ABSTRACT
Introduction
Nucleic acids represent a promising platform for creating vaccines. One disadvantage of this approach is its relatively low immunogenicity. Electroporation (EP) is an effective way to increase the DNA vaccines immunogenicity. However, due to the different configurations of devices used for EP, EP protocols optimization is required not only to enhance immunogenicity, but also to ensure greater safety and tolerability of the EP procedure.
Area covered
An data analysis for recent years on the DNA vaccines delivery against viral and parasitic infections using EP was carried out. The study of various EP physical characteristics, such as frequency, pulse duration, pulse interval, should be considered along with the immunogenic construct design and the site of delivery of the vaccine, through the study of the immunogenic and protective characteristics of the latter.
Expert opinion
Future research should focus on regulating the humoral and cellular response required for protection against infectious agents by modifying the EP protocol. Significant efforts will be directed to establishing the possibility of redirecting the immune response toward the Th1 or Th2 response by changing the EP physical parameters. It will allow for an individual selective approach during EP, depending on the pathogen type of an infectious disease.
1. Introduction
There have been many advances in the field of nucleic acid vaccines over the past few years. DNA vaccines are emerging as a compelling alternative to other types of live or inactivated virus vaccines, vector vaccines, subunit proteins, virus-like particles, and others. The first ZyCoV-D DNA vaccine for human administration was licensed in 2021 when Zydus Lifesciences (India) received approval for the limited use of this vaccine in emergency situations. Currently, many candidate vaccines against viral and bacterial diseases are at the stage of clinical trials, including vaccines against human immunodeficiency virus (HIV) [Citation1], influenza [Citation2], against hantaviruses [Citation3], alpha viruses [Citation4], Zika virus (ZIKV) [Citation5], VZN [Citation6], Ebola virus [Citation7,Citation8], (SARS-CoV-2) [Citation9,Citation10]. While other DNA vaccines, such as those against malaria, toxoplasmosis, Staphylococcus aureus, tuberculosis, are under development [Citation11–16].
Among the main advantages of DNA vaccines, the following should be noted:
The production of DNA vaccines is inexpensive, fast and scalable: Construction of a plasmid, obtaining a producer strain, fermentation and purification of a plasmid can be done within 2-4 weeks [Citation17]; The technology for the production of such vaccines does not require complex manipulations or work with dangerous pathogens, which greatly facilitates the process of their creation and reduces its overall cost.
DNA vaccines are relatively stable at ambient temperature, which distinguishes them favorably from mRNA vaccines that require storage at low temperatures [Citation18].
DNA vaccines use the mechanism of translation of the host cell for gene expression, which makes it possible to provide the native structure of these protein compounds due to post-translational modifications [Citation19].
Endogenous synthesis of immunogens leads to their proteasomal or lysosomal lysis and subsequent binding of short peptides to MHC I or MHC class II. Thus, both the humoral and cellular components of the immune system are activated.
Due to the ability to easily replace the target gene in a DNA vaccine without changing the production technology, it becomes possible to quickly respond to the emergence of new pandemic infections, which is especially important for highly variable viruses.
DNA vaccines allow the creation of constructs encoding artificial immunogens, for example, T-cell polyepitopes containing many sequences of T-helper and cytotoxic epitopes. This makes it possible to modulate the immune response, causing not only the reactions of the humoral, but also the T-cell immunity, which are closely related to the formation of immunological memory, the elimination of viral infection, and the formation of a long-term effective response [Citation20–22].
The use of DNA vaccines is safer than traditional approaches, for example, with live attenuated vaccines, the use of which is associated with the risk of reversal of the strain to the wild type and a high incidence of side effects. In this case, there is no antivector immune response [Citation19].
DNA-based vaccines have certain limitations for their use. When DNA vaccines first entered human clinical trials, there were concerns about the theoretical possibility that they might cause autoimmune diseases and questions about their safety, mainly related to the likelihood of stable integration transfected DNA into the genome of somatic or even germ cells, which can lead to dysregulation of gene expression and mutations [Citation23]. A certain disadvantage of DNA vaccines is their low immunogenicity when administered in the form of naked plasmid DNA [Citation19,Citation24,Citation25]. A wide range of strategies have been tried to increase the immunogenicity of DNA vaccines, including packaging into liposomes, various polycationic polymers, the use of adjuvant plasmids encoding cytokines, and delivery by gene gun, electroporation, or jet injection [Citation26–29].
It should be noted that great advances have been made in the delivery of DNA vaccines using jet injection. Jet injection is a physical delivery method in which the vaccine is injected in a fraction of a second using a high-speed jet through a narrow orifice under high pressure, effectively delivering the injection into intradermal, intramuscular or subcutaneous tissue without the need for a needle [Citation30–32]. Jet injection has many advantages, leading to the use of injectors in clinical trials of DNA vaccines, including against COVID-19. The world’s first approved DNA vaccine, ZyCoV-D, is administered using the PharmaJet Tropis® needle-free injection system, which is licensed by WHO for human use [Citation33,Citation34].
However, electroporation is of particular interest. This method allows to effectively activate the T-cell response, and many studies are being conducted aimed at the accessibility and ease of use of this method for mass purposes. Electroporation is based on the action of an electric current on the cell membrane in order to form membrane pores for enhanced absorption of nucleic acid by the cell, which leads to a significant increase in the immunogenicity of DNA vaccines. By combining conventional methods of administration with electroporation, researchers achieve an increase in the level of the immune response by several orders of magnitude [Citation35,Citation36].
Electroporation is a method of introducing nucleic acids into cells either in vivo or in vitro by applying short electrical pulses to induce temporary and reversible permeabilization of the cell membrane [Citation37]. Since the mid-1960s, the application of electric fields to living cells has been explored for the purpose of delivering macromolecules, including nucleic acids, into the cell [Citation38–40]. Since the pioneering publication of Neumann et al. in 1982, in vivo electropermeabilization (also called electroporation) emerged as a promising tool for the transfer of drugs and nucleic acids due to its low cost, safety in production and use [Citation41–43].
The vast majority of experiments conducted over the past four decades have focused on optimizing the electrical pulse protocol and other parameters, such as the use of hyaluronidase, evaluating the interval between injection of plasmid DNA and delivery of electrical pulses, electrode geometry, properties of skin layers that were thought to influence efficiency and safety of electrotransfection of skeletal muscles [Citation44–48].
This review is devoted to the analysis of literature data and the development trend in the use of electroporation in the development of DNA vaccines against viral, bacterial and parasitic infections using various electroporators over the past 5 years.
2. Methodology
The research and clinical articles were retrieved from the following databases: PubMed, Scopus, Google Scholars. Used key terms correspond to the topics that observed in this review: ‘DNA vaccine,’ ‘mRNA.’
The search process covered the results from 2018 until 2023.
It should be emphasized that a universal electroporation protocol does not currently exist, so we tried to describe the variety of published protocols. Researchers adapt the electroporation conditions in a particular experiment, taking into account the specific model and electrodes of the electroporator, the type of laboratory animal, the antigen used and the composition of the vaccine.
3. Electroporation in vivo
Electroporation is a method for introducing nucleic acids into cells either in vivo or in vitro by applying short electrical pulses to induce temporary and reversible cell membrane permeabilization [Citation49]. It is believed that temporary pores are formed during electroporation depending on the transmembrane voltage [Citation50,Citation51]. During the period of membrane destabilization, nucleic acids present in the extracellular environment surrounding target cells gain access to the intracellular environment [Citation52]. After the EP pulses, the membrane is slowly resealed on a time scale from seconds to minutes. The end result of this process is that more than 100–1000-fold increase in plasmid delivery and gene expression can be achieved compared to naked DNA delivery without electroporation.
Skin and muscles are attractive sites for the administration of DNA vaccines using EP. For intradermal injection, both plate electrodes and less invasive microneedle electrodes can be used [Citation53]. An important circumstance is the fact that there are many APCs in the skin. However, most often the DNA vaccine is injected into the muscles. Muscles are readily available for injection, have high transfection efficiency, and require smaller doses of DNA compared to other tissues. The syncytial nature of muscle fibers facilitates the spread of a vaccine from one site of entry to many neighboring nuclei within the same fiber. Despite the low content of APCs in muscles, it is believed that EP leads to the recruitment of immune cells and thereby initiates an immune response [Citation54].
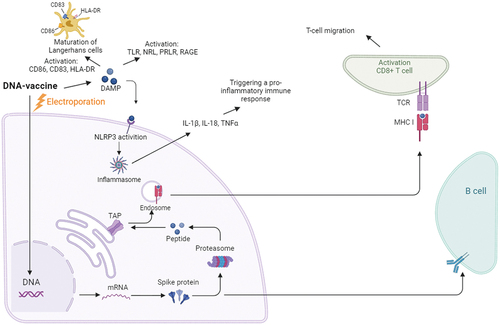
Immunization by electroporation leads to the activation of both humoral and cellular immune response, acting as an adjuvant, increasing local inflammation [Citation55]. The level of antibodies, including neutralizing ones, turns out to be several times higher than when immunized with the same doses of DNA using other delivery methods. This effect is associated, on the one hand, with an increase in the area of plasmid DNA delivery from the intercellular space, which increases the overall biosynthesis of the antigen and, accordingly, antibodies, and, on the other hand, the launch of a pro-inflammatory immune response through the secretion of IL-1β, TNFα [Citation56,Citation57] ().
An interesting mechanism is the migration of T cells and their precursors to the cathode, which, when using DC pulses of direct and reverse polarity of the same pulse length, possibly leads to an increase not only in the rate of migration, but also in the number of activated T cells [Citation58,Citation59]. Electroporation induces the maturation of Langerhans cells, as evidenced by the activation of CD86, CD83, and HLA-DR and their migration from the epidermis [Citation60].
