is a single-dose, live attenuated oral cholera vaccine licensed in the USA and Europe under the name Vaxchora vaccine [Citation47,Citation48]. Several other live attenuated oral vaccines are also under development, such as Peru-15, Cuban 638, and VA 1.4 () [Citation1,Citation28]. This review will focus on Vaxchora vaccine, including its development, characteristics, and clinical efficacy, with a focus on new studies completed since the last comprehensive review published in this journal by Levine et al. in 2017 [Citation49].
6. CVD 103-HgR characteristics
6.1. CVD 103-HgR design and development
CVD 103-HgR is a live attenuated V. cholerae serogroup O1, serotype Inaba, classical biotype, recombinant strain in which 94% of the toxigenic A subunit of cholera toxin is deleted, leaving only the nontoxic, immunogenic, binding B subunit [Citation33,Citation46]. The original commercial formulation of CVD 103-HgR, launched in 1993, was manufactured by Berna-Biotech (formerly Swiss Serum and Vaccine Institute) in Switzerland and licensed under the trade names Orochol vaccine or Mutacol vaccine (Switzerland, Austria, Finland, Australia, New Zealand, Canada, Sri Lanka, the Philippines, and several South American countries) for the protection of travelers [Citation50,Citation78]. For commercial reasons, the production of the vaccine ceased in 2001 when Berna Biotech was acquired by Crucell [Citation49,Citation73]. In 2009 PaxVax, Inc. (acquired by Emergent BioSolutions, Inc. in 2018) acquired the licensure rights to CVD 103-HgR, remanufactured it, and commercialized the reformulation as Vaxchora vaccine [Citation33,Citation78]. Vaxchora vaccine is approved for use in travelers in the USA (aged 2–64 years) and the EU (aged 2 years and older).
6.2. Chemistry
A single dose of Vaxchora vaccine is supplied as two foil packets: a buffer component and an active component. The active component contains lyophilized CVD 103-HgR, sucrose, ascorbic acid, lactose, and Hy-case SF [Citation33]. The buffer component contains sodium bicarbonate, sodium carbonate, ascorbic acid, and lactose [Citation33]. The buffer component is mixed with water before addition of the active component. The suspension is then stirred for 30 seconds and administered orally within 15 minutes [Citation48].
6.3. Storage
To simplify storage, the formulation has subsequently been developed so that the cold chain requirements are now less stringent. Following FDA approval of Vaxchora vaccine (frozen) in 2016, a prior approval supplement (PAS) was implemented to identify key process steps that could be optimized to improve the active component stability profile. Design-of-experiment studies were performed on fermentation steps and it was found that bacterial cultures were more stable when harvested at different stages of growth, as has been observed in other bacterial species [Citation51]. In addition, the curing process was optimized, and the lyophilization stabilization solution was changed to exclude sodium chloride and increase the sucrose concentration. After implementing the optimized fermentation, curing, and stabilization steps, Vaxchora vaccine demonstrated stability at 2–8°C. An additional study demonstrated that the vaccine and buffer can be stored for 5 days at 25°C, 12 hours at 30°C, and 6 hours at 32°C, and maintain potency once reconstituted. The PAS results were accepted by the FDA in 2019, and 2–8°C stable vaccine was approved in Europe by the European Medicines Agency (EMA) in 2020.
7. Clinical efficacy of CVD 103-HgR
7.1. Historical studies
As reviewed previously by Levine et al., 2017 [Citation49], the earlier formulation of CVD 103-HgR was evaluated in numerous studies (challenge, safety/immunogenicity, endemic, booster, pediatric, combination, and special populations; ). Overall, clinical trials with CVD 103-HgR involved more than 27,000 adults and children as young as 3 months of age, and more than 500,000 doses of the earlier formulation of CVD 103-HgR were sold for use in travelers aged 2 years or older between 1993 and 2003 [Citation49,Citation50,Citation73]. The vaccine was well-tolerated, with protection against moderate-to-severe diarrhea documented in challenge studies within as little as 8 days [Citation49]. The classical Inaba vaccine strain conferred cross-serotype and cross-biotype protection, as demonstrated in a field study of reactive vaccination with the Orochol vaccine E formulation during a large outbreak of El Tor Ogawa cholera in Micronesia, as well as in several challenge studies [Citation49].
Figure 1. Timeline of studies involving the previous formulation of the oral, live cholera vaccine CVD 103-HgR (Orochol, Mutachol) [Citation47–53,Citation54–69,Citation74Citation76Citation77]
![Figure 1. Timeline of studies involving the previous formulation of the oral, live cholera vaccine CVD 103-HgR (Orochol, Mutachol) [Citation47–53,Citation54–69,Citation74Citation76Citation77]](/cms/asset/8112a313-9f73-4338-bc88-0859c1d7868c/ierv_a_2003709_f0001_b.gif)
7.2. Prelicensure trials: establishing safety and efficacy
The safety and efficacy of the reformulated CVD 103-HgR vaccine was established in four randomized, placebo-controlled clinical trials [Citation49] that formed the basis for FDA approval: (1) a Phase 1 trial to establish safety and immunogenicity in healthy young adults [Citation46]; (2) a Phase 3 trial to establish clinical efficacy and safety in a human cholera challenge model [Citation30,Citation78]; (3) a Phase 3 lot-to-lot consistency trial [Citation70]; and (4) a Phase 3 trial to assess safety and immunogenicity in older adults between 46 and 64 years of age [Citation71].
7.2.1. Phase 1 safety and immunogenicity trial
The first step of the clinical development program was to conduct a randomized, double-blind, placebo-controlled, Phase 1 safety and immunogenicity trial in healthy adults at two sites in the USA [Citation46]. The results confirmed that the reformulated CVD 103-HgR, prepared from new master and working cell banks, produced a seroconversion rate among the 55 vaccine recipients that was similar to that of the original commercial vaccine and indicated that the new version was no more reactogenic, with SVA and anti-cholera toxin seroconversion rates of 89% and 57%, respectively [Citation46]. Shedding of the vaccine strain, evaluated in the first 7 days following vaccination, was detected in 11% of vaccinees and the incidence was highest on day 7. Stool or rectal swab cultures were obtained from household contacts of vaccinees at day 7 and there were no documented instances of CVD 103-HgR transmission.
7.2.2. Phase 3 challenge trial
The randomized (1:1), placebo-controlled, Phase 3 pivotal efficacy trial in healthy adults aged 18–45 years used a human infection model, with ingestion of virulent V. cholerae O1 El Tor Inaba strain N16961 10 days (N = 68) or 3 months (N = 66) after vaccination with a single dose of Vaxchora vaccine [Citation78]. The primary efficacy endpoint was the prevention of moderate (≥3.0 L) to severe (≥5.0 L) cholera diarrhea. The vaccine was well-tolerated, with protective efficacy of 90.3% (P < 0.0001 vs placebo) for study participants who were challenged at 10 days and 79.5% (P < 0.0001 vs placebo) challenged at 3 months. Of note, SVA seroconversion was seen in 79.8% of vaccine recipients in as little as 7 days, with cumulative seroconversion noted at day 28 in 90.4% of recipients. Ten days after vaccination, a robust SVA response was observed against all four serotypes/biotypes of V. cholerae tested (90.3%, 91.4%, 87.1%, and 89.2% against classical Inaba, El Tor Inaba, classical Ogawa, and El Tor Ogawa, respectively).
7.2.2.1. Correlates of protection with CVD 103-HgR
Data from the challenge trial were analyzed to establish a correlate of protection to serve as a bridge for inferring efficacy in populations in whom cholera challenge studies are not feasible, such as children and older adults. SVA seroconversion, defined as a fourfold or greater increase in titer above baseline within 10 days of vaccination, was tightly linked to efficacy in the challenge trial: when challenged 10 or 90 days after vaccination, the attack rate of moderate-to-severe diarrhea in vaccinees who did not seroconvert was 4/6 (67%; 95% confidence interval [CI] 22%–96%), while the attack rate in seroconverters was 2/62 (3%; 95% CI 0.4%–11%) [Citation30,Citation78]. Further analysis of the challenge study evaluated the role of vaccine-induced memory B cells in long-term immunity by analyzing the association between memory B-cell response and protection against moderate-to-severe diarrhea for the 66 vaccinees and placebo recipients who were challenged 3 months after administration of study therapy [Citation31]. Robust memory B-cell responses occurred in Vaxchora vaccine recipients and persisted for 6 months after vaccination, and there was a statistically significant association between vaccine-induced increases in LPS-specific IgA memory B cells and lower diarrheal stool volumes following challenge (r = −0.56, P < 0.001). These data support the hypothesis that memory B cells are mechanistically associated with protection against cholera [Citation31].
