ABSTRACT
Background
All European countries have national immunization programs (NIPs) to protect gainst infectious diseases. We aimed to estimate the individual lifetime cost of vaccination in 23 European countries, assuming full compliance with NIP schedules.
Research design and methods
We used publicly available data to estimate the individual lifetime cost of vaccination with the vaccines that are currently recommended and funded in each country for healthy individuals and for individuals with underlying medical conditions. We included a scenario analysis for healthy individuals in which all currently recommended vaccines were universally funded, and compared the annual costs per person of vaccination to the annual per-capita costs of all-cause hospitalization and anti-infective medications.
Results
The individual lifetime cost of vaccination was €592–3,504 for healthy individuals (median: €1,663; 13–20 diseases), €744–9,081 for individuals with underlying conditions (median: €2,992; 13–21 diseases), and €1,225–4,832 (median: €2,565; 21–22 diseases) in the scenario analysis, with median values for vaccine acquisition of €1,203, €1,731, and €1,788, respectively.
Conclusions
Our estimates show that the maximum potential cost of vaccination requires a relatively low level of investment assuming full compliance. These data could be useful for policymakers in future financial planning and evaluation of NIPs.
1. Introduction
The prevention of disease morbidity and mortality through vaccination is one of the greatest public health successes in human history [Citation1,Citation2]. Vaccination also has direct economic benefits through short- and long-term health care system cost savings, as well as reduced absences from school, work, and other socioeconomically important activities [Citation3–7]. Additional, less tangible, health and socioeconomic benefits of vaccination include promotion of healthy aging and protection of health care system capacity, especially during influenza and respiratory illness season [Citation3,Citation5,Citation6,Citation8]. As the World Health Organization highlighted in a recent report, prioritizing vaccination as an essential health service and strengthening routine vaccination programs is key to improving resiliency in primary health care in the context of the COVID-19 pandemic [Citation9]. The European Union (EU’s) One Health Action Plan against antimicrobial resistance also calls vaccination a solution with ‘great potential’ to prevent bacterial infections such as pneumococcal diseases, and thus reduce the use of antimicrobial drugs that accelerate the spread of antimicrobial resistance [Citation10].
Vaccination programs have consistently been found to have high benefit–cost ratios (BCRs). For example, an economic analysis of the 2009 United States (US) routine childhood vaccination schedule determined that the BCR of the program was 3.0 for direct health care costs (indicating that every dollar spent on the program saved $3 in health care costs) and 10.1 for overall societal costs [Citation11]. Similarly, a study focusing on the universal hepatitis B vaccination program in Italy estimated a BCR of 0.91 for direct costs over the first 10 years; however, the ratio increased to 2.47 after the first 20 years of the program, highlighting the need to consider long-term costs and benefits for vaccine-preventable diseases with chronic or delayed adverse clinical outcomes [Citation12]. A 50-year retrospective analysis of the US polio vaccination program calculated an overall net benefit of $180 billion (direct cost savings only) from an estimated $35 billion investment, but also reported that the cost-effectiveness ratio varied over time, highlighting the need for ongoing dynamic economic modeling [Citation13].
The BCRs of vaccination programs compare favorably to those of alternative preventive measures or treatment approaches. For instance, an analysis of six influenza prevention or control strategies in England, Wales, France, and Germany found that vaccination-based strategies were more cost-effective than those based on chemoprophylaxis or early treatment [Citation14]. Vaccines also have a lower median cost per quality-adjusted life-year gained than other preventive measures such as pharmaceutical replacement therapy and screening programs for cardiovascular disease, cancer, and osteoporosis [Citation15].
In response to concerns about insufficient vaccination coverage, the European Commission issued a set of 20 recommendations in 2018 to improve cooperation among member states with respect to preventable diseases [Citation16,Citation17]. The European Commission outlined a set of actions to increase vaccination coverage rates and develop sustainable vaccination policies, and included a recommendation to align vaccination schedules among all member states within 5–10 years [Citation16,Citation17]. However, these recommendations have been criticized by groups such as the European Academies Science Advisory Council and Federation of European Academies of Medicine as a ‘one-size-fits-all’ approach that does not account for the individual situation and needs of each member country [Citation18,Citation19]. A tailored approach to improving vaccination coverage and increasing funding for vaccinations in Europe will thus require separate economic analyses for each country. These efforts will require country-specific information on the individual lifetime cost of vaccination with the vaccines that are recommended and funded under current national immunization programs (NIPs), as well as of expanded NIPs that incorporate additional vaccines.