An important role in electroporation is also played by the release of damage-associated molecular pattern molecules (DAMPs), which are normally located intracellularly, but when exposed to an impulse, they can enter the intercellular space, activating the inflammatory response. There are two hypotheses of the reasons for the release of DAMP, one of which is apoptosis of a small number of cells, the other is the release of these molecules through the pores formed by pulses. In the extracellular space, DAMPs are recognized by the Tool-like receptor (TRL), NOD-like receptor (NLR), prolactin receptor (PRLR) and receptor for advanced glycation end products (RAGE) on immune cells, which stimulates the innate immune response () [Citation61,Citation62].
Thus, electroporation activates the innate immune response by regulating the synthesis of the memory T-cell population, which in turn forms a stable adaptive T- and B-cell response [Citation60,Citation63].
4. Issues of EP and ways to solve them
A certain issue of the large-scale use of the electroporation method can be the high cost of using this technology. The introduction of electroporation into medicine is associated with the purchase of expensive equipment, as well as the need to maintain a staff of specially trained personnel [Citation64,Citation65].
In the meantime, companies trying to commercialize electroporation technologies are conducting their research in order to reduce the disadvantages of this method of delivery associated with the discomfort of the procedure itself, by leveling certain pain sensations using various EP protocols [Citation66].
Currently, more and more companies are entering the market that produce devices for electroporation of nucleic acids in vivo, are developing to find an effective, cheap and convenient electroporator design. All this in the future may lead to the active spread of electroporation as a method of delivering DNA vaccines. The most famous of them are Inovio Pharmaceuticals, BTX, BEX CO, etc. These devices are used in many scientific studies, including clinical trials [Citation10,Citation60,Citation67–71]. Forms of the immune response (Th1 and Th2) to the same antigen (DNA) depending on various physical parameters, duration and intensity of EP are poorly studied. The data presented in the literature also indicate the variability of immune responses for different DNA constructs and different EP devices [Citation72]. Not all combinations of EP and DNA vaccines are likely to produce the same result due to their different vector construct designs, equipment design, and delivery parameters. The predicted effects of immunogenicity and protection after vaccination with different EP devices should be an important area of research in the next 5 years.
Thus, the issues discussed above of electroporation are potentially solvable. Over the past 30 years, the search for optimal electroporation protocols on various model objects has been carried out [Citation73], and protection during experimental and clinical studies will be the most important indicator of the effectiveness of vaccination by means of EP.
5. The use of EP in preclinical studies and clinical trials
Confirmation that the above problems of EP can be successfully solved are the publications with the results of clinical trials of DNA vaccines delivered using EP, presented in . These examples highlight the growing interest in developing vaccine delivery systems using EP.
Table 1. Use of EP to deliver DNA vaccines in clinical trials.
Many preclinical and clinical studies have been carried out over the past 10 years, some are still ongoing with optimization of protocols and improvement of the EP procedure. During clinical trials, it was shown that EP is an effective method of delivering DNA vaccines, which makes it possible to reduce the dose of the drug and significantly enhance the immune response, and, depending on the type of antigen, redirect this immune response to the most effective form of immunological response, with predominant production of neutralizing antibodies (Th2) or antigen-specific T cells (Th1). It was not possible to achieve a significant immune response in primates such as monkeys and humans immunized with naked DNA with a simple intramuscular injection of DNA vaccines, while booster DNA vaccination with EP allowed to multiply the desired form of immunological response.
One of the main parameters studied during clinical trials was the safety and tolerability of EP. It should be taken into account that no serious side effects associated with the delivery of DNA vaccines using optimized EP protocols have been identified during long-term preclinical studies in animal models. The question of the safety of using DNA vaccines in terms of the fear of integration of the plasmid into the host genome has not received significant confirmation. In turn, judging by biodistribution studies in animal tissues, exogenous DNA is present only at the injection site (usually in the skin and muscles).
In human clinical studies, there were also no serious side effects associated with the administration of a DNA vaccine with or without EP [Citation10,Citation60,Citation67,Citation90–93]. Nevertheless, despite such a large number of studies, it is worth noting the relative pain of the electroporation procedure itself, as well as possible damage to the skin and muscle tissue at the injection site [Citation94]. According to the results of surveys of participants in clinical studies, the level of pain that occurs after a standard needle injection is much lower than with electroporation [Citation67,Citation94,Citation95]. Discomfort can also haunt a recipient of DNA vaccines for two or more weeks after immunization due to the long recovery of histopathological lesions that inevitably appear after electroporation [Citation73]. In addition, partial damage to muscle tissue can reduce the effectiveness of DNA vaccine preparations due to the lack of expression of report genes in damaged cells [Citation73].
However, transient pain associated with the intramuscular (IM) EP procedure was noted to be significantly more intense compared to pain with intradermal (ID) EP applicators [Citation96], and therefore there is a trend toward ID-EP. The reduced invasiveness of DNA delivery, lower penetration depth, and lower current settings (Amps) for optimal delivery all result in a more tolerable procedure for the patient, confirming the observation that ID-EP vaccination procedures would be comparable to conventional intradermal or intramuscular injections vaccination carried out with a needle and syringe.
Of course, before using this delivery method in studies related to the assessment of the immunogenicity of a particular DNA construct, painstaking work is required to select the optimal protocol for this procedure. It should combine an acceptable level of pain for the subject, as well as minimal histopathological abnormalities at the site of electroporation [Citation97,Citation98].
6. Delivery of candidate DNA vaccines using EP
A lot of researches has been published on the development of candidate vaccines based on DNA against viral, bacterial and parasitic infections using EP at the moment. At the same time, the variety of forms and schemes for conducting EP (the number of pulses, their duration and interval) may be important in the safety and tolerability profile of the procedure, the formation of immunological reactions and protection against viral, bacterial and parasitic infections. Below we consider the activation of immunological mechanisms when using certain types of DNA vaccines and when they are administered using EP ().
Table 2. The use of EP in the delivery of DNA vaccines against viral infections.
6.1. Delivery of DNA vaccines against viral infections via EP
A BEX CUY21EDITII electroporator was used in the studies of Petkov et al and Lambert et al, BALB/c or CB6F1 mice were immunized, which were IM injected with DNA vaccines against HIV-1 and influenza, respectively [Citation99,Citation100]. The protocols of these studies differed significantly. A pulsed pre-shock of 400 volts was used in the studies of Petkov et al, with a very short duration of 0.05 ms, followed in various variations of the experiment by 8 pulses of 50 V/10 ms: 20 ms or 100 V/50 ms: 950 ms, at +/+ or ± polarity [Citation99]. The presence of a pre-impact can significantly reduce the specific resistance of the skin, which allows increasing the amount of DNA absorbed from the intercellular space, but at the same time it causes a strong destabilization of the bilipid membrane of the cell, which can lead to serious consequences, including necrosis and tissue burns. Lambert et al optimized their protocol without the use of a pulsed pre-shock by increasing the shock voltage to 150 V using two series of 5 pulses of ± polarity [Citation100]. BALB/c mice were immunized by Kisakov et al with 100 μg of pVAXrbd DNA using a LF 650P5 5 mm tweezers electrode according to the protocol of 3 pulses of direct and reverse polarity with a voltage of 12 V at intervals of 30 ms and 950 ms, with a current limit of 45 mA [Citation97]. As a result of the studies, high levels of cellular and humoral responses were revealed, including a high level of virus-neutralizing antibodies against SARS-CoV-2 virus. The protocol used by the authors made it possible to effectively induce a cellular response to DNA vaccines; however, this protocol leads to irreversible weak thermal damage to animal tissues.
The BTX electroporator has been widely used by a number of research groups [Citation101–105]. The BALB/c mice were the main models, but in the last two studies, Syrian hamsters and white rabbits were used, respectively. A DNA vaccine encoding the S, N, E, M proteins of SARS-CoV-2 was developed in the studies of Chen et al [Citation101]. Guan et al developed a DNA vaccine encoding non-structural protein 3 (NS3) of the hepatitis C virus and used a two-needle array of electrodes at a distance of 5 mm from each other, they applied 8 pulses 27.5 V/20 ms:1s [Citation102]. At the same time, an increase in the level of the humoral response was observed, including the titer of virus-neutralizing antibodies, as well as an increase in the level of the T-cell immune response, which in turn made it possible to create sufficient activation of immunity to protect against a pathogenic virus when vaccinated animals were infected. A different protocol and EP parameters were used, since a 2-pin electrode was used in the works of Tretyakova et al [Citation103]. Rectangular pulses of 100 v/50 ms:200 ms were used, leading to the synthesis of a high titer of virus-neutralizing antibodies and protection of vaccinated animals from viral infection. Perhaps this was not the most optimal, although effective protocol. Already 10 rectangular pulses were used in studies by Chai et al on BALB/c mice and Syrian hamsters, but with a reduced voltage to 75 V/50 ms:100 ms [Citation104]. This protocol made it possible to induce a high level of Th1 response, and, first of all, a significant production of IFN-γ. At the same time, the authors observed protection against viral infection and also a fairly high level of Th2 response and the concentration of virus-specific antibodies. Dormeshkin’s study used the BTX AgilePulse, a proprietary protocol consisting of several low to high voltage pulses with variable pulse length, which promotes greater cell membrane porosity [Citation105]. Unfortunately, the details of this vaccination protocol are not disclosed by the authors of the article; at the same time, a very high level of virus-specific humoral response is noted after vaccination.