7.2.3. Phase 3 lot consistency trial
To demonstrate the consistency of the manufacturing process, the immunologic equivalence of different production lots of Vaxchora vaccine was tested in a large, randomized, placebo-controlled, double-blind, Phase 3 study conducted at multiple sites in the United States and Australia [Citation70]. Safety and immunogenicity were assessed in more than 3000 healthy adults with a mean age of 29.9 years, randomized 8:1 to vaccine or placebo [Citation70]. A robust and consistent SVA response after vaccination was demonstrated across multiple production lots, with overall seroconversion rates of 94% and 4% in Vaxchora vaccine and placebo recipients, respectively. The safety analysis showed that the vaccine was well-tolerated and that there were no meaningful differences in the frequency and severity of solicited reactogenicity signs and symptoms (tiredness, headache, abdominal pain, nausea/vomiting, lack of appetite, diarrhea, and fever) between vaccine production lots. Reactogenicity signs and symptoms that were significantly more frequent in the vaccine group than in the placebo arm were headache (28.9% vs 23.3%; P = 0.0419) and diarrhea (3.9% vs 1.2%; P = 0.0079), of which most cases were mild. Severe diarrhea (≥6 stools per 24 hours) was reported by 22/2789 (0.8%) vaccine recipients, but no symptoms lasted longer than 2 days. Unsolicited adverse events recorded up to 28 days after vaccination were similar in the two groups, with 23.0% of Vaxchora vaccine recipients and 24.0% of placebo recipients reporting at least one event [Citation70]. There were no study-related serious adverse events.
7.2.4. Phase 3 trial of immunogenicity and safety in older adults
Aging is accompanied by a decline in immune function that can lead to decreased responses to vaccines [Citation72]. The lot-to-lot consistency trial was performed in a relatively young adult population, aged 18–45 years, in order to ensure sufficient uniformity to meet the narrow immune response criteria required to demonstrate lot consistency. Therefore, a separate randomized, placebo-controlled, double-blind, Phase 3 study was conducted to evaluate the safety and immunogenicity of a single dose of Vaxchora vaccine in older adults aged 46–64 years [Citation71].
7.2.4.1. Immunogenicity
To assess the relationship between age and SVA response, data from subjects aged 46–64 years in the older adult study were pooled with data from subjects aged 18–45 years in the lot consistency trial to create a large database of 2979 recipients spanning ages 18–64 years. The SVA seroconversion rate (95% CI) 10 days after vaccination (day 11) for the 46–64 years age group was 90.4% (86.4–93.5%), which was non-inferior to the 93.5% (92.5–94.4%) rate in the 18–45 years age group. Evaluation of immunogenicity on a continuous basis in the pooled population of almost 3000 subjects showed a trend toward decreased seroconversion and vibriocidal geometric mean titers (GMTs) with increasing age. However, the noninferiority of seroconversion – the correlate of protection established by the cholera challenge study – in older adults suggested that efficacy would be similar in travelers to that observed for younger adults. Significant post-vaccination increases in LPS-specific IgA, LPS-specific IgG, and cholera toxin-specific IgG memory B cells were also observed in older adults and lasted for 6 months. Unlike SVA seroconversion rates and GMTs, no relationship between age and memory B-cell response was observed across the 18–64 years age range, although the statistical power to detect a trend was much smaller since memory B cells were only assayed in subsets of the study populations.
7.2.4.2. Cross-strain protection
Ten days after vaccination, a robust SVA response in older adults was observed against all four serotypes/biotypes of V. cholerae tested (90.4%, 91.0%, 73.2%, and 71.4% against classical Inaba, El Tor Inaba, classical Ogawa, and El Tor Ogawa, respectively). The Vaxchora vaccine challenge study tested efficacy against the El Tor Inaba strain and efficacy against the other three strains can only be inferred. However, since the seroconversion rates were similar to those observed among adults aged 18–45 years in the challenge study, it is reasonable to infer similar protection against all four serotypes/biotypes in both younger and older adults.
7.2.4.3. Reactogenicity
The vaccine was well tolerated in older adults, with more postvaccination reactogenicity signs and symptoms reported by placebo recipients (50.5% vs 36.3% vaccine recipients, P = 0.0174). Diarrhea was reported with similar frequency in vaccine and placebo recipients (2.4% vs 2.0%, P = 1.0000) and was mostly mild in severity.
7.2.4.4. Vaxchora vaccine in older adults: summary
The study in older adults demonstrated a SVA seroconversion rate that was non-inferior to that seen in younger adults (90.4% vs 93.5%), as well as robust memory B-cell responses. There was a relatively continuous age-related decline in SVA titer, but in a more limited dataset no such trend was detected for specific memory B cells. Since SVA seroconversion is a strong correlate of protection against cholera-induced diarrhea in controlled clinical studies of cholera-naïve populations, it is expected that Vaxchora vaccine will provide protection against cholera in older adults from developed countries who are at increased risk of infection when traveling in or visiting at-risk countries.
7.3. Post-licensure trials
7.3.1. Phase 4 trial of safety and immunogenicity in children and adolescents
The four prelicensure trials established the efficacy and safety of Vaxchora vaccine in adults aged 18–64 years [Citation9]. However, a single-dose vaccine that could provide rapid protection in all age groups would be valuable for the protection of travelers to, and ideally inhabitants of, cholera-endemic countries. Therefore, a randomized, placebo-controlled, double-blind, multicenter Phase 4 study was performed post-licensure to assess the safety, immunogenicity, and tolerability of Vaxchora vaccine in children and adolescents aged 2–17 years [Citation73,Citation74]. Since challenge studies with V. cholerae are not feasible in children, SVA seroconversion within 10 days of vaccination was used as a correlate of protection to bridge immunogenicity in this population to the response observed in adults aged 18–45 years from the Phase 3 lot consistency trial [Citation70].
7.3.1.1. Study design
At nine study sites in the United States, a total of 550 participants were randomized 6:1 to either a single dose of Vaxchora vaccine or placebo. The study population was split into three cohorts: cohort 1, aged 12–17 years (N = 189); cohort 2, aged 6–11 years (N = 185); and cohort 3, aged 2–5 years (N = 176). To determine immunogenicity, titers of classical Inaba SVA were measured at baseline and 10 days after vaccination (day 11) in all participants. Safety was assessed by recording solicited reactogenicity (tiredness, headache, abdominal pain, nausea/vomiting, lack of appetite, and diarrhea) for 7 days after vaccination (day 8), unsolicited adverse events for 28 days (day 29), and serious adverse events for 180 days (day 181). Acceptability was evaluated by assessing the percentage of participants in each cohort able to complete dosing according to the protocol.
7.3.1.2. Immunogenicity and comparison with adults aged 18–45 years
The primary endpoint was the proportion of participants achieving SVA seroconversion 10 days post-vaccination (day 11), which occurred in 99.4%, 97.8%, and 98.1% of vaccine recipients in cohorts 1, 2, and 3, respectively. These rates were non-inferior to the 93.5% seroconversion rate in the adult bridging population aged 18–45 years.
7.3.1.3. Long-term immunogenicity
The phase 3 and 4 studies of Vaxchora vaccine measured SVA levels for 6 months following vaccination. (). A subset of adolescents in cohort 1 of the pediatric trial was followed up for 2 years, with SVA titer, vibriocidal GMTs, and geometric mean fold increase (GMFI) assessed on days 365, 547, and 730 [Citation75]. SVA titers were at least four times above baseline in 64.5% of Vaxchora vaccine recipients at day 730, while GMT and GMFI both peaked at day 11 and remained above baseline at all time points, including day 730 (). The duration of vaccine-derived protection produced by immunization with Vaxchora vaccine is unknown. The persistence of SVA antibodies for 2 years after vaccination in the pediatric trial, as well as a previous study of CVD 103-HgR demonstrating the presence of SVA antibodies 3.5 years after vaccination, suggest that protection could be long-term, although the degree of protection is in need of further study [Citation74].