The primary objective of this study was to estimate the individual lifetime cost of vaccination in full compliance with the published schedule in 23 European countries, based on the vaccines that were both recommended and funded in their most recent NIPs. We also aimed to explore the individual lifetime cost of vaccination in a scenario in which each country’s NIP for healthy individuals was expanded to include universal funding for all currently recommended vaccines. Finally, we contextualized the annual costs per person of vaccination by comparing them to the per-capita cost of all-cause hospitalization and anti-infective medications for each country with available data.
2. Methods
2.1. Study design
This was a cost modeling study based on secondary analysis of publicly available primary data. The geographic scope was restricted to 29 countries: the current 27 member states of the EU plus the UK and Switzerland. Public vaccine costs were not available for six member countries (Estonia, Hungary, Ireland, Italy, Lithuania, and Malta); these countries were therefore excluded from the analysis. The study was carried out using data from the remaining 23 countries.
2.2. Variables and data sources
The sources of input data for each country are summarized in Supplemental Table 1. The input data comprised information on national demographics (including population size by sex (Supplemental Table 2), birth rate (Supplemental Table 2), life expectancy by sex (Supplemental Table 3), official national vaccination schedules as of January 2022 (including information on funding status, target populations, and total lifetime number of vaccine doses per individual; Supplemental Table 4), vaccine acquisition costs (public prices as of January 2022, including value-added tax), and the direct costs of vaccine administration such as clinic visits. The administration costs did not include the costs of cold-chain supply or personnel costs that are not directly related to the administration of vaccines.
If more than one vaccine was approved and available for prevention of the same disease, the vaccine with the lower total vaccination cost (combined costs of vaccine acquisition and administration) was used in the analysis unless the use of the more expensive vaccine was specified in certain circumstances (e.g. for individuals with certain underlying conditions or other risk factors). The costs of vaccines not currently approved in Bulgaria were calculated following official national guidelines [Citation20]. For other countries, if no vaccine cost information was publicly available then the cost of the vaccine was estimated using as reference the cost of the same vaccine in Belgium (for which public prices were available for most vaccines), adjusted for the average price difference of vaccines in common between Belgium and the country of interest. The cost of tuberculosis (TB) vaccines was estimated using public vaccine cost data from Sweden and France as reference. For all countries, the administration costs for vaccines that are not currently approved or for which no administration cost data were available were assumed to be the same as for approved vaccines delivered to the same age or risk groups. For vaccines requiring regular boosters, the total number of lifetime doses per person was estimated for each sex using country- and sex-specific data on life expectancy at birth.
The annual costs per capita of all-cause hospitalization and of all anti-infective medications were obtained from the OECD.Stat database; annual total costs for the period 2017–2019 were considered to avoid bias related to the COVID-19 pandemic [Citation21]. For hospitalization costs, the following filters were applied to the OECD.Stat database: Financing scheme, All financing schemes; Function, Inpatient curative and rehabilitative care; Provider, Hospitals; Measure, Current prices. For anti-infective costs, the filters were Health → Pharmaceutical Market → Pharmaceutical consumption → (variable) J01–antibacterials for systemic use. All costs in euros for non-euro using countries were calculated using January 2022 exchange rates from the European Commission [Citation22].
2.3. Analysis
All analyses were performed in Microsoft Excel. Cost estimations assumed that all individuals were vaccinated with all vaccines for which they were eligible, in full compliance with the published NIP schedule. Individual lifetime costs for each vaccine in each country were calculated as cost per vaccination (combined vaccine acquisition and administration costs per dose) × number of lifetime vaccine doses per eligible recipient. Annual costs per person were then defined by dividing the lifetime values by the life expectancy at birth for each country.
