The first two COVID-19 vaccines, both of which contain messenger RNA (mRNA), BNT162b2 from Pfizer Inc/BioNTech and mRNA-1273 from Moderna and a third containing a recombinant replication-incompetent adenovirus type 26 (Ad26) vector, Ad26.COV2.S from Janssen Pharmaceuticals Companies of Johnson & Johnson, were authorized for emergency use in the United States by the Food and Drug Administration (FDA) in mid-December 2020 and at the end of February 2021, respectively [Citation1–3]. In the pre-emergency use authorization clinical trials for these vaccines, local and systemic reactions were the main types of adverse events (AE) observed. The Centers for Disease Control and Prevention (CDC) uses three systems to monitor the safety of COVID-19 vaccines: 1) the Vaccine Adverse Event Reporting System (VAERS), which is the front-line, national, spontaneous surveillance system [Citation4]; 2) v-safe, a new smartphone and Internet survey-based, after-vaccination health checker for people who receive COVID-19 vaccines [Citation5]; there is also the associated v-safe pregnancy registry which collects detailed pregnancy and medical history information from v-safe participants who report being pregnant around the time of vaccination [Citation6]; and 3) the Vaccine Safety Datalink (VSD) which is a large linked database system used for active surveillance and traditional epidemiologic research [Citation7]. These complementary systems are being used to actively monitor the safety of COVID-19 vaccines in the United States [Citation8]. The results of this unprecedented and comprehensive effort are communicated through frequent presentations at the meetings of the Advisory Committee on Immunization Practices (ACIP) and in several fast-tracked published reports.
1. Vaccine adverse events reporting system
VAERS is a national passive vaccine safety surveillance system, co-administered by the CDC and the FDA since 1990, that receives spontaneous reports of AEs from health-care providers, vaccine manufacturers, vaccine recipients, and other members of the public following vaccination [Citation4]. Before the first COVID-19 vaccines were authorized for use, the average number of reports received by VAERS was approximately 40,000–50,000 per year. However, during the first 9 months of 2021 (as of 30 September 2021), VAERS has received 582,750 reports for the COVID-19 vaccines alone. VAERS data are monitored in real time to detect new, unusual, or rare vaccine AEs as well as increases in known AEs. Monitoring of AEs in VAERS is especially important whenever a new vaccine is licensed and recommended for use in the US population, as is currently the case with the novel COVID-19 vaccines approved under the FDA’s emergency use authorization (EUA) provision. Strengths of VAERS include its broad national scope and its ability to rapidly detect and evaluate reported AEs, particularly rare AEs. Detection of potential AE signals in VAERS allows for focused investigative efforts conducted in more robust surveillance systems and targeted epidemiological studies; the Vaccine Safety Datalink (VSD) is ideally suited for conducting these types of follow-up investigations (see below). Limitations of VAERS include both under- and over-reporting (the latter due to heightened awareness, perhaps linked to media publicity around AEs), inconsistency in the quality and completeness of reports, and lack of a denominator [Citation4]. Importantly, VAERS also generally cannot assess causality between receipt of a vaccine and a reported AE [Citation4].
2. V-safe
V-safe is a safety monitoring system established by the CDC specifically for the COVID-19 vaccination program [Citation5]. V-safe participants voluntarily self-enroll and via a smartphone receive text reminders that include links to then complete a web-based survey(s). V-safe enrollees are asked to complete daily surveys for the first 7 days after vaccination, then weekly through 6 weeks, and finally at 3, 6, and 12 months post-vaccination. The health check-in schedule resets when a person receives a second dose of the vaccine. During the first week after vaccination, enrollees are asked to complete surveys inquiring about specific local injection site and systemic reactions and to rank them as mild, moderate, or severe. In addition, enrollees are asked health impact questions, i.e. if they were unable to perform normal daily activities, if they missed work or school, or if they received care from a medical professional because of their reported symptoms or health conditions. Enrollees who report seeking medical care are actively contacted via telephone and encouraged to complete a VAERS report, if indicated. Surveys of female vaccinees also include questions about pregnancy status at the time of vaccination (initial survey) and about whether they had a subsequent positive pregnancy test result during the active follow-up period. All pregnant persons identified who meet eligibility criteria are offered an opportunity to enroll in the v-safe pregnancy registry [Citation6]. People who consent to enroll in the registry are contacted and interviewed during each trimester, postpartum, and during their child’s early infancy. During these check-ins, they are asked questions about their pregnancy and medical history.