Figure 2. Time course plot of vibriocidal geometric mean titer against classical Inaba V. cholerae-immune substudy populations.
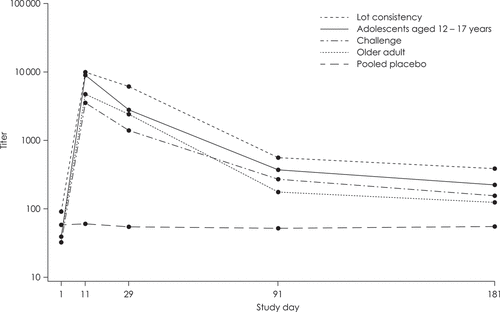
Table 3. Long-term immunogenicity of Vaxchora vaccine in adolescents
7.3.1.4. Safety and acceptability
Vaxchora vaccine was well tolerated in children and adolescents, with a safety profile similar to that seen in adults and also to the populations who received the previously marketed form of the vaccine. Most solicited reactogenicity was mild to moderate, with no significant differences between the vaccine and placebo groups. Unsolicited adverse events were reported by 23.9% of those who received the vaccine and 28.0% of placebo recipients; most were considered unrelated to study product. There were no vaccine-related serious adverse events. The study vaccine was well accepted, with 99.4%, 91.0%, and 82.7% of cohort 1, 2, and 3 recipients, respectively, taking at least 80% of the dose. The decrease in tolerance with age suggests that age may influence the ability to vaccinate young children.
7.4. Summary of clinical trial data
7.4.1. Efficacy
Vaxchora vaccine has been evaluated in 3703 participants in clinical trials (). In the pivotal Phase 3 study, most vaccine recipients seroconverted within 7 days and immunization resulted in 90.3% and 79.5% protective efficacy against cholera challenge at 10 and 90 days postvaccination, respectively [Citation78]. Further analysis established SVA seroconversion, defined as a fourfold or greater increase in SVA above baseline, as a robust correlate of protection in cholera-naïve populations [Citation29]. Additional Phase 3 and 4 safety and immunogenicity trials have demonstrated high rates of seroconversion in subjects aged 2–64 years, indicating likely protection against cholera [Citation71,Citation73,Citation74]. Vaxchora vaccine also induced cross-strain SVA responses, as well as a robust and persistent memory B-cell response which did not appear to be affected by age [Citation31].
Table 4. Summary of completed CVD 103-HgR (Vaxchora vaccine) clinical studies
7.4.2. Safety
Vaxchora vaccine is well tolerated in all age groups. Most of the solicited adverse reactions reported in the clinical trials were mild, lasted 2 days or less, and included tiredness, headache, abdominal pain, nausea/vomiting, lack of appetite, fever, and diarrhea, with only diarrhea, mostly mild, seen more frequently in vaccine vs placebo recipients. No vaccine-related serious adverse events were reported in any trial. In addition to these clinical trial data, 68,648 doses of Vaxchora vaccine have been sold in the United States since 2016 and no new safety signals have been identified.
7.4.3. Additional studies
7.4.3.1. Long-term immunogenicity
Long-term immunogenicity has been studied both after cholera challenge as well as following immunization. In a study of duration of infection-derived immunity 3 years after an initial cholera infection, four previously challenged volunteers and five cholera-naïve control subjects were challenged with 106 classical Ogawa organisms. None of the four cholera ‘veterans’ and four out of five control subjects developed diarrhea (P = 0.04). When compared to a control group of increased size comprising a pooled experience of challenge in cholera-naïve subjects, the difference in attack rates (0/4 vs 26/28) was highly significant (P < 0.0004) [Citation23]. This pivotal study suggested that a good approach to development of cholera vaccines might be to mimic natural immunity with orally administered, attenuated strains of V. cholerae.
Studies conducted with the previous formulation of CVD 103-HgR documented persistent antibody response that lasted up to 3.5 years, while evidence of protection against rechallenge with heterologous biotypes and serotypes of V. cholerae has been demonstrated up to 6 months after vaccination – the longest interval tested in a vaccine challenge study [Citation76,Citation77,Citation79,Citation80].
7.4.3.2. Reimmunization/booster data
Determination of the need for Vaxchora vaccine reimmunization is complicated by the presence of preexisting SVA and the persistence of local gut immunity, which may interfere with colonization of the small bowel following a second dose of vaccine [Citation81]. A study in a Thai population comparing a single dose of CVD 103-HgR with two doses 1 week apart showed no notable increase in the rate of seroconversion following the second dose [Citation82]. A similar study performed in Chilean infants and toddlers demonstrated SVA seroconversion rates of 61% and 68%, respectively, after one or two doses of CVD 103-HgR given 2 weeks apart [Citation83]. A Swiss study of adults who were given a booster dose of CVD 103-HgR between 15 and 24 months after the primary vaccination showed only a modest immune response compared with the primary immunization, with seroconversion rates to the homologous Inaba serotype seen in 81% and 28% of recipients, respectively, following the first and second dose [Citation81]. A study in Austria documented an 81% seroconversion rate following primary immunization and 57% and 65% rates upon reimmunization at 2.5 and 3.5 years, respectively [Citation76]. In each of these studies it is suggested that the presence of SVA, which appears to persist for at least 2 years in adolescents, and/or local immunity resulted in rapid clearance of the live attenuated vaccine by the primed immune system. Only after systemic and, more importantly, local immunity (likely mucosal IgA memory B cells) induced by prior vaccination (or in endemic areas, also prior exposure) have waned can the vaccine strain multiply in the small bowel and evoke an additional immune response [Citation76].
8. Regulatory affairs
Vaxchora vaccine was licensed by the FDA in 2016 for adults aged 18–64 years and remains the only cholera vaccine approved in the United States [Citation9,Citation47]. It was approved in the United States for pediatric use (2–17 years of age) in 2020 [Citation47]. The vaccine was licensed in Europe in April 2020 for adults and children from 6 years of age [Citation48] and recently approved by the EMA for use in children aged 2–5 years on the 26th of February, 2021 [Citation84]. Based on a usability study, Vaxchora vaccine was approved for home use in the EU, and more favorable storage requirements were approved in the United States and EU [Citation85]. Submissions are also planned in the United Kingdom, Canada, New Zealand, South Korea, and Australia.
9. Conclusion
A previous review of Vaxchora vaccine in 2017 noted that it was well-tolerated, elicited SVA seroconversion – a correlate of protection in controlled clinical studies – in approximately 90% of adults, and that protection against cholera challenge was evident as early as 10 days following ingestion of a single dose [Citation49]. The Phase 3 challenge study documented high levels of seroconversion as soon as 7 days after vaccination [Citation78]. The evidence base for Vaxchora vaccine continues to evolve with important product developments and the publication of data from more recent clinical trials. These studies have demonstrated the safety and immunogenicity of Vaxchora vaccine in a wide range of subjects aged 2–64 years. Vaxchora vaccine produces a robust memory B-cell response, which is associated with protection against diarrhea, as well as cross-strain vibriocidal antibodies. Vaxchora vaccine has gained approval for travelers aged 2–64 years in the USA, for travelers aged 2 years and older in Europe, and for home administration in Europe. To simplify storage, the formulation has been developed so that the cold chain requirements are now less stringent (+2°C to +8°C), and dose volumes have been reduced for children under 6 years of age to improve compliance. Thus, Vaxchora vaccine continues to offer promise for travelers as an alternative approach to inactivated vaccines in situations where rapid protection across most age groups is needed.