The first base-case analysis incorporated all universally funded vaccines from each country’s most recent NIP; i.e. all recommended and funded vaccines that would be received by a healthy person (). The second base case included all recommended and funded vaccines that would be received by an individual with all underlying medical conditions or other risk factors that would qualify them for additional funded vaccinations under each country’s NIP. These risk factors vary between countries and between vaccines but include age, sex, pregnancy, underlying conditions, and place of birth or residence. The individual lifetime cost in the second base-case analysis thus represents the maximum possible lifetime cost, for an individual who qualifies for all funded vaccines.
Table 1. Vaccines and number of lifetime doses included as of the January 2022 national immunization programs of 23 European countries*.
A number of assumptions were made. In some countries (Czech Republic, Finland, the Netherlands, Slovenia, and Sweden), TB vaccines are funded only for migrants to/from high-risk countries; no vaccination costs were included in the analyses for these countries, as the frequency of vaccination is very low. In countries where a separate monovalent hepatitis B (HepB) vaccine is funded for neonates at risk due to maternal infection, vaccination costs were not considered as the frequency of vaccination is very low (in addition, the model assumes that all mothers would have been fully vaccinated with a HepB vaccine).
A scenario for healthy individuals that assumed universal coverage with all recommended vaccines, regardless of current funding status, was also explored. For all countries, the scenario analysis included universal vaccination against diphtheria, tetanus, pertussis (collectively DTaP), adult Tdap booster, Haemophilus influenzae type B (Hib), hepatitis A (HepA), HepB, human papillomavirus (HPV), influenza (adult and pediatric), measles, meningococcal B (MenB), meningococcal C/ACWY (MenC/MenACWY), mumps, pneumococcal disease (pneumo; pediatric and adult), polio, rotavirus, rubella, varicella, and zoster (shingles). In addition, the scenario analysis included universal vaccination against tick-borne encephalitis (TBE) and TB in countries in which these vaccines are currently universally funded (). Vaccination against rabies is currently funded only in the Czech Republic, for people at risk. For the sake of simplification, the rabies vaccine was therefore not included in this study’s tables, but was included in the individual lifetime cost calculations for the Czech Republic. The calculation used the number of doses of each vaccine specified in each country’s most recent NIP, if available. If a schedule for a scenario analysis vaccine was not included in a country’s most recent NIP, the following numbers of lifetime doses were used (based on published guidelines): HepA, 1; HepB, 3; influenza (pediatric), 5; MenACWY, 1; MenB, 3; pneumococcal (adult), 1; rotavirus, 2 or 3; varicella, 2; zoster, 1; DT/Tdap adult booster, 1 dose every 10 years from age 25 onward [Citation23–31].
3. Results
3.1. Base cases
The most recent NIPs of the 23 countries included in the analysis varied in terms of the number of recommended and funded vaccines, and the number of lifetime doses of certain vaccines (). The costs per dose of vaccine acquisition and administration also varied between countries (), as did the recommended schedules for funded vaccines (Supplemental Table 4).
Table 2. Cost per dose of vaccine acquisition and administration in 23 European countries.
The NIPs of Greece and the UK incorporated universally funded vaccines against the most diseases (20 and 19 vaccines each, respectively), while those of Denmark, Romania, and Sweden incorporated the fewest (13 vaccines each; ). All countries universally funded vaccinations against diphtheria, Hib, measles, mumps, pertussis, pediatric pneumococcal, polio, rubella, and tetanus. Other commonly universally funded vaccines were HepB (21 countries), adult influenza (20), and HPV (19). Vaccines against TBE (1 country), HepA (2), and MenB (2) were universally funded in the fewest NIPs. The number of vaccines funded only for individuals with underlying conditions or other risk factors ranged from 0 (Austria, Bulgaria, Croatia, Cyprus, Germany, Romania, Sweden, UK) to 6 (Finland); the vaccines that were conditionally funded in the greatest number of countries were TB (6 countries), HepA (5), HPV (4 countries, all of which funded this vaccine for girls and women only), and MenB (4).
