KEYWORDS:
1. Anti-tick vaccine efficacy and effectiveness: the Subolesin model
Recent publications and particularly a recent paper by Ndawula, Jr [Citation1]. provided a comprehensive review on anti-tick vaccine research and results. In this review, the author addressed the limitations in tick vaccine research with emphasis on the methodology for the evaluation of vaccine efficacy and effectiveness and the need to advance in the characterization of the immunological mechanisms mediating vaccine efficacy for the control of both ixodid and argasid tick infestations. We agree on this proposal for the evaluation of vaccine efficacy and effectiveness. However, additional considerations disclosed here using our research based on the Subolesin tick antigen model are relevant for the development of effective and safe vaccines for the control of tick infestations and tick-borne diseases (TBD).
As disclosed in the review paper [Citation1], anti-tick vaccines constitute an environmentally sound effective intervention for the control of cattle ticks and TBD. Anti-tick vaccines are designed to reduce tick populations and the prevalence of TBD by reducing tick feeding, reproduction and development through antigen-specific antibodies that affect tick protein function and other immune mechanisms [Citation1,Citation2]. The application of Bm86/Bm95-based anti-tick vaccines have shown reduction in adult female ticks from different species (e.g. Rhipicephalus microplus, Rhipicephalus annulatus, Rhipicephalus decoloratus, Hyalomma dromedarii) in vaccinated versus control cattle and in the use of acaricides through lower application frequency (vaccine efficacy 41–100% [Citation2];). However, Bm86/Bm95-based vaccines were not effective against other tick species (e.g. Rhipicephalus appendiculatus) [Citation2]. This limitation led to the identification and characterization of other tick vaccine protective antigens such as Subolesin (also known as 4D8) [Citation3].
Subolesin is the tick ortholog of vertebrate Akirins [Citation3]. These regulatory cofactors are evolutionarily conserved throughout the metazoan without catalytic or DNA-binding capacity with a role in the regulation of multiple biological processes including immune response to pathogen infection [Citation3]. The main regulatory function of Subolesin/Akirin is mediated by protein–protein interactions with chromatin remodelers, transcription factors, histone acetyltransferases, RNA-associated proteins, and importins and possible implications of direct interactions with chromatin [Citation3–5].
Vaccination with Subolesin has shown protection against multiple tick species and pathogen infection/transmission [Citation3,Citation6]. The use of Subolesin-based vaccines alone or in combination with other antigens have reached 80–97% efficacy, similar or higher than that obtained with other tick antigens such as Bm86/Bm95, Metalloprotease, Ribosomal protein P0, Ferritin 2, and Aquaporin [Citation6] (reviewed by [Citation3]). Under field conditions, vaccination with the Subolesin-Anaplasma marginale Major surface protein 1a chimeric antigen resulted in reduction of tick infestations and pathogen infection/transmission in cattle and sheep [Citation7]. These results support the possibility of combining tick and pathogen derived antigens for the control of tick infestations and TBD. Furthermore, Subolesin has shown efficacy in the control of cattle tick infestations with an oral vaccine formulation with heat inactivated Mycobacterium bovis [Citation8] and the combination with Bm86 in a subcutaneous vaccine formulation.
Despite discussed challenges for the development of effective vaccines for the control of tick infestations and TBD [Citation1], the results with Subolesin support the possibility of developing vaccines for the control of multiple tick species and TBD, including the possibility of combining tick and pathogen derived antigens.
2. Methodological approaches for anti-tick vaccine development
The identification of candidate tick protective antigens is a major challenge for the development of effective anti-tick vaccines. Several methodological approaches have been proposed to address this challenge and ongoing research includes (a) a rational approach based on tick biology, (b) reverse genetics, (c) tick protein evolution, and (d) vaccinomics.
Vaccinomics is a platform for the identification of candidate protective antigens based on the characterization of tick-host-pathogen molecular interactions using omics technologies and data integration and analysis by a systems biology approach [Citation9]. To further advance on the identification of protective antigens, we proposed to combine vaccinomics, including interactomics, with intelligent Big Data analytic techniques [Citation10,Citation11]. The next step in the vaccinomics pipeline, was then the quantum vaccinomics based on the identification of protective epitopes or immunological quantum [Citation11,Citation12] (). Using the Subolesin model antigen, the application of quantum vaccinomics has validated the identification and combination of protective epitopes, suggesting the possibility of using this approach for the combination of tick and pathogen derived protective antigens [Citation11]. Additionally, modeling tick vaccines is also a key tool to improve the effectiveness of vaccines for the control of tick infestations and pathogen infection/transmission.
Figure 1. Vaccinomics approaches for anti-tick vaccine development. new approaches have been proposed to improve vaccine efficacy, effectiveness, and safety. vaccinomics includes the characterization of tick-host-pathogen molecular interactions through integration of multiple omics technologies and big data analytics for the identification of candidate protective antigens. here, we propose quantum vaccinomics as a step forward through the identification and combination of immune protective epitopes (immunological quantum) for the development of more effective and safe vaccines. vaccinomics strategy for anti-tick vaccines considers other biomolecules such as glycan-based protein post-translational modifications (e.g. glycan alpha-gal), combination of tick and pathogen derived antigens and the use of different vaccine platforms such as nanoparticles, virus-like particles, and probiotic-based vaccines.