One successful company producing electroporators is Inovio Pharmaceuticals. A number of studies performed on the CELLECTRA 2000 include many studies, the peculiarity of which is that the description of protocol variability is mostly not from voltage, but from current strength or electric field strength, the differences in these studies are in the number of pulses [Citation106–116]. For example, triangulated rectangular pulses with a power of 0.1 ampere/52 ms:1 s were used in studies by Choi et al on a DNA vaccine encoding the full-length sequence of the Mayaro virus envelope [Citation106]. This vaccination protocol leads to a high level of cellular immune response, which in turn allows for the formation of protection against infection by the virus. Rectangular pulses were used through a triangular three-electrode array, with a power of 0.1 amperes/52 ms: 1s for mice of the C57BL/6 line, and for rhesus monkeys, the current strength was increased to 0.2 amperes while maintaining the rest parameters in the studies of Zhao et al when developing a DNA vaccine for the prevention of COVID-19 [Citation107]. These modifications to the immunization protocol resulted in the production of a comparable high level of humoral and cellular response. Two triangulated rectangular pulses with a current of 0.1 A were used in BALB/c mice in the development of a DNA vaccine encoding the full-length membrane (prM) and envelope (E) precursor POWV by Choi et al. As a result of the analysis of the immune response, a high the level of humoral response after 2 immunizations, as well as a high level of cellular response, which together made it possible to form protection against the POWV virus [Citation108]. The group of Muthumani et al used two different protocols in mice and rhesus monkeys to study a ZIKV-prME DNA vaccine encoding a full-length membrane progenitor (prM) plus Env (E) and a construct encoding consensus capsid proteins [Citation109]. The EP protocol for mice included 2 rectangular DC pulses of 0.1 A each, duration 52 ms, and an interval of 1 s between pulses. Protocol for rhesus monkeys: 2 rectangular pulses of direct current of 0.2 Amp, duration 52 ms and an interval of 1 s between pulses. The increase in current strength was most likely caused by an increase in the size of the animal and a change in the resistivity of the skin. A DNA vaccine encoding SARS-CoV-2 S protein (full length), SARS-CoV-2 S protein (full length) fused to a short 5mer4 peptide was delivered by Babuadze et al. using a triangular three-electrode array consisting of solid 26-gauge stainless steel electrodes, immersed ~2 mm into the muscle. The protocol, unfortunately, was not specified in the study, but a high level of antibodies (including virus-neutralizing ones) was observed [Citation110]. Muthumani et al in the development of a DNA vaccine encoding HIV immunogens, Env, studies of synthetic DNA priming were carried out using double immunization with an interval of two weeks using adaptive EP, and booster immunization of the protein was performed at a similar interval to enhance the immune response [Citation111]. For this, paired pulses were applied at a constant current of 0.2 amps/52 ms:1s for intradermal delivery and 0.5 amps/52 ms:1s for intramuscular injection. In general, according to the results of the study, the booster immunization scheme led to a significant increase in the observed antibody titer and activation of the T-cell response. These protocols were refined and the result was the use of three pulses of 0.5 ampere/52 ms:1 s on white rabbits and rhesus monkeys in the studies of Jiang et al [Citation112]. This modification of the vaccination protocol led to earlier synthesis and enhancement of cellular and humoral responses in experimental animals. The DNA vaccine encoding the Ebola virus glycoprotein developed by Patel et al delivered by EP to rhesus monkeys without disclosing the protocol, reported protection from a lethal dose of the Ebola virus [Citation112]. Similar to the study by Patel et al [Citation112], the DNA vaccine encoding the precursor of the Lassa virus glycoprotein developed by Jiang et al does not provide EP protocols, but nevertheless reported on its results a high level of virus-neutralizing antibodies that protect mice and cynomolgus monkeys from the virus [Citation112]. By Cashman et al paired pulses were used on cynomolgus monkeys with a power of 0.2 amperes/52 ms:3 s, which formed a defense that could protect experimental animals from infection with a lethal dose of the virus [Citation114]. Research by Hirao et al [Citation115], use an immunization protocol similar to that of Zhao et al [Citation107], however, cynomolgus macaques are the object of study. The similar data were obtained on the activation of the immune response in vaccinated primates in this studies, in particular, a high level of virus-neutralizing antibodies was observed that could protect animals from experimental viral infection.
A number of researchers used a TERESA electroporator (Health Technology Co., LTD, Shanghai, China) in their studies on BALB/c mice and New Zealand white rabbits [Citation117–120]. This electroporator is interesting in that the pulse is applied through two silver electrodes spaced 6 mm apart. To perform electroporation in all the presented studies on this electroporator, one protocol was used: 6 pulses with a voltage of 36 V/10 ms:50 ms. Thanks to this protocol, impressive high levels of humoral response were obtained on this electroporator, and a high level of protection of vaccinated animals against infection with a lethal dose of the virus was also revealed.
It should also be noted that the titers of produced antibodies and the level of antiviral protection are often determined by the species of the infectious agent. For example, the causative agents of a number of acute viral infections are subject to faster and more effective neutralization in vivo, while some causative agents of chronic viral infections are more resistant to the action of immunological protection factors. Thus, when comparing the effectiveness of EP vaccination protocols, the species of the infectious agent should be taken into account. Evaluating the effectiveness of EP vaccination by one indicator of the immune response (for example, by serum antibody titer) also seems insufficiently justified, since only complex and balanced factors of immunological protection can determine the effectiveness of vaccination. It seems more reliable and objective to evaluate the effectiveness of EP vaccination by infecting experimental animals with a lethal dose of an infectious agent. It is important to achieve protection of vaccinated animals using low doses of antigen, which will also indicate the effectiveness of EP vaccination. The study of various physical characteristics of EP vaccination (frequency, duration of pulses, time between pulses) should be considered by researchers in the same way as the structure of the immunogenic construct and the site of vaccine delivery through the study of the immunogenic and protective properties of immunized animals.
The TriGrid delivery system electroporator (Ichor Medical System, San Diego, CA, U.S.A.) is used by many research [Citation64,Citation121–125]. The protocols in these studies were similar to those for BALB/c and ICR mice and for New Zealand white rabbits: stimulation of the injection site was carried out with an electrical impulse of 250 V/cm at a distance between the electrodes of 5 mm for 40 ms with an interval of 400 ms. In studies on cynomolgus monkeys, electroporation protocols were not provided, which somewhat limits the possibility of discussing the results of this work. Overall, this EP vaccination protocol activates the T-cell response, enhances the production of virus-neutralizing antibodies, and provides protection against lethal viral infection.
Our review presents studies by authors using electroporators from other manufacturers, including ELGEN-MID and ELGEN (IM-EP), Vet-ePorator™, Genedrive (IGEA, Carpi, Italy) and others [Citation125–127]. Cashman et al studying the immunogenicity of a plasmid vaccine encoding a Lassa virus glycoprotein precursor in guinea pigs, used ELGEN-MID to deliver the vaccine intradermally with three pulses of 50 V/100 ms:100 ms [Citation125]. The rectangular electrode configuration (distance 10 mm by 5 mm) and the penetration depth of 5 mm ensure the distribution of the electric field over a wider skin surface and its depth. Intramuscular delivery using ELGEN (IM-EP) uses a dual pulse of 60 V/0.25 amps/60 ms:60 ms. This EP immunization protocol provides protection for vaccinated guinea pigs from a lethal dose of the virus, even after a long time period from the moment of vaccination, re-infection with a lethal dose did not cause the death of immunized animals.
Martins et al using the Vet-ePorator™ in their ferret study, delivered plasmid DNA encoding the SARS-CoV-2 S protein using a protocol of eight 100 μs rectangular pulses at a frequency of 1 Hz [Citation126]. High-frequency pulses, like other EP methods, effectively activate both humoral and cellular immune responses. The use of this EP vaccination protocol helps to reduce the isolation of infectious forms of the virus by experimental animals in the case of an asymptomatic course of the disease.
In the development of plasmid DNA encoding the GPC and NP of the Crimean-Congo hemorrhagic fever virus, which was carried out as part of the study, the team of Hawman et al used a Genedrive electroporator (IGEA, Carpi, Italy) with a 0.6 ms 600 V/cm pulse followed by a 400 ms 60 V/cm pulse using a four-electrode array at a depth of 1 cm on cynomolgus monkeys contributes to the protection of vaccinated macaques from the disease of the Crimean-Congo hemorrhagic fever, prevents viremia and reduces the load of viral RNA in tissues in cases of asymptomatic course [Citation127].
All presented protocols were aimed at achieving a high level of immune response to DNA vaccine constructs through EP vaccination. This approach to evaluating the effectiveness of EP vaccination cannot be considered optimal and requires additional study and justification depending on the species of the infectious agent. It should be noted that there is a tendency to increase the safety and tolerability of the EP procedure, however, the solution of this issue has not been completed and still remains relevant and requires additional research.
6.2. Delivery of DNA vaccines against bacterial and parasitic infections via EP
Research on the delivery of DNA vaccines against bacterial and parasitic infections is of great importance in medicine and science [Citation14,Citation128]. The problem of bacterial resistance to antibiotics becomes more acute, which leads to the search for new methods to combat them every year. A distinctive feature of the use of DNA vaccines is the ability to prevent infection due to the formation of stable anti-infective immunity.
There are quite a few studies on vaccination by means of EP, in which the most commonly used are electroporators from BTX, CELLECTRA 2000, TriGrid, presented in .idass
Table 3. The use of EP in the delivery of DNA vaccines against bacterial and parasitic infections.