The individual lifetime vaccine acquisition costs for both base cases, as well as the individual lifetime vaccination costs (combined costs of vaccine acquisition and administration), are presented in and . For a healthy individual receiving all universally funded vaccines, the median lifetime cost of vaccine acquisition across all 23 countries was €1,203. The acquisition cost was highest in Germany (€3,049), Greece (€1,724), Luxembourg (€1,704), and Switzerland (€1,531) and lowest in Poland (€350) and Romania (€511). The median lifetime cost of vaccination was €1,663, with Germany (€3,504), Switzerland (€3,335), and Luxembourg (€2,431) having the highest costs and Romania (€592) and Poland (€717) the lowest.
Figure 1. Individual lifetime costs of A) vaccines and B) vaccination (combined vaccine acquisition and administration costs) in 23 European countries in the two base cases and scenario analysis. Base case, healthy individual: includes all universally funded vaccines from each country’s most recent national immunization program. Base case, individual with underlying conditions: includes all funded vaccines from each country’s most recent national immunization program. Scenario analysis, healthy individual: for all countries, includes universal vaccination against diphtheria, tetanus, pertussis, Haemophilus influenzae type B, hepatitis A, hepatitis B, human papillomavirus, influenza, measles, meningococcal B, meningococcal C/ACWY, mumps, pneumococcal disease, polio, rotavirus, rubella, varicella, and zoster. Universal vaccination against tick-borne encephalitis and tuberculosis was included for countries in which these vaccines were universally funded in the most recent national immunization program. Rabies vaccination was included for the Czech Republic. Overall costs in (B) comprise costs of vaccines plus costs of administration such as clinic visits, national immunization program administration costs, etc.
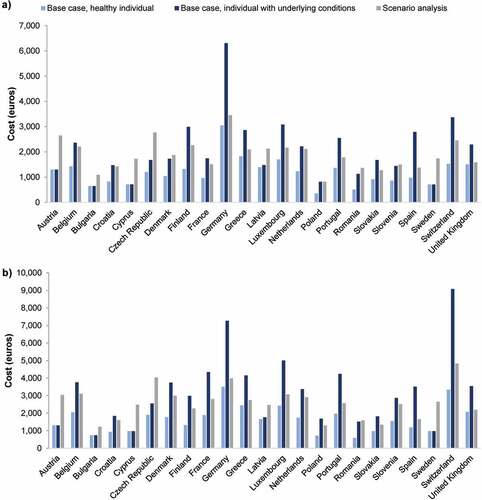
Table 3. Individual lifetime cost of vaccination in 23 European countries in the two base cases and scenario analysis.
For an individual with underlying conditions or other risk factors receiving all funded vaccines, the lifetime cost of vaccine acquisition was highest in Germany (€6,306), Switzerland (€3,371), and Luxembourg (€3,082) and lowest in Bulgaria (€648) and Sweden (€711), with a median cost of €1,731; the lifetime vaccination cost was highest in Switzerland (€9,081), Germany (€7,270), and Luxembourg (€5,006) and lowest in Bulgaria (€744) and Cyprus (€973), with a median cost of €2,992.
The annual cost per person of vaccine acquisition ranged from €5 (Poland) to €38 (Germany) for healthy individuals (median: €15) and from €9 (Bulgaria, Cyprus, Sweden) to €78 (Germany) for individuals with underlying conditions (median: €21; ). The annual cost per person of vaccination ranged from €8 (Romania) to €43 (Germany) for healthy individuals (median: €21) and from €10 (Bulgaria) to €109 (Switzerland) for individuals with underlying conditions (median: €36).
Table 4. Annual per-person cost of vaccine acquisition and vaccination and per-capita costs of all-cause hospitalization and anti-infective medications in 23 European countries.
3.2. Scenario analysis
In the scenario of universal coverage with all recommended vaccines, the individual lifetime cost of vaccine acquisition for a healthy individual would be highest in Germany (€3,454), the Czech Republic (€2,766), and Austria (€2,645) and lowest in Poland (€819) and Bulgaria (€1,091; and ). The individual lifetime cost of vaccination would be highest in Switzerland (€4,832), Czech Republic (€4,037) and Germany (€3,981), and lowest in Bulgaria (€1,225) and Poland (€1,299). The median values were €1,788 for vaccine acquisition and €2,565 for vaccination. The annual cost per person of vaccine acquisition would range from €11 (Poland) to €43 (Germany) with a median of €22 (, Supplemental Figure 1). The annual cost per person of vaccination would range from €17 (Bulgaria, Poland, Slovakia) to €58 (Switzerland) with a median of €32.