Strengths of the v-safe system include the following: its national scope and active nature, its usefulness for assessing local and systemic reactogenicity, and its capability through the v-safe pregnancy registry to assess early pregnancy loss and birth defect outcomes. Limitations of this system include being resource intensive, reliance on voluntary participation and self-reporting, and registration information not being uniformly available in all vaccination locations. Participants may also be misclassified as pregnant due to errors made in completing the v-safe health survey. In addition, the sample size in the pregnancy registry may be inadequate to study rare events.
3. Vaccine safety datalink
Established in 1990, the VSD is a collaboration between the Immunization Safety Office (ISO) at the CDC and nine integrated health-care systems [Citation7]. The VSD conducts surveillance and research studies on rare AEs following immunization and has complete vaccination and health outcome data on approximately 12 million persons with a birth cohort of 100,000 annually. The VSD includes demographic and medical information, such as age, sex, race/ethnicity, health plan enrollment, vaccinations, hospitalizations, emergency room and outpatient encounters, and mortality data, and vaccine safety evaluations conducted utilize both traditional and novel epidemiologic methods. Importantly, the VSD investigators, in addition to the automated data outlined above, also have access to patients’ electronic health record (EHR), which is key for verifying diagnoses and capturing information unavailable through standardized datasets. Since the start of the U.S. COVID-19 vaccination program, VSD has conducted near real-time safety surveillance using Rapid Cycle Analysis (RCA) to monitor and evaluate data for 23 pre-specified outcomes following COVID-19 vaccination. Weekly analyses are conducted, and if a statistical signal is found, it may trigger an additional investigation which may include chart review of selected outcomes. As of 30 August 2021, none of the outcome events being monitored in the United States have signaled in the primary sequential RCA analysis which compares events in the 21-day risk interval after COVID-19 vaccination with the number of expected events derived from vaccinated concurrent comparators who were in a comparison interval (days 22–42) after COVID-19 vaccination; these comparisons are adjusted for age group, sex, race/ethnicity, VSD site, as well as calendar date [Citation9,Citation10]. The VSD has also planned several safety studies of COVID-19 vaccines including in pregnant women. Limitations of the VSD include the following: (1) the VSD largely consists of an insured population and thus findings may not be generalizable to the entire population of the United States; (2) COVID-19 vaccine rollout including among pregnant persons has varied widely in various parts of the country, and for this reason, evaluation of rare medically attended outcomes will take time until there is adequate power for conducting interim analyses; and (3) not all outcomes are well suited for real-time analyses because of longer risk windows or because they are very nonspecific, and these therefore may need to be studied using more traditional retrospective epidemiologic methods that can take longer to complete.
The first systems to provide post-authorization safety data on COVID-19 vaccines were VAERS and v-safe, which reflects their strengths in being national in scope. The first month of surveillance data in VAERS and v-safe showed that most AEs were local and systemic reactions, which was consistent with data from pre-authorization clinical trials [Citation11]. Moreover, the v-safe pregnancy registry and VAERS promptly provided valuable and reassuring preliminary data on maternal COVID-19 vaccine safety [Citation12]. As of 13 September 2021, the v-safe COVID-19 pregnancy registry had enrolled 5096 pregnant persons [Citation13].
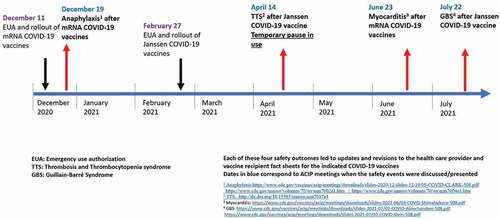
Following media reports from the United Kingdom of a few cases of anaphylaxisa following the BNT162b2 vaccine, VAERS was the first US monitoring system to show increased reporting of this rare AE [Citation14]. Prompt detection of this potentially life-threatening AE had important management implications, and clinical guidance was rapidly communicated to providers and the public by CDC [Citation15]. Recently, data from the VSD has shown that the rate of post-vaccination anaphylaxis after mRNA vaccines is approximately five cases per million doses administer [Citation10]
Another important signal detected first in VAERS was thrombosis and thrombocytopenia syndrome (TTS)b following the Ad26.COV2.S vaccine which resulted in a temporary pause in the administration of this vaccine beginning on 13 April 2021 [Citation16]. Risk–benefit analysis done shortly afterward demonstrated the benefits of vaccination with the Ad26.COV2.S vaccine despite this rare AE, and ACIP reaffirmed its interim recommendation for its use in persons ≥18 years [Citation17]. These findings led to an update of the clinical considerations by the CDC [Citation18]. The FDA also added a warning to the Ad26.COV2.S vaccine EUA fact sheets for health-care providers and separately for vaccine recipients regarding this rare AE, which included a new warning for rare clotting events among women aged 18–49 years [Citation3]. This was an example of the VAERS system performing as intended, in promptly detecting this very rare but clinically extremely serious AE which required nationwide notification of clinicians to avoid heparin therapy in managing these patients.