DNA vaccines against various bacterial and parasitic diseases were delivered via EP using a BTX electroporator in BALB/c, C57BL/6 mice and guinea pigs in studies [Citation37,Citation129,Citation130]. Saljoughian et al used the protocol on mice of the BALB/c line with a voltage of 63–66 V with a pulse duration of 20.9 ms, the number of pulses 8, and with an interval of 200 ms [Citation37]. This method caused an increase in the cellular and humoral response, as well as a decrease in the rate of reproduction of the Leishmania donovani parasite in experimental animals. Jain et al demonstrated enhancement of the humoral response (IgG, IgG1, IgG2a) in BALB/c mice, activation of the T-cell immune response and protection against Brucella abortus infection, due to the application of six pulses of 50 ms duration and with an interval between pulses of 200 ms at a voltage of 100 V [Citation129]. Cao et al without disclosing the details of the vaccination protocol In studies in BALB/c and C57BL/6 mice, showed impressive results in the form of a significant increase in the level of specific antibodies, and especially in the C57BL/6 mouse model. Protection against Mycobacterium tuberculosis and a decrease in the activity of disease transmission through EP vaccination were found [Citation130]. Kim et al in their studies, in addition to mice, also used guinea pigs, according to the protocol, they used 90 V/mm, a pulse duration of 25 ms, 3 pulses with a change in polarity after each pulse, which led to an increase in humoral and cellular responses of Th2 and Th1-type, as well as protection from fatal infection when guinea pigs are infected with Bacillus anthracis [Citation131].
As mentioned earlier, a major role in the development of modern electroporation belongs to Inovio Pharmaceuticals with the CELLECTRA electroporator. Ferraro et al used rectangular pulses with a constant current of 0.1 A, each pulse lasted 52 ms with a delay of 1 s between pulses [Citation132]. This vaccination protocol contributed to an increase in the level of humoral and cellular against P. falciparum. An important aspect of EP vaccination is a high level of protection after a long time from the moment of immunization. This protocol was modified in studies by Vijayachari et al: in particular, the current strength was increased to 0.5 A, but also 3 pulses of 52 ms duration and 1 sec intervals between pulses were applied, and this led to a significant increase in the Th1-type cellular response, as well as to a significant stimulation of humoral immunity for the prevention of Leptospirosis [Citation133].
Studies by Liang et al were carried out on a TERESA electroporator with a voltage of 36 V and a frequency of 25 Hz; six pulses with a duration of 10 ms were applied at a depth of 3 mm [Citation134]. This protocol is high frequency and results in a significant increase in cellular response mediated by both CD4+ and CD8+ cells. A number of significant works, presented by researcher’s groups use the TriGrid delivery system and apply Electrode arrays (8 mm/15.5 mm/7.5 mm) with a strength of 250 V/cm [Citation135–137]. By Chen et al through EP, there is a significant increase in antibody levels, even at a low dose, of an administered DNA vaccine encoding the surface antigens of Plasmodium falciparum gametocytes and gametes, as well as a decrease in infectivity [Citation133]. In the studies of two other authors, who are also working on the creation of a vaccine against Plasmodium falciparum, the formation of high levels of antibodies is also observed, which may indicate the effectiveness of the use of EP [Citation136,Citation137].
An important work in the presented table is the study by Donate et al dedicated to the creation and delivery of a DNA vaccine encoding the Bacillus anthracis toxin-associated protein gene [Citation138]. In this study, they used a microelectrode array (MEA), which transmits an impulse through two types of electrodes: round and four-plate. The EP protocol for a round electrode consists of 8 pulses, which were applied with a voltage from 5 to 45 V, with a pulse duration of 150 ms and a frequency of 6.67 Hz. In turn, the protocol for a four-plate electrode: 8 pulses, 4 at a time, 60 V, duration 150 ms and frequency 6.67 Hz. As a result of these experiments on mice of the BALB/c line, an increase in the level of the humoral response against Bacillus anthracis was revealed.
EP protocols for the delivery of DNA vaccines against bacterial and parasitic infections in most cases are aimed at achieving significant activation of T-lymphocytes, including regulatory antigen-specific CD4+ lymphocytes and cytotoxic CD8+ cells. In addition, a high level of humoral immune response was observed, which in turn led to the formation of vaccine-mediated protection and a decrease in the infectivity of the pathogen under experimental conditions.
7. Redirecting the immune response toward the Th1 or Th2 type is an important goal of vaccination with EP
Vaccine-mediated mechanisms of immunological protection are largely determined by the species of the infectious agent. In a number of infections, the production of neutralizing antibodies is an absolutely sufficient condition for the mechanisms of vaccine-mediated protection; in other infections, joint activity of T-cells and antibodies is necessary for protection. The main task of modern research on EP is to establish the protocol and physical parameters of EP, in which there is a selective redirection of the immune response toward Th1 or Th2 responses, when using the same antigen for EP. The most promising to date are studies with EP, including clinical work, in acute viral infections, where immunological mechanisms of protection and immune correlates of protections have already been determined. The task of EP in this case is to induce the synthesis of virus-neutralizing antibodies at an earlier time and at a higher level. In the case of chronic bacterial and viral infections, vaccine-mediated protection will depend not only on humoral factors, but also on the effectiveness of the cellular immunity (CD8+ CTL). Thus, by changing the mode and the EP protocol, it is necessary to develop ways to selectively redirect the immune response and its amplitude in response to a DNA vaccine. The data presented in the review indicate this possibility. It is possible to redirect the desired response to the humoral or cellular level using the DNA vaccine design itself, the encoded antigen, and regulatory elements. For example, the attachment of a secretory domain to a target antigen will ensure its secretion from the cell and, accordingly, induce the synthesis of antibodies. Therefore, depending on the tasks set when creating vaccines, it is possible to shift the emphasis toward T-cell or humoral immunity by modifying the design and strengthening it with the help of EP.
Analyzing the presented data on the delivery of DNA vaccines against viral and parasitic infections using EP, it should be noted that this method of delivery makes it possible to induce both cellular and humoral immune responses. Using intramuscular or intradermal EP protocols, it is also possible to regulate the immune response to a DNA vaccine, either in the direction of preferential induction of an antibody response (virus-neutralizing antibodies) or a cell-mediated response (CD4+ T cells, CD8+ CTL) as the most important factors of protection against viral and bacterial infections as well as against protozoa. A number of researchers believe that ID EP is considered as a methodology that more actively induces cellular responses, while IM EP predominantly activates humoral immunity [Citation139]. Using the combined intramuscular or intradermal administration of prime-boost vaccines, it is also possible to regulate the shape and amplitude of the immune response.
The researches can be singled out as EP modes that led to effective activation of the humoral immune response [Citation97,Citation99,Citation101,Citation129]. Petkov et al used a 400 V pulse pre-shock, of a very short duration of 0.05 ms, followed by 8 pulses of 50 V/10 ms:20 ms or 100 V/50 ms:950 ms, at +/+ or ± polarity. This approach made it possible to significantly increase the induction of both links of immunity upon delivery of a DNA vaccine encoding the HIV-1 viral protease [Citation99]. The EP protocol was optimized for the delivery of the pVAXrbd plasmid encoding the SARS-CoV-2 receptor-binding domain to reduce pain and traumatic consequences, while a significant level of activation of humoral and T-cell immunity was established in the research by Kisakov et al [Citation97]. The Pfs25 DNA vaccine against Plasmodium falciparum was delivered as an immunogen using the TriGrid delivery system and Electrode arrays (8 mm/15.5 mm/7.5 mm) with an intensity of 250 V/cm in study by Chen et al [Citation101]. Jain et al in their studies on DNA vaccine encoding ribosomal protein L9 of Brucella abortus used a BTX ECM830 electroporator with an electroporation protocol: 6 pulses of 50 ms duration with an interval between pulses of 200 ms at a voltage of 100 V [Citation129]. As we can see, these researchers have been working on the creation of DNA vaccines against parasites and gram-negative bacteria. As a result, there was a significant increase in the level of antibodies that determine protection in these infections.
Liang et al was used the following protocol for the DNA vaccine against Mycobacterium tuberculosis ag85a/b bacteria: voltage 36 V and frequency 25 Hz, six pulses with a duration of 10 ms at a depth of 3 mm, which led to preferential activation of T cells [Citation134].
Thus, the presented researches show that by varying the designs and method of administering a DNA vaccine followed by EP with optimized protocols, it is possible to change and redirect the immune response in the required direction (Th1 or Th2) depending on the type of infectious agent and the requirements of protective immunity.
8. Conclusion
The data presented in this article are limited by the timing of advances in electroporation applications over the past 5 years. From the presented data, it follows that electroporation is an effective and promising method for intramuscular and intradermal delivery of DNA vaccines, in which, to date, there is a significant variety of protocols used. For example, voltage, current strength, pulse duration and frequency vary within significant limits: 10–200 V, 0.1–0.5 A, 10–52 ms10–200 ms, 10–200 Hz. The variety of protocols depends on a number of reasons. One of them is to use different types of animals, which may have different thickness of the skin and muscle tissue. The second is the presence of a number of manufacturers of electroporation devices, such as Inovio Pharmaceuticals, BTX, BEX CO, etc., and accordingly the devices have some differences. The third reason is the desire of protocol developers to optimize them in such a way as to obtain not only a high immune response, but also to achieve tolerability of the procedure itself. The data presented in the literature indicate some variability of immune responses when using DNA constructs and EP devices [Citation72]. However, there are certain criteria that protocols must meet: a certain duration and shape of the electrical impulse is required to reach a certain threshold of transmembrane voltage, allowing the phenomenon of EP to manifest itself. Thus, only cells or sections of the cell membrane that have reached the electric field threshold of this magnitude will be subjected to electroporation. It is important that these parameters have minimal trauma and low pain effect, which were displayed in the studies presented in and this review.