3.3. Comparators
The annual cost per capita of all-cause hospitalization ranged from €180 (Croatia) to €2,364 (Switzerland) with a median of €585, and the annual cost per capita of all anti-infective medications ranged from €12 (Portugal) to €64 (Greece) with a median of €33 (, Supplemental Figure 1). Anti-infective medication costs were not available for all countries.
4. Discussion
In this study, we estimated the individual lifetime cost of vaccination with the vaccines that are recommended and funded in the January 2022 NIPs of 23 European countries using two base cases: healthy individuals and individuals with underlying medical conditions or other risk factors. We also included a scenario analysis for healthy individuals of expanded NIPs with universal funding for all recommended vaccines. The lifetime individual cost of vaccination in all three analyses varied substantially between countries. This high degree of variation means that the European Commission’s recommendation to align vaccination schedules among its member states within 5–10 years [Citation16,Citation17] would have varying economic impacts in different nations. In all countries, annual vaccination costs per person were small compared to the annual cost per capita of all-cause hospitalization, and in most countries the annual vaccine acquisition costs per person were lower than the annual cost per capita of all anti-infective medications in countries for which data were available. For a healthy individual, the median annual cost per person of vaccination was 27.9-fold lower than the equivalent cost of hospitalization and the median annual vaccine acquisition costs per person was 2.2-fold lower than the equivalent cost of anti-infective medications; for an individual with underlying medical conditions, the median annual cost per person of vaccination was 16.3-fold lower than the per-capita cost of hospitalization and the median annual vaccine acquisition costs per person was 1.6-fold lower than the per-capita cost of anti-infective medications.
A 2016 analysis of the NIPs of seven European countries (England, France, Germany, Italy, Portugal, Spain, and Sweden) also reported large ranges in the lifetime individual costs of vaccination – from €443 for a healthy man in Sweden to €3,395 for a woman in England with underlying medical conditions – as well as in the per-dose costs of vaccine acquisition and administration [Citation32]. From seven years of age onwards the lifetime cost of vaccination was higher for women than for men due to the relatively high cost of the HPV vaccine (which at the time of analysis was administered only to women in some countries) as well as greater life expectancy at birth, necessitating more Tdap booster doses and annual influenza vaccines [Citation32]. This trend is also reflected in our data (Supplemental Tables 5 & 6). An expanded and updated analysis that also included Poland found that annual per-capita spending on vaccines also varied greatly, ranging from €3 in Spain in 2013 to €21 in Sweden in 2015 [Citation33]. The proportion of national healthcare spending that was dedicated to vaccines in each of these eight countries in 2005–2016 ranged from 0.15% in France and Spain to 0.62% in Germany [Citation33].
The lifetime individual costs we estimated for France were higher than published estimates based on previous versions of the French NIP in the healthy individual base case, but lower than the published figures in the base case for an individual with underlying conditions [Citation32,Citation34]. Both of our base-case estimates for Portugal were lower than published estimates based on an earlier NIP, but the opposite was true for Germany and Sweden (for Swedish values estimated in the local currency) [Citation32]. These differences are likely due to changes in NIPs and in vaccine prices. The costs we estimated for Spain were very similar to those calculated by Ethgen et al. in both base cases, but higher than those estimated by Soler Soneira et al.; the latter study used net vaccine prices, whereas ours included value-added taxes, which may explain these differences [Citation32,Citation35]. Overall, these differences between studies highlight the importance of dynamic economic modeling with standardized parameters to understand the main drivers of NIP cost changes over time.