10. Expert opinion
Cholera remains a significant public health issue in many developing countries, particularly in displaced communities living in overcrowded and unsanitary conditions [Citation1,Citation86]. The intensity of the 2010 outbreak in Haiti underscores how devastating epidemics can be when ignited in a population lacking background immunity [Citation28]. Travelers from nonendemic areas to areas with ongoing cholera transmission are also at risk of infection [Citation1]. The risk for international travelers is generally considered to be low [Citation20] but underreporting is likely as mild cases are treated as acute travelers’ diarrhea with no laboratory evaluation [Citation1,Citation87]. Individuals who are likely to have direct contact with cholera patients (healthcare or aid workers) or who are staying for prolonged periods in close contact with the local population, such as those visiting friends or relatives, are at an increased risk [Citation1,Citation20]. Cholera gravis is more likely in travelers with hypochlorhydria, blood group O, or with underlying bowel, cardiac, or renal disease [Citation1,Citation10,Citation49]. Young children may be at higher risk of infection due to their inability to adhere to hygiene measures and their propensity to put objects into their mouths [Citation87]. Risk to travelers is also related to their destination and its distance from a healthcare facility [Citation49]. The reformulated CVD 103-HgR vaccine, Vaxchora vaccine, providing rapid protection within 7–10 days of a single dose, has become an established option for cholera prevention in travelers since its introduction in the United States in 2016 [Citation1,Citation33,Citation78].
In endemic areas, cholera incidence is greatest among children under 5 years of age, whereas all ages are vulnerable to infection in ‘virgin soil’ epidemics [Citation88]. Killed whole-cell vaccines, such as Dukoral vaccine and Shanchol vaccine, have been used effectively in developing countries to minimize seasonal epidemics and outbreaks. In a randomized, placebo-controlled trial in India, a two-dose regimen of Shanchol vaccine was shown to be safe and provided 67%, 66%, and 65% protection against cholera at 2, 3, and 5 years of follow-up, respectively [Citation39–41]. Another randomized, placebo-controlled trial of whole-cell recombinant-B-subunit vaccine (Dukoral vaccine) performed in military recruits in Peru demonstrated short-term protective efficacy of 86% against symptomatic cholera but not against asymptomatic infection [Citation89]. In these studies, killed WC cholera vaccines required two doses given weeks apart to provide their peak protection, which could limit their use when a reactive public health response is needed to diminish the spread of explosive outbreaks, but killed vaccines have also been shown to provide significant protection after a single dose. A double-blind, placebo-controlled study of single-dose Shanchol vaccine in a cholera-endemic area of Bangladesh demonstrated overall protective efficacy of 40% during 6 months of follow-up, which persisted for at least 2 years [Citation42,Citation43]. However, no protection was seen in children less than 5 years of age, and this has been attributed to lower preexisting natural immunity [Citation38]. In a case–control study during a cholera outbreak in South Sudan, a single dose of Shanchol vaccine provided an adjusted short-term protective efficacy of 87%, while another matched case–control study of single-dose Shanchol vaccine during an outbreak in Zambia documented efficacy of 89% at 7 weeks [Citation90,Citation91]. When vaccine supplies are limited, reactive single-dose vaccination campaigns have been proposed as a short-term strategy to limit the spread of disease both by increasing the number of vaccine recipients and also by providing herd immunity [Citation92–95]. Some limitations of killed whole –cell vaccines include cost, cold chain requirements, and the need for buffer and potable water for administration of B-subunit-containing vaccines, although these can be minimized with the use of killed whole-cell vaccines which do not contain the cholera toxin B subunit [Citation38]. A study in Bangladesh demonstrated that Shanchol vaccine maintains safety and immunogenicity when stored under ambient temperatures of up to 42°C for up to 14 days [Citation96].
In theory, live vaccines that can be administered as a single dose and elicit rapid protection may have the potential to fill the public health gap for infants and young children in endemic regions and in emergency outbreak situations when timely deployment of vaccines is critical in ‘virgin soil’ epidemics. CVD 103-HgR has been studied in cholera endemic regions and was noted to require 1 log higher dosing to optimize serologic response, likely because of interference with intestinal vaccine replication caused by environmental enteropathy as well as previous exposures to wild-type cholera, which may interfere with replication of the vaccine bacteria in the gut [Citation49]. In a WHO-implemented reactive mass vaccination program to control an explosive cholera epidemic in Micronesia, which documented the logistical feasibility of single-dose use of CVD 103-HgR in a reactive vaccination program, protective efficacy of the high-dose formulation (Orochol E), containing ~2–10 × 109 colony forming units (CFU), under field conditions was 79.2% (95% CI 71.9–84.6) [Citation97], although this was a retrospective cohort study with limited information available on participants. This study contrasts with a large prospective, randomized, double-blind, placebo-controlled field trial of CVD 103-HgR performed in Indonesia, which demonstrated a protective efficacy of only 14% [Citation98]. The study was done before the ability of cholera vaccines to elicit indirect protection was known, and the unexpectedly low incidence of cholera in the placebo population may have been secondary to inadvertent indirect protection of that group from an overall reduction in the incidence of cholera in the community secondary to mass vaccination, as well as a lack of cluster randomization [Citation49]. Modeling studies of disease transmission have suggested that immunization may reduce cholera by up to 89% in unvaccinated people [Citation94]. In a more recent study, CVD 103-HgR doses were evaluated in a study of Malian participants randomized to ingest a single standard dose (≥2 × 108 CFU; n = 50) or high dose (≥2 × 109 CFU; n = 50) of Vaxchora vaccine or Shanchol vaccine (n = 50) [Citation99]. Two weeks after vaccination, the rates of seroconversion with the high-dose CVD 103-HgR vaccine (83.3%) were greater than the standard-dose vaccine (71.7%) and significantly greater than a single dose of Shanchol vaccine (56.0%; P = 0.003). While these results generated enthusiasm to evaluate the use of a high-dose formulation of Vaxchora vaccine as a potential future tool for reactive vaccination in explosive ‘virgin soil’ epidemics in developing countries [Citation99], the clinical significance of these findings is uncertain, and prospective, randomized trials evaluating protective efficacy in endemic and epidemic situations are needed.
In summary, given the rapid immunologic response following a single dose, in cholera-naïve subjects as well as inhabitants of endemic countries [Citation78,Citation99], a potential role for Vaxchora vaccine may be rapid immunization of a cholera-naïve population during a ‘virgin soil’ epidemic, such as occurred in Haiti, and also immunization of children <5 years of age in endemic areas. One potential for consideration is initial vaccination with Vaxchora vaccine followed by booster doses of a less expensive killed OCV. Further study is needed, including prospective, double-blind comparative field trials.
10.1. Future areas of study
Several questions remain regarding Vaxchora vaccine. Key among these are the uncertainties regarding the duration of immunity following single-dose vaccination and the need for, and timing of, revaccination. Can Vaxchora vaccine replicate the strong and long-lasting protection induced by wild-type V. cholerae infection in the previous study of volunteers who were rechallenged after 3 years, or does a stronger immune response induced by natural infection lead to a longer period of protection [Citation23]? While SVA seroconversion is a useful marker of short-term protection in cholera-naïve populations, the actual duration of protection after vaccination is unknown and is likely mediated by other mechanisms, such as mucosal secretory IgA memory B cells in the small intestine. One way to measure the long-term duration of protection is via challenge studies, but these are difficult to perform, expensive, and not without risk [Citation49]. Another method would be by way of efficacy trials during cholera outbreaks, although the ethics of using a placebo group in such situations are questionable, given the availability of effective vaccines for protection. More likely, a non-inferiority trial comparing other vaccines would be required. Similarly, the need for, and optimal timing of, revaccination is unclear. Studies are needed which measure SVA seroconversion – the best correlate of protection in controlled clinical studies of cholera-naïve populations – after revaccination at various intervals following initial vaccination, and which also measure other biomarkers of protection. Given the presence of SVA antibodies at least 2 years following immunization, as noted in the adolescent substudy, it is possible that some level of protection remains at this time point, which might blunt vaccine response. Revaccination studies could be performed 2, 3, and 4 years after initial vaccination.