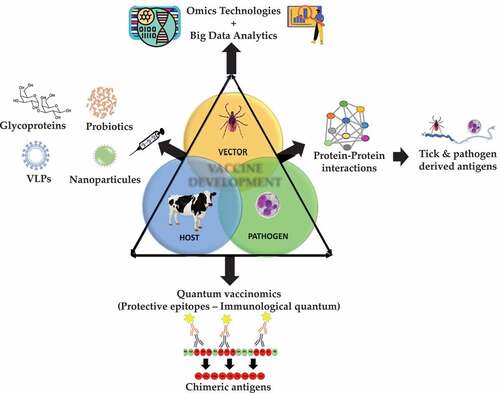
Another area of interest for anti-tick vaccines is the possibility of including other biomolecules such as glycan-based protein post-translational modifications [Citation12]. In particular, the glycan alpha-gal (Galα1-3Galβ1-(3)4GlcNAc-R) present on tick and pathogen derived glycoproteins has been associated with the alpha-gal syndrome in some individuals exposed to tick bites that results in the production of anti-alpha-gal IgE antibodies, which may cause delayed anaphylaxis to mammalian meat consumption or immediate anaphylaxis to tick bites, xenotransplantation and certain drugs such as cetuximab. As a trade-off in result to ‘catastrophic-selection,’ the anti-alpha-gal IgM/IgG antibody and cellular response in humans protect against pathogens with alpha-gal modifications on surface proteins [Citation12]. Consequently, the immune response to alpha-gal has been proposed to function in the control of ticks and TBD [Citation13,Citation14].
3. Conclusions and future directions
The control of ticks and TBD represent a challenge for human and animal health worldwide [Citation1]. To address this challenge, a multidisciplinary approach is required by combining different control measures including cattle management, rational use of acaracides and anti-tick vaccines. Application of novel translational biotechnology approaches will advance research on developing effective and safe vaccines for the control of tick infestations and TBD. These approaches include and are not limited to Big Data analytics, quantum vaccinomics, combination of tick and pathogen derived antigens including glycoproteins, targeting tick antibody evasion, epigenetic regulatory mechanisms and cement biomolecules, activation of trained immunity mechanisms and use of different vaccine platforms such as nanoparticles, virus-like particles, and probiotic-based vaccines [Citation15] (). As stressed during the COVID-19 pandemic, a One Health perspective with control strategies based on the geo-economic situations and target tick vectors and pathogens is necessary for the effective control of tick infestations and TBD.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
We thank members of our laboratory for their contribution and support to this research. Part of the research included in this paper was supported by the Ministerio de Ciencia e Innovación/Agencia Estatal de Investigación MCIN/AEI/10.13039/501100011033, Spain and EU-FEDER (Grant BIOGAL PID2020-116761GB-I00) and the Consejería de Educación, Cultura y Deportes, JCCM, Spain, project CCM17-PIC-036 (SBPLY/17/180501/000185).
Additional information
Funding
References
- Ndawula C Jr. from bench to field: a guide to formulating and evaluating Anti-Tick vaccines delving beyond efficacy to effectiveness. Vaccines (Basel). 2021;9(10):1185.
- de la Fuente J, Kocan KM. Advances in the identification and characterization of protective antigens for recombinant vaccines against tick infestations. Expert Rev Vaccines. 2003;2(4):583–593.
- Artigas-Jerónimo S, Villar M, Cabezas-Cruz A, et al. Functional evolution of Subolesin/Akirin. Front Physiol. 2018;9:1612.
- Shaw DK, Wang X, Brown LJ, et al. Infection-derived lipids elicit an immune deficiency circuit in arthropods. Nat Commun. 2017;8(1):14401.
- Goto A, Matsushita K, Gesellchen V, et al. Akirins are highly conserved nuclear proteins required for NF-kappaB-dependent gene expression in drosophila and mice. Nat Immunol. 2008;9(1):97–104.
- Kasaija PD, Contreras M, Kabi F, et al. Vaccination with recombinant subolesin antigens provides cross-Tick species protection in Bos indicus and crossbred cattle in Uganda. Vaccines (Basel). 2020;8(2):319.
- Torina A, Moreno-Cid JA, Blanda V, et al. Control of tick infestations and pathogen prevalence in cattle and sheep farms vaccinated with the recombinant Subolesin-Major surface protein 1a chimeric antigen. Parasit Vectors. 2014;7(1):10.
- Contreras M, Kasaija PD, Merino O, et al. Oral vaccination with a formulation combining Rhipicephalus microplus Subolesin with heat inactivated Mycobacterium bovis reduces tick infestations in cattle. Front Cell Infect Microbiol. 2019;9:45.
- Contreras M, Villar M, Alberdi P, et al. Vaccinomics approach to tick vaccine development. Methods Mol Biol. 2016;1404:275–286.
- de la Fuente J, Villar M, Estrada-Peña A, et al. High throughput discovery and characterization of tick and pathogen vaccine protective antigens using vaccinomics with intelligent big data analytic techniques. Expert Rev Vaccines. 2018;17(7):569–576.
- Artigas-Jerónimo S, Comín J, Villar M, et al. A novel combined scientific and artistic approach for the advanced characterization of interactomes: the Akirin/Subolesin model. Vaccines (Basel). 2020;8(1):77.
- Galili U. Evolution in primates by “catastrophic-selection” interplay between enveloped virus epidemics, mutated genes of enzymes synthesizing carbohydrate antigens, and natural anti-carbohydrate antibodies. Am J Phys Anthropol. 2019;168(2):352–363.
- Cabezas Cruz A, Valdés JJ, de la Fuente J. Control of vector-borne infectious diseases by human immunity against α-Gal. Expert Rev Vaccines. 2016;15(8):953–955.
- Cabezas-Cruz A, Hodžić A, Mateos-Hernández L, et al. Tick-human interactions: from allergic klendusity to the α-Gal syndrome. Biochem J. 2021;478(9):1783–1794.
- Ferreira Leal B, Sánchez Ferreira CA. Ticks and antibodies: may parasite density and tick evasion influence the outcomes following immunization protocols? Vet Parasitol. 2021;300:109610.