This was one of the most comprehensive analyses to date of the current individual lifetime costs of vaccination in multiple European countries. Study limitations are noted. Different countries use different vaccine procurement and funding schemes; GDP per capita also affects the purchasing power of each nation. These differences preclude direct NIP cost comparisons between countries. The use of public list prices rather than confidential actual costs may have overestimated vaccine acquisition costs; conversely, the exclusion of indirect costs of vaccine administration such as cold-chain management and transportation may have underestimated vaccine administration costs. Comprehensive official government data were not available for all metrics for all countries, and some of the inputs into the model were therefore estimates. Our estimates were based on conservative assumptions and rigorous cross-referencing against the closest equivalent available data. Nevertheless, six EU member countries had to be excluded from the analysis due to lack of suitable data. We did not perform a formal analysis of the most important drivers of the differences in costs between countries; this will be an important aspect of future research on this topic. Finally, none of our models included the costs of vaccination against SARS-CoV-2; these data will be included in an updated analysis once the governments of the 23 countries finalize the details of their long-term COVID-19-prevention and management programs.
5. Conclusions
The most recent NIPs of European countries differ in the number of funded and recommended vaccines and the number of lifetime doses of some vaccines, contributing to substantial variation between countries in the lifetime individual cost of vaccination for healthy individuals and for individuals with underlying conditions and other risk factors. These differences illustrate the importance of ongoing monitoring of vaccine-related costs to account for updated NIPs and vaccine schedules, changing population demographics, inflation, fluctuating exchange rates, the availability of new vaccines, and other changes that are known to contribute to fluctuations in the cost-effectiveness of NIPs over time [Citation13]. Overall, the costs of vaccine acquisition were half of the total costs of anti-infective medications and the costs of vaccination in all countries were 16.3–27.9-folder lower than the costs of all-cause hospitalization. Our study will serve as the basis for detailed analyses of the economic costs and benefits of expanding current NIPs to include universal funding for all vaccines that are recommended by each national government, as well as of financial planning and evaluation of vaccination programs among European countries.
Declaration of interests
A Bento-Abreu, U Sabale, E Tsoumani, V Laigle, S Salomonsson and G Salomonsson are employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA and may own stocks and/or stock options in Merck & Co., Inc., Rahway, NJ, USA. O Ethgen and N Dauby provided analytical recommendations and critical review but were not compensated by MSD in that role. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or material discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have received an honorarium for their review work. Peer reviewers on this manuscript have no other relevant financial or other relationships to disclose.
Ethical approval
This was a cost modeling study based on secondary analysis of publicly available primary data. This study was not considered human subjects research and was exempt from human subjects committee review and the need for informed consent.
Author contributions
All authors have (1) substantially contributed to the conception and design of the review article and interpreting the relevant literature, and (2) been involved in writing the review article or revised it for intellectual content.
Supplemental Material
Download MS Word (182.3 KB)Acknowledgments
The authors thank Cath Ennis, PhD in collaboration with ScribCo for medical writing assistance.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14760584.2023.2157266
Additional information
Funding
References
- Andre FE, Booy R, Bock HL Vaccination greatly reduces disease, disability, death and inequity worldwide. Bull World Health Organ. 2008 Feb;86(2):140–146.
- Greenwood B. The contribution of vaccination to global health: past, present and future. Philos Trans R Soc Lond B Biol Sci. 2014;369(1645):20130433.
- Carroll S, Rojas AJ, Glenngard AH Vaccination: short- to long-term benefits from investment. J Mark Access Health Policy. 2015;3.
- Largeron N, Levy P, Wasem J Role of vaccination in the sustainability of healthcare systems. J Mark Access Health Policy. 2015;3.
- Postma MJ, Carroll S, Brandao A. The societal role of lifelong vaccination. J Mark Access Health Policy. 2015;3.
- Quilici S, Smith R, Signorelli C. Role of vaccination in economic growth. J Mark Access Health Policy. 2015;3.
- Remy V, Zollner Y, Heckmann U. Vaccination: the cornerstone of an efficient healthcare system. J Mark Access Health Policy. 2015;3.
- Bonanni P, Picazo JJ, Remy V. The intangible benefits of vaccination - what is the true economic value of vaccination? J Mark Access Health Policy. 2015;3.
- World Health Organization. Guiding Principles for recovering, building resiliency, and strengthening of immunization in 2022 and beyond. 2022.
- European Commission. A European one health action plan against antimicrobial resistance (AMR). 2017.
- Zhou F, Shefer A, Wenger J Economic evaluation of the routine childhood immunization program in the United States, 2009. Pediatrics. 2014 Apr;133(4):577–585.