Studies in Haiti [Citation100] and Mozambique [Citation101] suggest that cholera is more likely to affect individuals with human immunodeficiency virus (HIV) infection, although it is unclear if travelers with HIV from developed countries are at increased risk of the disease. A small study in Mali demonstrated that CVD 103-HgR is immunogenic in individuals with HIV, although less so than in those without HIV, with no increase in adverse events [Citation102]. Overall, the safety and effectiveness of Vaxchora vaccine have not been established in immunocompromised individuals, which, as a live bacterial vaccine, might limit its use in reactive vaccination campaigns in areas with high rates of HIV-infected individuals, some of whom may be severely immunocompromised. Future studies might further evaluate the use of Vaxchora vaccine in individuals with HIV or other forms of immunosuppression living in industrialized countries. Another area of further study might be use of the Vaxchora vaccine in individuals with chronic gastrointestinal conditions, such as inflammatory bowel disease, malabsorption, or Helicobacter pylori gastric infection. For example, a small study in Mali noted a higher CVD 103-HgR seroconversion rate in H. pylori seropositive than seronegative volunteers [Citation103, Citation104].
Cholera during pregnancy is associated with miscarriage, fetal death, and premature delivery, and pregnant women are considered especially vulnerable to V. cholerae infection [Citation28]. While healthcare providers are often reluctant to use vaccines during pregnancy, a recent meta-analysis showed no evidence of an association between oral cholera vaccination and adverse pregnancy outcome in endemic regions [104]. While use of the previous marketed version of CVD 103-HgR (Orochol) in over 500,000 individuals resulted in no reports of adverse pregnancy outcomes, data on the use of Vaxchora vaccine in pregnant women in developed countries is limited. To address this issue, a pregnancy registry has been ongoing in the United States since 2016, albeit with limited enrollment, and will likely continue in Europe.
An ongoing evaluation from the adolescent substudy of the Vaxchora vaccine pediatric trial will measure the impact of vaccination on circulating memory B cells, a possible correlate of long-term protection, with results available in the second half of 2021. An upcoming clinical trial in 2022 will evaluate the safety, tolerability, and immunogenicity of Vaxchora vaccine in infants aged 6–24 months in the United States, using a vaccine volume reduced to 10 mL to improve acceptability. Infants are at particularly high risk for complications of cholera, and a safe and effective vaccine is needed for this very vulnerable age group. Given the lower protective efficacy of inactivated cholera vaccines in children younger than 5 years of age [Citation37] (particularly infants younger than 2 years of age), additional studies evaluating the safety and immunogenicity of Vaxchora vaccine in younger pediatric subjects should be considered, to optimize protection of both those traveling to and residing in areas where cholera is endemic. Finally, additional ongoing clinical trials are studying the long-term immunogenicity of Vaxchora vaccine over 8 years postvaccination, and the effects of Vaxchora vaccine given with or without Vivotif typhoid vaccine on the immune system and intestinal microbiota (Clinical trials registration: clinicaltrials.gov NCT03724357; NCT03705585).
Article highlights
The live oral cholera vaccine, Vaxchora vaccine, was recently licensed in Europe for home administration in adults and children 2 years of age and older traveling to at-risk countries, while the age indication in the United States was recently expanded to include travelers 2-64 years of age.
The Vaxchora vaccine dose volume has been reduced in children to improve acceptability, and cold chain requirements are now less stringent at +2°C to +8°C.
Vaxchora vaccine induces SVA seroconversion in as little as 7 days and is 90% protective at 10 days and 80% protective at 90 days postvaccination against cholera challenge. The long-term duration of protection provided by Vaxchora vaccine is unknown and in need of further study.
Vaxchora vaccine induces robust memory B-cell responses which last for at least 6 months, and there is a statistically significant association between vaccine-induced increases in LPS-specific IgA memory B cells and lower diarrheal stool volumes following challenge.
Determination of the need for Vaxchora vaccine reimmunization is complicated by the presence of pre-existing SVA and the persistence of local gut immunity, which may interfere with colonization of the small bowel following a second dose of vaccine.
While killed cholera vaccines have demonstrated efficacy in endemic and epidemic situations, they require multiple doses and provided limited protection after one dose, especially in children less than 5 years of age. Vaxchora vaccine might one day provide a useful alternative in at-risk infants and children – further study is needed.
Declaration of interest
J McCarty, PA de Lame, M Lock, and D Haney are paid consultants for Emergent Travel Health Inc. L Bedell and D Cassie are employees of Emergent Travel Health Inc. S Bennett is an employee of Adjuvance Technologies Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Gabutti G, Rossanese A, Tomasi A, et al. Cholera, the current status of cholera vaccines and recommendations for travellers. Vaccines (Basel). 2020Oct14;8(4). PubMed PMID: 33066373; eng. doi:https://doi.org/10.3390/vaccines8040606
- Legros D. Global cholera epidemiology: opportunities to reduce the burden of cholera by 2030. J Infect Dis. 2018Oct15;218(suppl_3):S137–s140. PubMed PMID: 30184102; PubMed Central PMCID: PMCPMC6207143; eng.
- Cholera vaccines: WHO position paper – August 2017. Wkly Epidemiol Rec. 2017Aug25;92(34):477–498. PubMed PMID: 28845659; eng.
- WHO. Cholera: key facts 2019 [ cited 2020 Accessed24 November 2021 24 November]. cited: https://www.who.int/news-room/fact-sheets/detail/cholera
- Reidl J, Klose KE. Vibrio cholerae and cholera: out of the water and into the host. FEMS Microbiol Rev. 2002Jun;26(2):125–139. PubMed PMID: 12069878; eng.
- Mutreja A, Dougan G. Molecular epidemiology and intercontinental spread of cholera. Vaccine. 2020Feb29;38(Suppl 1):A46–a51. PubMed PMID: 31345641; eng.
- Weill FX, Domman D, Njamkepo E, et al. Genomic history of the seventh pandemic of cholera in Africa. Science. 2017Nov10;358(6364):785–789. PubMed PMID: 29123067; eng.
- Nair GB, Takeda Y. Cholera outbreaks. Preface. Curr Top Microbiol Immunol. 2014;379:v–vii. PubMed PMID: 25299007; eng.
- Wong KK, Burdette E, and Mahon BE, et al. Recommendations of the advisory committee on immunization practices for use of cholera vaccine. MMWR Morb Mortal Wkly Rep. 2017May12;66(18):482–485. PubMed PMID: 28493859; PubMed Central PMCID: PMCPMC5657988; eng.
- Centers for Disease Control and Prevention. Chapter 4: travel-related infectious diseases 2020. Yellow Book. June 24, 2019 [cited 2020 Nov 24].
- Ganesan D, Gupta SS, Legros D. Cholera surveillance and estimation of burden of cholera. Vaccine. 2020Feb29;38(Suppl 1):A13–A17. PubMed PMID: 31326254; eng.
- Ali M, Nelson AR, and Lopez AL, et al. Updated global burden of cholera in endemic countries. PLoS Negl Trop Dis. 2015;9(6):e0003832. PubMed PMID: 26043000; PubMed Central PMCID: PMCPMC4455997; eng.
- WHO. Cholera, 2019. Weekly Epidemiological Rec. 2020;37:441–448.
- WHO. Ending cholera: a global roadmap to 2030. 2017.
- WHO. Current major event: arbovirus diagnostics EQAP. Weekly Epidemiological Monitor. 2019;12(50).
- Connor BA, Dawood R, and Riddle MS, et al. Cholera in travellers: a systematic review. J Travel Med. 2019Dec23;26(8):taz085. PubMed PMID: 31804684; PubMed Central PMCID: PMCPMC6927393; eng.
- Mascarello M, Deiana ML, Maurel C, et al. Cholera with severe renal failure in an Italian tourist returning from Cuba, July 2013. Euro Surveill. 2013Aug29;18(35):20572. PubMed PMID: 24008229; eng.
- Slesak G, Fleck R, Jacob D, et al. Imported cholera with acute renal failure after a short business-trip to the Philippines, Germany, October 2015. Euro Surveill. 2016;21(1). PubMed PMID: 26767388; eng. DOI:https://doi.org/10.2807/1560-7917.es.2016.21.1.30099
- Reyes-Corcho A, Pinsker RW, Sarkar S, et al. Cholera gravis associated with acute renal failure in a traveler from Haiti to the United States. Travel Med Infect Dis. 2012Sep;10(5–6):236–239. PubMed PMID: 23137437; eng.