9. Expert opinions
Over the past two decades, electroporation has been used in a large number of clinical trials for the delivery of nucleic acid-based vaccines. Current research refutes early concerns about post-vaccination effects of EP in humans. Over the past 5 years, the use of EP in the delivery of DNA vaccines has most often been done using intramuscular administration. However, recently there has been a trend toward a more accessible target for delivery – intradermal. The skin is saturated with an abundance of immunocompetent cells, which makes it possible to reduce the doses used while maintaining effectiveness.
Undoubtedly, the next 5 years will see the continued introduction of nucleic acid-based vaccines as an effective preventive and therapeutic strategy. The number of developments of DNA vaccines against various infectious diseases will increase. A notable example was the COVID-19 pandemic, which launched the first approved DNA vaccine for humans. As a result, the total number of DNA vaccines approved for human vaccination will increase in the future. We believe that the scalability and cost-effectiveness of producing this type of vaccine allows the platform to be competitive with other vaccination strategies for both therapeutic and prophylactic vaccines, and for their application in developing or resource-poor countries.
However, due to the low immunogenicity of DNA vaccines, the problem of increasing their effectiveness will become increasingly urgent. The use of effective DNA vaccine delivery technology will be important. In this regard, there will be active development of various methods to improve efficiency.
Electroporation is considered one of the most effective delivery methods, which also has an adjuvant effect. Among all delivery methods, EP has a number of advantages, since it effectively delivers the DNA plasmid into cells, which makes it possible to reduce the dose and frequency of administration. The use of EP leads to the activation of a cascade of reactions that enhance the immune response, which may be the key to solving the low immunogenicity of DNA vaccines.
Ongoing research should be aimed at developing a universal and effective EP protocol with reduced procedural sequelae. One of the important tasks is to obtain cheap and accessible equipment for mass vaccinations. In studies, there are different conformations of electrodes (surface, needle, microneedles) acting on the site of drug administration. This causes varying degrees of activation of immune responses. Future studies may be aimed at regulating the levels of humoral and cellular responses required for protection by modifying the EP protocol. Significant efforts will be aimed at establishing the possibility of redirecting the immune response toward a Th1 or Th2 response by changing the physical parameters of the EP. This will allow for an individual and selective approach when conducting EP, depending on the type of infectious disease agent. The use of optimized protocols for delivering DNA vaccines using EP, which are less invasive and traumatic, will allow this method to become a leader among other methods of vaccine delivery.
The study of a selective prime-boost strategy will continue, with EP used to selectively activate cellular or humoral immunity and regulate the amplitude of the immune response. The use of various methods together with EP will expand the possibilities taking into account the characteristics of infectious diseases.
A promising area of application of EP will be the development of vaccines against cancer. Various approaches to performing reversible and irreversible EP, the use of DNA constructs encoding cytokines and the ability to vary the immune response will be one of the possible keys to creating drugs. However, the most important direction will be the development of EP as a method of delivering individual DNA vaccines (personal medicine), taking into account the individual characteristics of the formation of post-vaccination immunity in people with immunodeficiencies, tumor and systemic diseases. Interest in such vaccines is growing as the number of people with similar diseases in the world grows every year.
Article highlights
An advantage of electroporation is that more than 100-1000-fold increases in plasmid delivery and gene expression can be achieved compared to delivery of naked DNA without electroporation.
Immunization by electroporation leads to the activation of both humoral and cellular immune response, acting as an adjuvant, increasing local inflammation.
Depending on the tasks set when creating vaccines, the emphasis can be shifted toward T-cell or humoral immunity by modifying the design and enhancing it with EP.
There is a tendency to increase the safety and tolerability of the EP procedure, however, the solution of this problem has not been completed and still remains relevant and requires additional research.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or material discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or mending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Elizaga ML, Li SS, Kochar NK, et al. Safety and tolerability of HIV-1 multiantigen pDNA vaccine given with IL-12 plasmid DNA via electroporation, boosted with a recombinant vesicular stomatitis virus HIV gag vaccine in healthy volunteers in a randomized, controlled clinical trial. PLoS One. 2018;13(9):e0202753. doi: 10.1371/journal.pone.0202753
- Houser KV, Chen GL, Carter C, et al. Safety and immunogenicity of a ferritin nanoparticle H2 influenza vaccine in healthy adults: a phase 1 trial. Nat Med. 2022;28(2):383–391. doi: 10.1038/s41591-021-01660-8
- Hooper J, Paolino KM, Mills K, et al. A phase 2a randomized, double-blind, dose-optimizing study to evaluate the immunogenicity and safety of a bivalent DNA vaccine for hemorrhagic fever with renal syndrome delivered by intramuscular electroporation. Vaccines (Basel). 2020;8(3):1–21. doi: 10.3390/vaccines8030377
- Hannaman D, Dupuy LC, Ellefsen B, et al. A phase 1 clinical trial of a DNA vaccine for Venezuelan equine encephalitis delivered by intramuscular or intradermal electroporation. Vaccine. 2016;34(31):3607–3612. doi: 10.1016/j.vaccine.2016.04.077
- Gaudinski MR, Houser KV, Morabito KM, et al. Safety, tolerability, and immunogenicity of two Zika virus DNA vaccine candidates in healthy adults: randomised, open-label, phase 1 clinical trials. Lancet. 2018;391(10120):552–562. doi: 10.1016/S0140-6736(17)33105-7
- Ledgerwood JE, Pierson TC, Hubka SA, et al. A west Nile virus DNA vaccine utilizing a modified promoter induces neutralizing antibody in younger and older healthy adults in a phase I clinical trial. J Infect Dis. 2011;203(10):1396–1404. doi: 10.1093/infdis/jir054
- Sarwar UN, Costner P, Enama ME, et al. Safety and immunogenicity of DNA vaccines encoding ebolavirus and marburgvirus wild-type glycoproteins in a phase i clinical trial. J Infect Dis. 2015;211(4):549–557. doi: 10.1093/infdis/jiu511
- Tebas P, Kraynyak KA, Patel A, et al. Intradermal SynCon® Ebola GP DNA vaccine is temperature stable and safely demonstrates cellular and humoral immunogenicity advantages in healthy volunteers. J Infect Dis. 2019;220(3):400–410. doi: 10.1093/infdis/jiz132
- Ahn JY, Lee J, Suh YS, et al. Safety and immunogenicity of two recombinant DNA COVID-19 vaccines containing the coding regions of the spike or spike and nucleocapsid proteins: an interim analysis of two open-label, non-randomised, phase 1 trials in healthy adults. Lancet Microbe. 2022;3(3):e173–e183. doi: 10.1016/S2666-5247(21)00358-X
- Kraynyak KA, Blackwood E, Agnes J, et al. SARS-CoV-2 DNA vaccine INO-4800 induces durable immune responses capable of being boosted in a phase 1 open-label trial. J Infect Dis. 2022;225(11):1923–1932. doi: 10.1093/infdis/jiac016
- Jenkins M, Kerr D, Fayer R, et al. Serum and colostrum antibody responses induced by jet-injection of sheep with DNA encoding a Cryptosporidium parvum antigen. Vaccine. 1995;13(17):1658–1664. doi: 10.1016/0264-410X(95)00121-G
- Gül C, Karakavuk T, Karakavuk M, et al. An overview of DNA vaccines development studies against Toxoplasma gondii. Turkiye Parazitoloji Dergisi. 2022;46:253–270. doi: 10.4274/tpd.galenos.2022.02486
- Sefidi-Heris Y, Jahangiri A, Mokhtarzadeh A, et al. Recent progress in the design of DNA vaccines against tuberculosis. Drug Discov Today. 2020;25(11):1971–1987. doi: 10.1016/j.drudis.2020.09.005
- Lee J, Arun Kumar S, Jhan YY, et al. Engineering DNA vaccines against infectious diseases. Acta Biomater. 2018;80:31–47. doi: 10.1016/j.actbio.2018.08.033
- Carter EW, Kerr DE. Optimization of DNA-based vaccination in cows using green fluorescent protein and protein a as a prelude to immunization against staphylococcal mastitis. J Dairy Sci. 2003;86(4):1177–1186. doi: 10.3168/jds.S0022-0302(03)73701-1
- Scheiblhofer S, Weiss R, Thalhamer J. Genetic vaccination approaches against malaria based on the circumsporozoite protein. Wiener Klinische Wochenschrift, Supplement. 2006;118(S3):9–17. doi: 10.1007/s00508-006-0676-0
- Cai Y, Rodriguez S, Hebel H. DNA vaccine manufacture: Scale and quality. Expert Rev Vaccines. 2009;8(9):1277–1291. doi: 10.1586/erv.09.84
- Shafaati M, Saidijam M, Soleimani M, et al. A brief review on DNA vaccines in the era of COVID-19. Future Virol. 2022;17(1):49–66. doi: 10.2217/fvl-2021-0170
- Li L, Petrovsky N. Molecular mechanisms for enhanced DNA vaccine immunogenicity. Expert Rev Vaccines. 2016;15(3):313–329. doi: 10.1586/14760584.2016.1124762
- Bazhan SI, Antonets DV, Starostina EV, et al. In silico design of influenza a virus artificial epitope-based T-cell antigens and the evaluation of their immunogenicity in mice. J Biomol Struct Dyn. 2022;40(7):3196–3212. doi: 10.1080/07391102.2020.1845978
- Bazhan SI, Antonets DV, Karpenko LI, et al. In silico designed Ebola virus T-cell multi-epitope DNA vaccine constructions are immunogenic in mice. Vaccines (Basel). 2019;7.