Since April 2021, increased reports of myocarditis/pericarditisc after mRNA COVID-19 vaccines were reported in the United States. Initial investigation in VAERS suggests that the observed reporting rate of myopericarditis exceeds the expected or background rate of myopericarditis. Although as of 30 August 2021, the VSD primary RCA analysis demonstrated no signals for myocarditis/pericarditis or for any other outcome after both mRNA doses in the overall VSD population including all ages ≥12 years, in subgroup analyses, both mRNA vaccines were associated with myocarditis/pericarditis in persons aged 12–39 years during days 0–21 after vaccination and especially during days 0–7 [Citation10]. Although the longer-term implications of this outcome are unknown, the early data suggests that it affects predominantly young men, most frequently follows the second vaccine dose, and the majority of the cases have early resolution of their symptoms following conservative management [Citation19]. These findings led to an update of the clinical considerations by the CDC [Citation20] and the revision to the health-care provider and vaccine recipient fact sheets for the mRNA-1273 and BNT162b2 vaccines [Citation21].
By July 2021, the number of observed reports of Guillain–Barré syndrome (GBS) after the Ad26.COV2.S vaccine exceeded by fivefold the expected number of reports of GBS in VAERS [Citation22]. Although in the VSD the primary RCA analysis did not signal for GBS, the chart confirmed unadjusted incidence rate of GBS during the 21 days after the Ad26.COV2.S vaccine was noted to be much higher than during the 21 days after mRNA vaccines [Citation23]. The finding of this association led to the addition of a warning to the FDA’s emergency use authorization fact sheets [Citation3].
These three safety outcomes were rapidly detected by the surveillance systems in place with prompt policy actions taken ().
4. Comment
We have reviewed the three systems being used for monitoring the safety of COVID-19 vaccines: (1) VAERS, (2) v-safe and the v-safe pregnancy registry, and (3) VSD. These systems have together provided early post-authorization safety data for very rare and serious AEs of anaphylaxis, TTS, myocarditis/pericarditis, and GBS, which have led to policy changes and updates in the vaccines’ EUA information statements for health-care providers and vaccine recipients. These three systems, each with its unique set of strengths and limitations, are providing complementary and critical data for the ongoing monitoring of the safety of COVID-19 vaccines.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC). Mention of a product or company name is for identification purposes only and does not constitute endorsement by the CDC.
Author contributions
All authors substantially contributed to the conception and design of this article and the interpretation of the relevant literature, as well as to its writing and revision for intellectual content.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Safety events after COVID-19 vaccines
aAnaphylaxis is a severe, potentially life-threatening allergic reaction that rarely occurs after vaccination.
bThrombosis and thrombocytopenia syndrome (TTS) – Thrombosis is the formation of blood clots that block blood vessels and thrombocytopenia refers to a low blood platelet count (<150,000 per microliter).
cMyocarditis is inflammation of the heart muscle and pericarditis is inflammation of the lining outside the heart.
dGuillain–Barré syndrome (GBS) is an acute, immune-mediated paralytic disorder of the peripheral nervous system.
Acknowledgments
We thank Ms. Christina Banister for review and editing assistance of the manuscript. We also thank the staff of the Immunization Safety Office and General Dynamics Information Technology for their work and dedication to public health during the COVID-19 pandemic.
Additional information
Funding
References
- Food and Drug Administration. Fact sheet for healthcare providers administering vaccine (vaccination providers): emergency use authorization (EUA) of the Pfizer BioNTech COVID-19 vaccine to prevent coronavirus disease 2019 (COVID-19); 2021;1(12):2021 Available from: https://www.fda.gov/media/144413/download
- Food and Drug Administration. Fact sheet for healthcare providers administering vaccine (vaccination providers): emergency use authorization (EUA) of the Moderna COVID-19 vaccine to prevent coronavirus disease 2019 (COVID-19); 2021 1 12 2021. Available from: https://www.fda.gov/media/144637/download
- Food and Drug Administration. Fact sheet for healthcare providers administering vaccine (vaccination providers) emergency use authorization (EUA) of the Janssen COVID-19 vaccine to prevent coronavirus disease 2019 (COVID-19); 2021 1 12 2021. Available from: https://www.fda.gov/media/146304/download
- Shimabukuro TT, Nguyen M, Martin D, et al. Safety monitoring in the Vaccine Adverse Event Reporting System (VAERS). Vaccine. 2015;33:4398–4405.