- Boccalini S, Taddei C, Ceccherini V Economic analysis of the first 20 years of universal hepatitis B vaccination program in Italy: an a posteriori evaluation and forecast of future benefits. Hum Vaccin Immunother. 2013 May;9(5):1119–1128.
- Thompson KM, Tebbens RJ. Retrospective cost-effectiveness analyses for polio vaccination in the United States. Risk Anal. 2006 Dec;26(6):1423–1440.
- Scuffham PA, West PA. Economic evaluation of strategies for the control and management of influenza in Europe. Vaccine. 2002 Jun 7;20(19–20):2562–2578.
- Orenstein W, Offit P, Edwards KM Plotkin’s vaccines. 7th Edition ed.: Elsevier; 2017.
- Vaccination: Commission calls for stronger EU cooperation against preventable diseases [Internet]. 2018. Available from: https://ec.europa.eu/commission/presscorner/detail/en/IP_18_3457
- European Commission. Strengthened cooperation against vaccine preventable diseases. 2017.
- Courvoisier T, Charpentier B, van der Meer JWM Vaccination in Europe: an EASAC and FEAM commentary on the EC Roadmap ‘Strengthened cooperation against vaccine preventable diseases’
- The Lancet. Addressing decreasing vaccine coverage in the EU. Lancet. 2018 Apr 28;391(10131):1638.
- Republic of Bulgaria National Council on Prices and Reimbursement of Medicinal Products. Ordinance on the terms, rules and procedure for regulation and registration of prices for medicinal products 2021. Available from: https://ncpr.bg/en/regulations/bulgarian-legislation/regulations.html
- Organisation for economic co-operation and development. OECD.Stat. Available from: https://stats.oecd.org/Index.aspx?ThemeTreeId=9
- European Commission. Exchange rate (InforEuro). Available from: https://ec.europa.eu/info/funding-tenders/procedures-guidelines-tenders/information-contractors-and-beneficiaries/exchange-rate-inforeuro_en
- European Medicines Agency. Zostavax 2016. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/zostavax
- Datapharm. VARIVAX 2022. Available from: https://www.medicines.org.uk/emc/product/5582/smpc
- European Medicines Agency. Use of Varilrix (live attenuated varicella virus [Oka strain]) to be harmonised in the EU 2021. Available from: https://www.ema.europa.eu/en/documents/referral/varilrix-article-30-referral-use-varilrix-live-attenuated-varicella-virus-oka-strain-be-harmonized_en.pdf
- European Medicines Agency. Menveo, INN-meningococcal Group A, C, W135 and Y conjugate vaccine. Available from: https://www.ema.europa.eu/en/documents/product-information/menveo-epar-product-information_en.pdf
- European Medicines Agency. Nimenrix 2022. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/nimenrix
- European Medicines Agency. Bexsero 2022. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/bexsero
- European Medicines Agency. Trumenba, INN-Meningococcal group B vaccine. Available from: https://www.ema.europa.eu/en/documents/product-information/trumenba-epar-product-information_en.pdf
- GlaxoSmithKline. Havrix junior vaccine 2020. Available from: https://gskpro.com/content/dam/global/hcpportal/en_CY/PDF/Homepage/Products/Havrix/smpc-havrix-720-junior-12-08-2020-en.pdf
- European Medicines Agency. HBVaxPro 2011. Available from: https://www.ema.europa.eu/en/documents/overview/hbvaxpro-epar-summary-public_en.pdf
- Ethgen O, Cornier M, Chriv E The cost of vaccination throughout life: a Western European overview. Hum Vaccin Immunother. 2016 Aug 2;12(8):2029–2037.
- Ethgen O, Remy V, Wargo K. Vaccination budget in Europe: an update. Hum Vaccin Immunother. 2018;14(12):2911–2915.
- Baron-Papillon F, Cornier M, Remy V What are the lifelong costs of vaccinating one individual? The French case. Value Health. 2014 Nov;17(7):A672.
- Soler Soneira M, Olmedo Lucerón C, Sánchez-Cambronero Cejudo L El coste de vacunar a lo largo de toda la vida en España. Rev Esp Salud Pública. 2020;94:e202002005.