- Karpenko LI, Bazhan SI, Eroshkin AM, et al. Artificial epitope-based immunogens in HIV-vaccine design. In: Adv HIV AIDS Control. IntechOpen; 2018. p. 205–225. doi: 10.5772/intechopen.77031
- Liu L. A comparison of plasmid DNA and mRNA as vaccine technologies. Vaccines (Basel). 2019;7(2):37. doi: 10.3390/vaccines7020037
- Braathen R, Spång HCL, Hinke DM, et al. A DNA vaccine that encodes an antigen-presenting cell-specific heterodimeric protein protects against cancer and influenza. Mol Ther Methods Clin Dev. 2020;17:378–392. doi: 10.1016/j.omtm.2020.01.007
- Kim D, Wu Y, Kim YB, et al. Advances in vaccine delivery systems against viral infectious diseases. Drug Deliv Transl Res. 2021;11(4):1401–1419. doi: 10.1007/s13346-021-00945-2
- Karpenko LI, Apartsin EK, Dudko SG, et al. Cationic polymers for the delivery of the Ebola DNA vaccine encoding artificial t-cell immunogen. Vaccines (Basel). 2020;8(4):8. doi: 10.3390/vaccines8040718
- Franck CO, Fanslau L, Bistrovic Popov A, et al. Biopolymer-based carriers for DNA vaccine design. Angew Chem Int Ed. 2021;133(24):13333–13351. doi: 10.1002/ange.202010282
- Suschak JJ, Williams JA, Schmaljohn CS. Advancements in DNA vaccine vectors, non-mechanical delivery methods, and molecular adjuvants to increase immunogenicity. Hum Vaccin Immunother. 2017;13(12):2837–2848. doi: 10.1080/21645515.2017.1330236
- Gary EN, Weiner DB. DNA vaccines: prime time is now. Curr Opin Immunol. 2020;65:21–27. doi: 10.1016/j.coi.2020.01.006
- Weniger BG, Papania MJ. Alternative vaccine delivery methods. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines: Sixth Edition; 2013. p. 1200–1231.
- Mitragotri S. Current status and future prospects of needle-free liquid jet injectors. Nat Rev Drug Discov. 2006;5(7):543–548. doi: 10.1038/nrd2076
- Wang R, Bian Q, Xu Y, et al. Recent advances in mechanical force-assisted transdermal delivery of macromolecular drugs. Int J Pharm. 2021;602:602. doi: 10.1016/j.ijpharm.2021.120598
- Momin T, Kansagra K, Patel H, et al. Safety and immunogenicity of a DNA SARS-CoV-2 vaccine (ZyCoV-D): results of an open-label, non-randomized phase I part of phase I/II clinical study by intradermal route in healthy subjects in India. EClin Med. 2021;38:101020. doi: 10.1016/j.eclinm.2021.101020
- Mallapaty S. India’s DNA COVID vaccine is a world first – more are coming. Nature. 2021;597(7875):161–162. doi: 10.1038/d41586-021-02385-x
- Petkov SP, Heuts F, Krotova OA, et al. Evaluation of immunogen delivery by DNA immunization using non-invasive bioluminescence imaging. Hum Vaccin Immunother. 2013;9(10):2228–2236. doi: 10.4161/hv.25561
- Eusébio D, Neves AR, Costa D, et al. Methods to improve the immunogenicity of plasmid DNA vaccines. Drug Discov Today. 2021;26(11):2575–2592. doi: 10.1016/j.drudis.2021.06.008
- Saljoughian N, Zahedifard F, Doroud D, et al. Cationic solid–lipid nanoparticles are as efficient as electroporation in DNA vaccination against visceral leishmaniasis in mice. Parasite Immunol. 2013;35(12):397–408. doi: 10.1111/pim.12042
- Coster HGL. A quantitative analysis of the voltage-current relationships of fixed charge membranes and the associated property of “punch-through”. Biophys J. 1965;5(5):669–686. doi: 10.1016/S0006-3495(65)86745-5
- Coster HGL, George EP, Simons R. The electrical characteristics of fixed charge membranes. Biophys J. 1969;9(5):666–684. doi: 10.1016/S0006-3495(69)86411-8
- Sale AJH, Hamilton WA. Effects of high electric fields on micro-organisms. III. Lysis of erythrocytes and protoplasts. BBA - Biomembr. 1968;163(1):37–43. doi: 10.1016/0005-2736(68)90030-8
- Neumann E, Schaefer-Ridder M, Wang Y, et al. Gene transfer into mouse lyoma cells by electroporation in high electric fields. EMBO J. 1982;1(7):841–845. doi: 10.1002/j.1460-2075.1982.tb01257.x
- Davis HL, Whalen RG, Demeneix BA. Direct gene transfer into skeletal muscle in vivo: factors affecting efficiency of transfer and stability of expression. Hum Gene Ther. 1993;4(2):151–159. doi: 10.1089/hum.1993.4.2-151
- Neumann E, Kakorin S, Tœnsing K. Fundamentals of electroporative delivery of drugs and genes. Bioelectrochem Bioenerg. 1999;48(1):3–16. doi: 10.1016/S0302-4598(99)00008-2
- McMahon JM, Signori E, Wells KE, et al. Optimisation of electrotransfer of plasmid into skeletal muscle by pretreatment with hyaluronidase – increased expression with reduced muscle damage. Gene Ther. 2001;8(16):1264–1270. doi: 10.1038/sj.gt.3301522
- Mennuni C, Calvaruso F, Zampaglione I, et al. Hyaluronidase increases electrogene transfer efficiency in skeletal muscle. Hum Gene Ther. 2002;13(3):355–365. doi: 10.1089/10430340252792495
- Vilquin JT, Kennel PF, Paturneau-Jouas M, et al. Electrotransfer of naked DNA in the skeletal muscles of animal models of muscular dystrophies. Gene Ther. 2001;8(14):1097–1107. doi: 10.1038/sj.gt.3301484
- Gollins H, McMahon J, Wells KE, et al. High-efficiency plasmid gene transfer into dystrophic muscle. Gene Ther. 2003;10(6):504–512. doi: 10.1038/sj.gt.3301927
- Vicat JM, Boisseau S, Jourdes P, et al. Brief report: muscle transfection by electroporation with high-voltage and short-pulse currents provides high-level and long-lasting gene expression. Hum Gene Ther. 2000;11(6):909–916. doi: 10.1089/10430340050015518
- Sardesai NY, Weiner DB. Electroporation delivery of DNA vaccines: prospects for success. Curr Opin Immunol. 2011;23(3):421–429. . doi: 10.1016/j.coi.2011.03.008
- Cukjati D, Batiuskaite D, André F, et al. Real time electroporation control for accurate and safe in vivo non-viral gene therapy. Bioelectrochemistry. 2007;70(2):501–507. doi: 10.1016/j.bioelechem.2006.11.001
- Trollet C, Bloquel C, Scherman D, et al. Electrotransfer into Skeletal Muscle for Protein Expression. Curr Gene Ther. 2006;6. doi: 10.1186/1472-6750-6-16
- Becker SM, Kuznetsov AV. Local temperature rises influence in vivo electroporation pore development: A numerical stratum corneum lipid phase transition model. J Biomech Eng. 2007;129(5):712–721. doi: 10.1115/1.2768380
- Daugimont L, Baron N, Vandermeulen G, et al. Hollow microneedle arrays for intradermal drug delivery and DNA electroporation. J Membr Biol. 2010;236(1):117–125. doi: 10.1007/s00232-010-9283-0
- Gothelf A, Gehl J. What you always needed to know about electroporation based DNA vaccines. Hum Vaccin Immunother. 2012;8(11):1694–1702. doi: 10.4161/hv.22062
- Adam L, Tchitchek N, Todorova B, et al. Innate molecular and cellular signature in the skin preceding long-lasting T cell responses after electroporated DNA vaccination. J Immunol. 2020;204(12):3375–3388. doi: 10.4049/jimmunol.1900517
- Nold-Petry CA, Nold MF, Zepp JA, et al. IL-32–dependent effects of IL-1β on endothelial cell functions. Proc Natl Acad Sci U S A Internet. 2009 [cited 2023 Oct 6];106(10):3883. 10.1073/pnas.0813334106
- Dolgachev V, Panicker S, Balijepalli S, et al. Electroporation-mediated delivery of FER gene enhances innate immune response and improves survival in a murine model of pneumonia. Gene Ther. 2018;25(5):359–375. doi: 10.1038/s41434-018-0022-y
- Arnold CE, Rajnicek AM, Hoare JI, et al. Physiological strength electric fields modulate human T cell activation and polarisation. Sci Rep. 2019;9(1). doi: 10.1038/s41598-019-53898-5
- Chiarella P, Massi E, De Robertis M, et al. Electroporation of skeletal muscle induces danger signal release and antigen-presenting cell recruitment independently of DNA vaccine administration. Expert Opin Biol Ther. 2008;8(11):1645–1657. doi: 10.1517/14712598.8.11.1645
- Tebas P, Yang SP, Boyer JD, et al. Safety and immunogenicity of INO-4800 DNA vaccine against SARS-CoV-2: a preliminary report of an open-label, phase 1 clinical trial. EClinicalMedicine. 2021;31:31. doi: 10.1016/j.eclinm.2020.100689