- Vsafe after vaccination health checker 1 12 2021. Available from: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/vsafe.html
- Centers for Disease Control and Prevention. V-safe COVID-19 vaccine pregnancy registry 1 12 2021. Available from: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/vsafepregnancyregistry.html
- McNeil MM, Gee J, Weintraub ES, et al. The vaccine safety datalink: successes and challenges monitoring vaccine safety. Vaccine. 2014;32:5390–5398.
- Centers for Disease Control and Prevention. Vaccine safety monitoring 1 12 2021. Available from: https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/index.html
- Rapid Cycle Analysis (RCA) to monitor the safety of COVID-19 vaccines in near real-time within the vaccine safety datalink 1 12 2021. Available from: https://www.cdc.gov/vaccinesafety/pdf/VSD-1342-COVID19-RCA-Protocol_FinalV1.1_508.pdf.
- Klein N. Rapid cycle analysis to monitor the safety of COVID-19 vaccines in near real-time within the vaccine safety datalink: myocarditis and anaphylaxis 1 12 2021. Available form: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-08-30/04-COVID-Klein-508.pdf
- Gee J, Marquez P, Su J, et al. First month of COVID-19 vaccine safety monitoring – United States, December 14, 2020–January 13, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:283–288.
- Shimabukuro TT, Kim SY, Myers TR, et al. Preliminary findings of mRNA COVID-19 vaccine safety in pregnant persons. N Engl J Med. 2021 Apr 21:NEJMoa2104983. DOI:https://doi.org/10.1056/NEJMoa2104983. Epub ahead of print. PMID: 33882218; PMCID: PMC8117969. •• Of considerable importance: the first post-authorization safety data published on mRNA COVID-19 vaccines in pregnant persons.
- Olson C. COVID-19 vaccine safety in pregnancy: updates from the v-safe COVID-19 vaccine pregnancy registry 1 12 2021. Available from: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-09-22/09-COVID-Olson-508.pdf
- Shimabukuro TT, Cole M, Su JR. Reports of anaphylaxis after receipt of mRNA COVID-19 vaccines in the US – December 14, 2020-January 18, 2021. JAMA. 2021;325(11):1101–1102.
- Centers for Disease Control and Prevention. Interim clinical considerations for use of COVID-19 vaccines currently authorized in the United States 1 12 2021. Available from: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html
- See I, Su JR, Lale A, et al. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. JAMA. 2021;325(24):2448–2456.
- MacNeil JR, Su JR, Broder KR, et al. Updated recommendations from the advisory committee on immunization practices for use of the Janssen (Johnson & Johnson) COVID-19 vaccine after reports of thrombosis with thrombocytopenia syndrome among vaccine recipients – United States, April 2021. MMWR Morb Mortal Wkly Rep. 2021;70:651–656.
- Centers for Disease Control and Prevention. Clinical care considerations for COVID-19 vaccination 1 12 2021. Available from: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/index.html
- Advisory Committee on Immunization Practices (ACIP). COVID-19 vaccine safety updates, June 23, 2021. Available from: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-06/03-COVID-Shimabukuro-508.pdf
- Centers for Disease Control and Prevention. Clinical considerations: myocarditis and pericarditis after receipt of mRNA COVID-19 vaccines among adolescents and young adults 1 12 2021. Available from: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/myocarditis.html
- Food and Drug Administration. Coronavirus (COVID-19) Update: June 25, 2021. Available from: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-june-25-2021
- Advisory Committee on Immunization Practices (ACIP). Guillain-Barré syndrome (GBS) after Janssen COVID-19 vaccine: Vaccine Adverse Event Reporting System (VAERS); [ cited 2021 Jul 22]. Available from: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-07/02-COVID-Alimchandani-508.pdf
- Advisory Committee on Immunization Practices (ACIP). Rapid Cycle Analysis (RCA) to monitor the safety of COVID-19 vaccines in near real-time within the vaccine safety datalink: Guillain-Barré syndrome (GBS); [ cited 2021 Jul 22]. Available from: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-07/03-COVID-Klein-508.pdf