- WHO. Advice for international travel and trade in relation to cholera. 2018.
- Harris JB. Cholera: immunity and prospects in vaccine development. J Infect Dis. 2018Oct15;218(suppl_3):S141–s146. PubMed PMID: 30184117; PubMed Central PMCID: PMCPMC6188552; eng.
- Leung T, Matrajt L. Immune responses to cholera following natural infection: a review. medRxiv. 2020 2020 07 27;20163139. DOI:https://doi.org/10.1101/2020.07.27.20163139
- Levine MM, Black RE, Clements ML, et al. Duration of infection-derived immunity to cholera. PubMed PMID: 7252264; eng, J Infect Dis. 1981 Jun;143(6):818–820.
- Glass RI, Becker S, Huq MI, et al. Endemic cholera in rural Bangladesh, 1966-1980. Am J Epidemiol. 1982Dec;116(6):959–970. PubMed PMID: 7148820; eng.
- Ali M, Emch M, and Park JK, et al. Natural cholera infection-derived immunity in an endemic setting. J Infect Dis. 2011Sep15;204(6):912–918. PubMed PMID: 21849288; PubMed Central PMCID: PMCPMC3156915; eng.
- Harris AM, Bhuiyan MS, Chowdhury F, et al. Antigen-specific memory B-cell responses to Vibrio cholerae O1 infection in Bangladesh. PubMed PMID: 19528207; PubMed Central PMCID: PMCPMC2738048. eng, Infect Immun. 2009 Sep;77(9):3850–3856.
- Harris JB. Cholera: immunity and Prospects in Vaccine Development. J Infect Dis. 2018Oct15;218(suppl_3):S141–s146. PubMed PMID: 30184117; PubMed Central PMCID: PMCPMC6188552; eng.
- Shaikh H, Lynch J, Kim J, et al. Current and future cholera vaccines. Vaccine. 2020Feb29;38(Suppl 1):A118–a126. PubMed PMID: 31879125; eng.
- Islam K, Hossain M, and Kelly M, et al. Anti-O-specific polysaccharide (OSP) immune responses following vaccination with oral cholera vaccine CVD 103-HgR correlate with protection against cholera after infection with wild-type Vibrio cholerae O1 El Tor Inaba in North American volunteers. PLoS Negl Trop Dis. 2018Apr;12(4):e0006376. PubMed PMID: 29624592; PubMed Central PMCID: PMCPMC5906022; eng.
- Haney DJ, Lock MD, and Simon JK, et al. Antibody-based correlates of protection against cholera: analysis of a challenge study in a cholera-naïve population. Clin Vaccine Immunol. 2017May31;24(8). PubMed PMID: 28566334; PubMed Central PMCID: PMCPMC5583470; eng. doi:https://doi.org/10.1128/cvi.00098-17
- Haney DJ, Lock MD, and Gurwith M, et al. Lipopolysaccharide-specific memory B cell responses to an attenuated live cholera vaccine are associated with protection against Vibrio cholerae infection. Vaccine. 2018May11;36(20):2768–2773. PubMed PMID: 29655627; PubMed Central PMCID: PMCPMC5922764; eng.
- Alam MM, Riyadh MA, and Fatema K, et al. Antigen-specific memory B-cell responses in Bangladeshi adults after one- or two-dose oral killed cholera vaccination and comparison with responses in patients with naturally acquired cholera. Clin Vaccine Immunol. 2011May;18(5):844–850. PubMed PMID: 21346055; PubMed Central PMCID: PMCPMC3122537; eng.
- Saluja T, Mogasale VV, and Excler JL, et al. An overview of Vaxchora(TM), a live attenuated oral cholera vaccine. Hum Vaccin Immunother. 2020;16(1):42–50. PubMed PMID: 31339792; PubMed Central PMCID: PMCPMC7012186; eng.
- Cholera vaccines: WHO position paper. Wkly Epidemiol Rec. 2010Mar26;85(13):117–128. PubMed PMID: 20349546.
- WHO. Cholera vaccine stockpiles [9 June 2021 Acessed24 November 2021]. Available from: https://www.who.int/groups/icg/cholera/stockpiles
- Pezzoli L. Oral cholera vaccine working group of the global task force on cholera C. Global oral cholera vaccine use, 2013-2018. Vaccine. 2020Feb29;38(Suppl 1):A132–A140. PubMed PMID: 31519444.
- Bi Q, Ferreras E, and Pezzoli L, et al. Protection against cholera from killed whole-cell oral cholera vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2017Oct;17(10):1080–1088. PubMed PMID: 28729167; PubMed Central PMCID: PMCPMC5639147; eng.
- Deen J, Clemens JD. Licensed and recommended inactivated oral cholera vaccines: From development to innovative deployment. Trop Med Infect Dis. 2021 Mar 9. 6(1). PubMed PMID: 33803390; PubMed Central PMCID: PMCPMC8005943. DOI: https://doi.org/10.3390/tropicalmed6010032
- Sur D, Lopez AL, Kanungo S, et al. Efficacy and safety of a modified killed-whole-cell oral cholera vaccine in India: an interim analysis of a cluster-randomised, double-blind, placebo-controlled trial. Lancet. 2009Nov14;374(9702):1694–1702. PubMed PMID: 19819004.
- Sur D, Kanungo S, Sah B, et al. Efficacy of a low-cost, inactivated whole-cell oral cholera vaccine: results from 3 years of follow-up of a randomized, controlled trial. PLoS Negl Trop Dis. 2011Oct;5(10):e1289. PubMed PMID: 22028938; PubMed Central PMCID: PMCPMC3196468.
- Bhattacharya SK, Sur D, Ali M, et al. 5 year efficacy of a bivalent killed whole-cell oral cholera vaccine in Kolkata, India: a cluster-randomised, double-blind, placebo-controlled trial. Lancet Infect Dis. 2013Dec;13(12):1050–1056. PubMed PMID: 24140390.
- Qadri F, Wierzba TF, Ali M, et al. Efficacy of a single-dose, inactivated oral cholera vaccine in Bangladesh. N Engl J Med. 2016May5;374(18):1723–1732. PubMed PMID: 27144848.
- Qadri F, Ali M, Lynch J, et al. Efficacy of a single-dose regimen of inactivated whole-cell oral cholera vaccine: results from 2 years of follow-up of a randomised trial. Lancet Infect Dis. 2018Jun;18(6):666–674. PubMed PMID: 29550406; eng.
- Lopez AL, Deen J, and Azman AS, et al. Immunogenicity and protection from a single dose of internationally available killed oral cholera vaccine: a systematic review and meta-analysis. Clin Infect Dis. 2018Jun1;66(12):1960–1971. PubMed PMID: 29177437; PubMed Central PMCID: PMCPMC5982790.
- Mayo-Smith LM, Simon JK, and Chen WH, et al. The live attenuated cholera vaccine CVD 103-hgr primes responses to the toxin-coregulated pilus antigen TCPA in subjects challenged with wild-type vibrio cholerae. Clin Vaccine Immunol. 2017Jan;24(1):e00470–16. PubMed PMID: 27847368; PubMed Central PMCID: PMCPMC5216439; eng.
- Chen WH, Greenberg RN, and Pasetti MF, et al. Safety and immunogenicity of single-dose live oral cholera vaccine strain CVD 103-HgR, prepared from new master and working cell banks. Clin Vaccine Immunol. 2014Jan;21(1):66–73. PubMed PMID: 24173028; PubMed Central PMCID: PMCPMC3910924; eng.
- Pax Vax. Vaxchora Prescribing Information. 2016 Accessed24 November 2021. Available from: https://www.fda.gov/media/98688/download
- Emergent BioSolutions. Vaxchora Summary of Product Characteristics 2020 [cited 2020 Nov 30].
- Levine MM, Chen WH, Kaper JB, et al. PaxVax CVD 103-HgR single-dose live oral cholera vaccine. Expert Rev Vaccines. 2017Mar;16(3):197–213. PubMed PMID: 28165831; eng.