- Polajzer T, Jarm T, Miklavcic D. Analysis of damage-associated molecular pattern molecules due to electroporation of cells in vitro. Radiol Oncol. 2020;54(3):317–328. doi: 10.2478/raon-2020-0047
- Schultheis K, Smith TRF, Kiosses WB, et al. Delineating the cellular mechanisms associated with skin electroporation. Hum Gene Ther Methods. 2018;29(4):177–188. doi: 10.1089/hgtb.2017.105
- Emming S, Bianchi N, Polletti S, et al. A molecular network regulating the proinflammatory phenotype of human memory T lymphocytes. Nat Immunol. 2020;21(4):388–399. doi: 10.1038/s41590-020-0622-8
- Peletta A, Prompetchara E, Tharakhet K, et al. DNA vaccine administered by cationic lipoplexes or by in vivo electroporation induces comparable antibody responses against sars-cov-2 in mice. Vaccines (Basel). 2021;9(8):874. doi: 10.3390/vaccines9080874
- Rakoczy K, Kisielewska M, Sędzik M, et al. Electroporation in clinical applications—the potential of Gene Electrotransfer and Electrochemotherapy. Appl Sci (Switzerland). 2022;12(21):10821. doi: 10.3390/app122110821
- Saade F, Petrovsky N. Technologies for enhanced efficacy of DNA vaccines. Expert Rev Vaccines. 2012;11(2):189–209. doi: 10.1586/erv.11.188
- Modjarrad K, Roberts CC, Mills KT, et al. Safety and immunogenicity of an anti-middle East respiratory syndrome coronavirus DNA vaccine: a phase 1, open-label, single-arm, dose-escalation trial. Lancet Infect Dis. 2019;19(9):1013–1022. doi: 10.1016/S1473-3099(19)30266-X
- Andrade VM, Christensen-Quick A, Agnes J, et al. INO-4800 DNA vaccine induces neutralizing antibodies and T cell activity against global SARS-CoV-2 variants. NPJ Vaccin. 2021;6(1):6. doi: 10.1038/s41541-021-00384-7
- Krotova O, Starodubova E, Petkov S, et al. Consensus HIV-1 FSU-A integrase gene variants electroporated into mice induce polyfunctional antigen-specific CD4+ and CD8+ T cells. PLoS One. 2013;8(5):e62720. doi: 10.1371/journal.pone.0062720
- Latanova AA, Petkov S, Kilpelainen A, et al. Codon optimization and improved delivery/immunization regimen enhance the immune response against wild-Type and drug-resistant HIV-1 reverse transcriptase, preserving its Th2-polarity. Sci Rep. 2018;8(1). doi: 10.1038/s41598-018-26281-z
- Leroy LA, Mac DA, Kandlur A, et al. Cytokine adjuvants IL-7 and IL-15 improve humoral responses of a SHIV LentiDNA vaccine in animal models. Vaccines (Basel). 2022;10(3):10. doi: 10.3390/vaccines10030461
- Brisse M, Vrba SM, Kirk N, et al. Emerging concepts and technologies in vaccine development. Front Immunol. 2020;11: doi: 10.3389/fimmu.2020.583077
- Sokołowska E, Błachnio-Zabielska AU. A critical review of electroporation as a plasmid delivery system in mouse skeletal muscle. Int J Mol Sci. 2019;20(11):2776. doi: 10.3390/ijms20112776
- VGX-3100 and electroporation in treating Patients with HIV-Positive high-grade anal lesions | ClinicalTrials.Gov [Internet]. [cited 2023 Oct 6]. Available from: https://clinicaltrials.gov/study/NCT03603808?tab=table.
- Safety, Tolerability and Immunogenicity of INO-4800 for COVID-19 in Healthy Volunteers | ClinicalTrials.Gov [Internet]. [cited 2023 Oct 6]. Available from: https://clinicaltrials.gov/study/NCT04336410?tab=table.
- A study to evaluate Safety & immunogenicity of SARS-CoV-2 DNA vaccine delivered intramuscularly followed by electroporation for COVID-19 | ClinicalTrials.Gov [Internet]. [cited 2023 Oct 6]. Available from: https://clinicaltrials.gov/study/NCT05102643?tab=table.
- A first-in-human study to evaluate Safety, tolerability, reactogenicity, and immunogenicity of JNJ-64300535, a DNA vaccine, administered by electroporation-mediated intramuscular injection, in participants with chronic hepatitis B who are on stable Nucleos(t)Ide therapy and virologically suppressed | ClinicalTrials.Gov [Internet]. [cited 2023 Oct 6]. Available from: https://clinicaltrials.gov/study/NCT03463369?tab=table.
- Study of PENNVAXTM-B (gag, Pol, Env) + electroporation in HIV-1 infected Adult participants | ClinicalTrials.Gov [Internet]. [cited 2023 Oct 6].
- Safety, tolerability and immunogenicity of INO-4800 followed by electroporation in healthy volunteers for COVID19 | ClinicalTrials.Gov [Internet]. [cited 2023 Oct 6]. Available from: https://clinicaltrials.gov/study/NCT04447781?tab=table.
- Safety and immunogenicity of HIV DNA-C CN54ENV and recombinant HIV CN54gp140 vaccines in healthy volunteers | ClinicalTrials.Gov [Internet]. [cited 2023 Oct 6]. Available from: https://clinicaltrials.gov/study/NCT02589795?tab=table.
- GLS-5700 in Healthy Volunteers | ClinicalTrials.gov [Internet]. [cited 2023 Nov 15]. Available from: https://clinicaltrials.gov/study/NCT02809443.
- Dose-finding, Safety study of plasmid DNA Therapeutic vaccine to treat cervical intraepithelial neoplasia | ClinicalTrials.Gov [Internet]. [cited 2023 Nov 15]. Available from: https://clinicaltrials.gov/study/NCT02139267.
- The Safety and immunogenicity of the DNA-GTU vaccine administered to HIV-infected Patients on ART vs placebo | ClinicalTrials.Gov [Internet]. [cited 2023 Nov 15]. Available from: https://clinicaltrials.gov/study/NCT02457689?tab=table.
- Study of a DNA-based Venezuelan equine encephalitis virus DNA vaccine administered by electroporation in healthy volunteers | ClinicalTrials.Gov [Internet]. [cited 2023 Nov 15]. Available from: https://clinicaltrials.gov/study/NCT01984983?tab=table.
- A phase II trial to assess the Safety and immunogenicity of DNA priming administered by the ID Zetajet® with or without ID derma VaxTM electroporation followed by IM MVA boosting in healthy volunteers in Tanzania and Mozambique | ClinicalTrials.Gov [Internet]. [cited 2023 Nov 15]. Available from: https://clinicaltrials.gov/study/NCT01697007?tab=table.
- Evaluating the Safety of and immune response to an HIV vaccine followed by booster, administered by two devices, in HIV-Uninfected adults | ClinicalTrials.Gov [Internet]. [cited 2023 Nov 15]. Available from: https://clinicaltrials.gov/study/NCT01260727.
- A study of DNA vaccine with electroporation for the prevention of disease caused by H1 and H5 influenza virus | ClinicalTrials.Gov [Internet]. [cited 2023 Oct 6]. Available from: https://clinicaltrials.gov/study/NCT01405885?tab=table.
- Study of PENNVAXTM-B (gag, Pol, Env) + electroporation in HIV-1 infected Adult participants | ClinicalTrials.Gov [Internet]. [cited 2023 Nov 15]. Available from: https://clinicaltrials.gov/study/NCT01082692?tab=table.
- Study of VGX-3400X, H5N1 avian influenza virus DNA plasmid + electroporation in healthy adults | ClinicalTrials.Gov [Internet]. [cited 2023 Nov 15]. Available from: https://clinicaltrials.gov/study/NCT01142362.
- Alter G, Yu J, Liu J, et al. Immunogenicity of Ad26.COV2.S vaccine against SARS-CoV-2 variants in humans. Nature. 2021;596(7871):268–272. doi: 10.1038/s41586-021-03681-2
- Martin JE, Sullivan NJ, Enama ME, et al. A DNA vaccine for Ebola virus is safe and immunogenic in a phase I clinical trial. Clin Vaccin Immunol. 2006;13(11):1267–1277. doi: 10.1128/CVI.00162-06
- Poland GA, Ovsyannikova IG, Kennedy RB. SARS-CoV-2 immunity: review and applications to phase 3 vaccine candidates. Lancet. 2020;396(10262):1595–1606. doi: 10.1016/S0140-6736(20)32137-1
- Haidari G, Day S, Wood M, et al. The Safety and immunogenicity of GTU®MultiHIV DNA vaccine delivered by Transcutaneous and intramuscular injection with or without electroporation in HIV-1 positive subjects on suppressive ART. Front Immunol. 2019;10:10. doi: 10.3389/fimmu.2019.02911
- Therapeutic vaccination in treated HIV disease | ClinicalTrials.Gov [Internet]. [cited 2023 Oct 6]. Available from: https://clinicaltrials.gov/study/NCT03606213?a=12.