- Herzog C. Successful comeback of the single-dose live oral cholera vaccine CVD 103-HgR. Travel Med Infect Dis. 2016Jul-Aug;14(4):373–377. PubMed PMID: 27425792; eng.
- Jaishankar J, and Srivastava P. Molecular basis of stationary phase survival and applications. Front Microbiol. 2017;8:2000. PubMed PMID: 29085349; PubMed Central PMCID: PMCPMC5650638; eng.
- Migasena S, Pitisuttitham P, and Prayurahong B, et al. Preliminary assessment of the safety and immunogenicity of live oral cholera vaccine strain CVD 103-HgR in healthy Thai adults. Infect Immun. 1989Nov;57(11):3261–3264. PubMed PMID: 2807523; PubMed Central PMCID: PMCPMC259791; eng.
- Cryz SJ Jr., Levine MM, Kaper JB, et al. Randomized double-blind placebo controlled trial to evaluate the safety and immunogenicity of the live oral cholera vaccine strain CVD 103-HgR in Swiss adults. Vaccine. 1990Dec8;(6):577–580. PubMed PMID: 2087879; eng. doi:https://doi.org/10.1016/0264-410x(90)90012-b
- Kotloff KL, Wasserman SS, and O’Donnell S, et al. Safety and immunogenicity in North Americans of a single dose of live oral cholera vaccine CVD 103-HgR: results of a randomized, placebo-controlled, double-blind crossover trial. Infect Immun. 1992Oct;60(10):4430–4432. PubMed PMID: 1398956; PubMed Central PMCID: PMCPMC257485; eng.
- Suharyono SC, Simanjuntak C, and Witham, N, et al. Safety and immunogenicity of single-dose live oral cholera vaccine CVD 103-HgR in 5-9-year-old Indonesian children. Lancet. 1992Sep19;340(8821):689–694. PubMed PMID: 1355798; eng.
- Losonsky GA, Tacket CO, and Wasserman SS, et al. Secondary Vibrio cholerae-specific cellular antibody responses following wild-type homologous challenge in people vaccinated with CVD 103-HgR live oral cholera vaccine: changes with time and lack of correlation with protection. Infect Immun. 1993Feb;61(2):729–733. PubMed PMID: 8423098; PubMed Central PMCID: PMCPMC302786; eng.
- Wasserman SS, Kotloff KL, Losonsky GA, et al. Immunologic response to oral cholera vaccination in a crossover study: a novel placebo effect. Am J Epidemiol. 1993Dec1;138(11):988–993. PubMed PMID: 8256784; eng.
- Simanjuntak CH, O’Hanley P, Punjabi NH, et al. Safety, immunogenicity, and transmissibility of single-dose live oral cholera vaccine strain CVD 103-HgR in 24- to 59-month-old Indonesian children. J Infect Dis. 1993Nov;168(5):1169–1176. PubMed PMID: 8228350; eng.
- Gotuzzo E, Butron B, and Seas C, et al. Safety, immunogenicity, and excretion pattern of single-dose live oral cholera vaccine CVD 103-HgR in Peruvian adults of high and low socioeconomic levels. Infect Immun. 1993Sep;61(9):3994–3997. PubMed PMID: 8359923; PubMed Central PMCID: PMCPMC281106; eng.
- Lagos R, Avendaño A, Horwitz I, et al. [Tolerance and immunogenicity of an oral dose of CVD 103-HgR, a live attenuated Vibrio cholerae 01 strain: a double-blind study of Chilean adults]. Rev Med Chil. 1993Aug; 1218: 857–863. PubMed PMID: 8296092; spa.
- Wasserman SS, Losonsky GA, Noriega F, et al. Kinetics of the vibriocidal antibody response to live oral cholera vaccines. Vaccine. 1994Aug;12(11):1000–1003. PubMed PMID: 7975839; eng.
- Lagos R, Avendaño A, and Prado V, et al. Attenuated live cholera vaccine strain CVD 103-HgR elicits significantly higher serum vibriocidal antibody titers in persons of blood group O. Infect Immun. 1995Feb;63(2):707–709. PubMed PMID: 7822046; PubMed Central PMCID: PMCPMC173056; eng.
- Cryz SJ Jr., Que JU, and Levine MM, et al. Safety and immunogenicity of a live oral bivalent typhoid fever (Salmonella typhi Ty21a)-cholera (Vibrio cholerae CVD 103-HgR) vaccine in healthy adults. Infect Immun. 1995Apr;63(4):1336–1339. PubMed PMID: 7890391; PubMed Central PMCID: PMCPMC173155; eng.
- Kollaritsch H, Furer E, and Herzog C, et al. Randomized, double-blind placebo-controlled trial to evaluate the safety and immunogenicity of combined Salmonella typhi Ty21a and Vibrio cholerae CVD 103-HgR live oral vaccines. Infect Immun. 1996Apr;64(4):1454–1457. PubMed PMID: 8606118; PubMed Central PMCID: PMCPMC173943; eng.
- Lagos R, Losonsky G, Abrego P, et al. Tolerencia, immunogenicidad, excresion y transmision de la vacuna anti-cholera oral viva-atenuada, CVD 103-HgR estudio pareado de doble ciego en ninos chilenos de 24 a 59 meses. Bol Hosp Infant Mex. 1996;53:214–220.
- Kollaritsch H, Que JU, Kunz C, et al. Safety and immunogenicity of live oral cholera and typhoid vaccines administered alone or in combination with antimalarial drugs, oral polio vaccine, or yellow fever vaccine. J Infect Dis. 1997Apr;175(4):871–875. PubMed PMID: 9086143; eng.
- Pax Vax. Data on file. BB-IND 2112.
- Lagos R, Fasano A, Wasserman SS, et al. Effect of small bowel bacterial overgrowth on the immunogenicity of single-dose live oral cholera vaccine CVD 103-HgR. J Infect Dis. 1999Nov;180(5):1709–1712. PubMed PMID: 10515838; eng.
- Cooper PJ, Chico ME, Losonsky G, et al. Albendazole treatment of children with ascariasis enhances the vibriocidal antibody response to the live attenuated oral cholera vaccine CVD 103-HgR. J Infect Dis. 2000Oct;182(4):1199–1206. PubMed PMID: 10979918; eng.
- McCarty JM, Lock MD, Hunt KM, et al. Safety and immunogenicity of single-dose live oral cholera vaccine strain CVD 103-HgR in healthy adults age 18-45. Vaccine. 2018Feb1;36(6):833–840. PubMed PMID: 29317118; eng.
- McCarty JM, Lock MD, Bennett S, et al. Age-related immunogenicity and reactogenicity of live oral cholera vaccine CVD 103-HgR in a randomized, controlled clinical trial. Vaccine. 2019Mar7;37(11):1389–1397. PubMed PMID: 30772070; eng.
- Gruver AL, Hudson LL, and Sempowski GD. Immunosenescence of ageing. J Pathol. 2007Jan;211(2):144–156. PubMed PMID: 17200946; PubMed Central PMCID: PMCPMC1931833; eng.
- McCarty JM, Gierman EC, and Bedell L, et al. Safety and immunogenicity of live oral cholera vaccine CVD 103-HgR in children and adolescents aged 6-17 years. Am J Trop Med Hyg. 2020Jan;102(1):48–57. PubMed PMID: 31769402; PubMed Central PMCID: PMCPMC6947768; eng.
- McCarty JM, Cassie D, Bedell L, et al. Safety and immunogenicity of live oral cholera vaccine CVD 103-HgR in children aged 2-5 years in the United States. Am J Trop Med Hyg. 2021;104(3):861–865. PubMed PMID: 33319739; eng.
- McCarty JM, Cassie D, and Bedell L, et al. Long term immunogenicity of live oral cholera vaccine CVD 103-HgR in adolescents 12-17 years of age in the US. Am J Trop Med Hyg. 2021 104 5 1758–1760. .
- Kollaritsch H, Cryz SJ Jr., Lang AB, et al. Local and systemic immune responses to combined vibrio cholerae CVD103-HgR and salmonella typhi ty21a live oral vaccines after primary immunization and reimmunization. Vaccine. 2000Jul1;18(26):3031–3039. PubMed PMID: 10825607; eng.
- Tacket CO, Losonsky G, Nataro JP, et al. Onset and duration of protective immunity in challenged volunteers after vaccination with live oral cholera vaccine CVD 103-HgR. PubMed PMID: 1527420; eng, J Infect Dis. 1992 Oct;166(4):837–841.
- Chen WH, Cohen MB, and Kirkpatrick BD, et al. Single-dose live oral cholera vaccine CVD 103-HgR protects against human experimental infection with vibrio cholerae o1 El Tor. Clin Infect Dis. 2016Jun1;62(11):1329–1335. PubMed PMID: 27001804; PubMed Central PMCID: PMCPMC4872293; eng.
- Levine MM, Kaper JB, Herrington D, et al. Safety, immunogenicity, and efficacy of recombinant live oral cholera vaccines, CVD 103 and CVD 103-HgR. Lancet. 1988Aug27;2(8609):467–470. PubMed PMID: 2900401; eng.
- Tacket CO, Cohen MB, and Wasserman SS, et al. Randomized, double-blind, placebo-controlled, multicentered trial of the efficacy of a single dose of live oral cholera vaccine CVD 103-HgR in preventing cholera following challenge with Vibrio cholerae O1 El tor Inaba three months after vaccination. Infect Immun. 1999Dec; 6712: 6341–6345. PubMed PMID: 10569747; PubMed Central PMCID: PMCPMC97039; eng.
- Cryz SJ Jr., Levine MM, and Losonsky G, et al. Safety and immunogenicity of a booster dose of Vibrio cholerae CVD 103-HgR live oral cholera vaccine in Swiss adults. Infect Immun. 1992Sep;60(9):3916–3917. PubMed PMID: 1500200; PubMed Central PMCID: PMCPMC257409; eng.
- Su-Arehawaratana P, Singharaj P, Taylor DN, et al. Safety and immunogenicity of different immunization regimens of CVD 103-HgR live oral cholera vaccine in soldiers and civilians in Thailand. J Infect Dis. 1992Jun;165(6):1042–1048. PubMed PMID: 1583321; eng.
- Lagos R, San Martin O, Wasserman SS, et al. Palatability, reactogenicity and immunogenicity of engineered live oral cholera vaccine CVD 103-HgR in Chilean infants and toddlers. Pediatr Infect Dis J. 1999Jul;18(7):624–630. PubMed PMID: 10440439; eng.
- Agency EM. Summary of opinion (post authorisation) - Vaxchora cholera vaccine, oral, live 2021 [Accessed24 November 2021]. Available from: https://www.ema.europa.eu/en/documents/smop/chmp-post-authorisation-summary-positive-opinion-vaxchora-ii-03-g_en.pdf
- Emergent Travel Health. Data on file. 2020.
- WHO. Cholera: key facts. 2019 Jan 17 2019 [cited 2020 Jan 17]. Available from: https://www.who.int/en/news-room/fact-sheets/detail/cholera
- Leung AKC, Leung AAM, Wong AHC, et al. Travelers’ diarrhea: a clinical review. Recent Pat Inflamm Allergy Drug Discov. 2019;13(1):38–48. PubMed PMID: 31084597; eng.
- Wierzba TF. Oral cholera vaccines and their impact on the global burden of disease. Hum Vaccin Immunother. 2019;15(6):1294–1301. PubMed PMID: 30183486; PubMed Central PMCID: PMCPMC6663124; eng.
- Sanchez JL, Vasquez B, Begue RE, et al. Protective efficacy of oral whole-cell/recombinant-B-subunit cholera vaccine in Peruvian military recruits. Lancet. 1994Nov5;344(8932):1273–1276. PubMed PMID: 7967990.
- Azman AS, Parker LA, Rumunu J, et al. Effectiveness of one dose of oral cholera vaccine in response to an outbreak: a case-cohort study. Lancet Glob Health. 2016Nov4;(11):e856–e863. PubMed PMID: 27765293. doi:https://doi.org/10.1016/S2214-109X(16)30211-X
- Ferreras E, Chizema-Kawesha E, Blake A, et al. Single-dose cholera vaccine in response to an outbreak in Zambia. N Engl J Med. 2018Feb8;378(6):577–579. PubMed PMID: 29414267.
- Ali M, Emch M, von Seidlein L, et al. Herd immunity conferred by killed oral cholera vaccines in Bangladesh: a reanalysis. Lancet. 2005Jul2-8;366(9479):44–49. PubMed PMID: 15993232.
- Khatib AM, Ali M, von Seidlein L, et al. Effectiveness of an oral cholera vaccine in Zanzibar: findings from a mass vaccination campaign and observational cohort study. Lancet Infect Dis. 2012Nov;12(11):837–844. PubMed PMID: 22954655.
- Longini IM Jr., Nizam A, Ali M, et al. Controlling endemic cholera with oral vaccines. PLoS Med. 2007Nov27;4(11):e336. PubMed PMID: 18044983; PubMed Central PMCID: PMCPMC2082648.
- Azman AS, Luquero FJ, Ciglenecki I, et al. The impact of a one-dose versus two-dose oral cholera vaccine regimen in outbreak settings: a modeling study. PLoS Med. 2015Aug;12(8):e1001867. PubMed PMID: 26305226; PubMed Central PMCID: PMCPMC4549326.
- Saha A, Khan A, Salma U, et al. The oral cholera vaccine Shanchol when stored at elevated temperatures maintains the safety and immunogenicity profile in Bangladeshi participants. Vaccine. 2016Mar18;34(13):1551–1558. PubMed PMID: 26896684.
- Calain P, Chaine JP, Johnson E, et al. Can oral cholera vaccination play a role in controlling a cholera outbreak? Vaccine. 2004Jun23;22(19):2444–2451. PubMed PMID: 15193408; eng.
- Richie EE, Punjabi NH, Sidharta YY, et al. Efficacy trial of single-dose live oral cholera vaccine CVD 103-HgR in North Jakarta, Indonesia, a cholera-endemic area. Vaccine. 2000May8;18(22):2399–2410. PubMed PMID: 10738097; eng.
- Sow SO, Tapia MD, and Chen WH, et al. Randomized, placebo-controlled, double-blind phase 2 trial comparing the reactogenicity and immunogenicity of a single standard dose to those of a high dose of CVD 103-HgR live attenuated oral cholera vaccine, with shanchol inactivated oral vaccine as an open-label immunologic comparator. Clin Vaccine Immunol. 2017Dec;24(12):e00265–17. PubMed PMID: 29021299; PubMed Central PMCID: PMCPMC5717191; eng.
- Valcin CL, Severe K, and Riche CT, et al. Predictors of disease severity in patients admitted to a cholera treatment center in urban Haiti. Am J Trop Med Hyg. 2013Oct;89(4):625–632. PubMed PMID: 24106188; PubMed Central PMCID: PMCPMC3795091; eng.
- von Seidlein L, Wang XY, Macuamule A, et al. Is HIV infection associated with an increased risk for cholera? Findings from a case-control study in Mozambique. Trop Med Int Health. 2008May;13(5):683–688. PubMed PMID: 18331384; eng.
- Perry RT, Plowe CV, and Koumaré B, et al. A single dose of live oral cholera vaccine CVD 103-HgR is safe and immunogenic in HIV-infected and HIV-noninfected adults in Mali. Bull World Health Organ. 1998;76(1):63–71. PubMed PMID: 9615498; PubMed Central PMCID: PMCPMC2305629; eng.
- Muhsen K, Sow SO, Tapia MD, et al. Pre-existing Helicobacter pylori serum IgG enhances the vibriocidal antibody response to CVD 103-HgR live oral cholera vaccine in Malian adults. Sci Rep. 2020Oct9;10(1):16871. PubMed PMID: 33037244; PubMed Central PMCID: PMCPMC7547695 Emergent Biosolutions, Gaithersburg, MD as Vaxchora. The authors declare no competing interests. eng.
- Zhang Y, Zhang H, Wang B, et al. Pregnancy outcomes after a mass vaccination campaign with an oral cholera vaccine: a systematic review and meta-analysis. Bjog. 2020Aug;127(9):1066–1073. PubMed PMID: 32289871; eng.