- Hettinga J, Carlisle R. Vaccination into the dermal compartment: Techniques, challenges, and prospects. Vaccines (Basel). 2020;8(3):534. doi: 10.3390/vaccines8030534
- Roos AK, Eriksson F, Timmons JA, et al. Skin electroporation: Effects on transgene expression, DNA persistence and local tissue environment. PLoS One. 2009;4(9):4. doi: 10.1371/journal.pone.0007226
- Kisakov DN, Kisakova LA, Borgoyakova MB, et al. Optimization of in vivo electroporation conditions and delivery of DNA vaccine encoding SARS-CoV-2 RBD using the determined protocol. Pharmaceutics. 2022;14(11):14. doi: 10.3390/pharmaceutics14112259
- Fusco R, Di Bernardo E, D’Alessio V, et al. Reduction of muscle contraction and pain in electroporation-based treatments: an overview. World J Clin Oncol. 2021;12(5):367–381. doi: 10.5306/wjco.v12.i5.367
- Petkov S, Kilpeläinen A, Bayurova E, et al. HIV-1 protease as DNA immunogen against Drug resistance in HIV-1 infection: DNA Immunization with Drug resistant HIV-1 protease protects mice from challenge with protease-expressing cells. Cancers (Basel). 2022;15(1):15. doi: 10.3390/cancers15010238
- Lambert L, Kinnear E, McDonald JU, et al. DNA vaccines encoding antigen targeted to MHC class II induce influenza-specific CD8+ T cell responses, enabling faster resolution of influenza disease. Front Immunol. 2016;7: doi: 10.3389/fimmu.2016.00321
- Chen J, Huang B, Deng Y, et al. Synergistic immunity and protection in mice by co-immunization with DNA vaccines encoding the spike protein and other structural proteins of SARS-CoV-2. Vaccines (Basel). 2023;11(2):11. doi: 10.3390/vaccines11020243
- Guan J, Deng Y, Chen H, et al. Priming with two DNA vaccines expressing hepatitis C virus NS3 protein targeting dendritic cells elicits superior heterologous protective potential in mice. Arch Virol. 2015;160(10):2517–2524. doi: 10.1007/s00705-015-2535-7
- Tretyakova I, Hearn J, Wang E, et al. DNA vaccine initiates replication of live attenuated chikungunya virus in vitro and elicits protective immune response in mice. J Infect Dis. 2014;209(12):1882–1890. doi: 10.1093/infdis/jiu114
- Chai KM, Tzeng TT, Shen KY, et al. DNA vaccination induced protective immunity against sars cov-2 infection in hamsterss. PLoS Negl Trop Dis. 2021;15(5):15. doi: 10.1371/journal.pntd.0009374
- Dormeshkin D, Katsin M, Stegantseva M, et al. Design and immunogenicity of SARS-CoV-2 DNA vaccine encoding RBD-PVXCP fusion protein. Vaccines (Basel). 2023;11(6):11. doi: 10.3390/vaccines11061014
- Choi H, Kudchodkar SB, Reuschel EL, et al. Protective immunity by an engineered DNA vaccine for Mayaro virus. PLoS Negl Trop Dis. 2019;13(2):e0007042. doi: 10.1371/journal.pntd.0007042
- Zhao G, Zhang Z, Ding Y, et al. A DNA vaccine encoding the full-length spike protein of beta variant (B.1.351) elicited broader cross-reactive immune responses against other SARS-CoV-2 variants. Vaccines (Basel). 2023;11(3):11. doi: 10.3390/vaccines11030513
- Choi H, Kudchodkar SB, Ho M, et al. A novel synthetic DNA vaccine elicits protective immune responses against Powassan virus. PLoS Negl Trop Dis. 2020;14(10):14. doi: 10.1371/journal.pntd.0008788
- Muthumani K, Griffin BD, Agarwal S, et al. In vivo protection against ZIKV infection and pathogenesis through passive antibody transfer and active immunisation with a prMenv DNA vaccine. NPJ Vaccin. 2016;1(1):1. doi: 10.1038/npjvaccines.2016.21
- Babuadze GG, Fausther-Bovendo H, de LaVega MA, et al. Two DNA vaccines protect against severe disease and pathology due to SARS-CoV-2 in Syrian hamsters. NPJ Vaccin. 2022;7(1). doi: 10.1038/s41541-022-00461-5
- Muthumani K, Wise MC, Broderick KE, et al. HIV-1 Env DNA vaccine plus protein boost delivered by EP expands B- and T-cell responses and neutralizing phenotype in vivo. PLoS One. 2013;8(12):e84234. doi: 10.1371/journal.pone.0084234
- Jiang J, Ramos SJ, Bangalore P, et al. Integration of needle-free jet injection with advanced electroporation delivery enhances the magnitude, kinetics, and persistence of engineered DNA vaccine induced immune responses. Vaccine. 2019;37(29):3832–3839. doi: 10.1016/j.vaccine.2019.05.054
- Patel A, Reuschel EL, Kraynyak KA, et al. Protective efficacy and long-term immunogenicity in cynomolgus macaques by Ebola virus glycoprotein synthetic DNA vaccines. J Infect Dis. 2019;219(4):544–555. doi: 10.1093/infdis/jiy537
- Cashman KA, Wilkinson ER, Shaia CI, et al. A DNA vaccine delivered by dermal electroporation fully protects cynomolgus macaques against Lassa fever. Hum Vaccin Immunother. 2017;13(12):2902–2911. doi: 10.1080/21645515.2017.1356500
- Hirao LA, Draghia-Akli R, Prigge JT, et al. Multivalent smallpox DNA vaccine delivered by intradermal electroporation drives protective immunity in nonhuman primates against lethal monkeypox challenge. J Infect Dis. 2011;203(1):95–102. doi: 10.1093/infdis/jiq017
- Jiang J, Banglore P, Cashman KA, et al. Immunogenicity of a protective intradermal DNA vaccine against Lassa virus in cynomolgus macaques. Hum Vaccin Immunother. 2019;15(9):2066–2074. doi: 10.1080/21645515.2019.1616499
- Xie P, Li Y, Li Y, et al. Immune effect of a Newcastle disease virus DNA vaccine with IL-12 as a molecular adjuvant delivered by electroporation. Arch Virol. 2020;165(9):1959–1968. doi: 10.1007/s00705-020-04669-5
- Sheng Z, Chen H, Feng K, et al. Electroporation-mediated Immunization of a candidate DNA vaccine expressing Dengue virus serotype 4 prM-E antigen confers long-term protection in mice. Virol Sin. 2019;34(1):88–96. doi: 10.1007/s12250-019-00090-8
- Sheng Z, Gao N, Cui X, et al. Electroporation enhances protective immune response of a DNA vaccine against Japanese encephalitis in mice and pigs. Vaccine. 2016;34(47):5751–5757. doi: 10.1016/j.vaccine.2016.10.001
- Chen H, Zheng X, Wang R, et al. Immunization with electroporation enhances the protective effect of a DNA vaccine candidate expressing prME antigen against dengue virus serotype 2 infection. Clin Immunol. 2016;171:41–49. doi: 10.1016/j.clim.2016.08.021
- Prompetchara E, Ketloy C, Keelapang P, et al. Induction of neutralizing antibody response against four dengue viruses in mice by intramuscular electroporation of tetravalent DNA vaccines. PLoS One. 2014;9(6):e92643. doi: 10.1371/journal.pone.0092643
- Prompetchara E, Ketloy C, Tharakhet K, et al. DNA vaccine candidate encoding SARS-CoV-2 spike proteins elicited potent humoral and Th1 cell-mediated immune responses in mice. PLoS One. 2021;16(3):e0248007. doi: 10.1371/journal.pone.0248007
- Mucker EM, Golden JW, Hammerbeck CD, et al. A nucleic acid-based orthopoxvirus vaccine targeting the vaccinia virus L1, A27, B5, and A33 proteins protects rabbits against lethal rabbitpox virus aerosol challenge. J Virol. 2022;96(3). doi: 10.1128/JVI.01504-21
- De PD, Van GE, Chen A, et al. A therapeutic hepatitis b virus DNA vaccine induces specific immune responses in mice and non-human primates. Vaccines (Basel). 2021;9(9):969. doi: 10.3390/vaccines9090969
- Cashman K, Broderick K, Wilkinson E, et al. Enhanced efficacy of a codon-optimized DNA vaccine encoding the glycoprotein precursor gene of Lassa virus in a Guinea pig disease model when delivered by Dermal Electroporation. Vaccines (Basel). 2013;1(3):262–277. doi: 10.3390/vaccines1030262
- Martins M, Do Nascimento GM, Conforti A, et al. A linear SARS-CoV-2 DNA vaccine candidate reduces virus shedding in ferrets. Arch Virol. 2023;168(4):168. doi: 10.1007/s00705-023-05746-1
- Hawman DW, Ahlén G, Appelberg KS, et al. A DNA-based vaccine protects against Crimean-Congo haemorrhagic fever virus disease in a cynomolgus macaque model. Nat Microbiol. 2020;6(2):187–195. doi: 10.1038/s41564-020-00815-6
- Ivory C, Chadee K. DNA vaccines: designing strategies against parasitic infections. Genet Vaccines Ther. 2004;2(1):17. doi: 10.1186/1479-0556-2-17
- Jain S, Afley P, Dohre SK, et al. Evaluation of immunogenicity and protective efficacy of a plasmid DNA vaccine encoding ribosomal protein L9 of Brucella abortus in BALB/c mice. Vaccine. 2014;32(35):4537–4542. doi: 10.1016/j.vaccine.2014.06.012
- Cao Y, Hayashi CTH, Zavala F, et al. Effective functional immunogenicity of a DNA vaccine combination delivered via in vivo electroporation targeting malaria infection and transmission. Vaccines (Basel). 2022;10(7):10. doi: 10.3390/vaccines10071134
- Kim NY, Chang DS, Kim Y, et al. Enhanced immune response to DNA vaccine encoding Bacillus anthracis PA-D4 protects mice against anthrax spore challenge. PLoS One. 2015;10(10):10. doi: 10.1371/journal.pone.0139671
- Ferraro B, Talbott KT, Balakrishnan A, et al. Inducing humoral and cellular responses to multiple sporozoite and liver-stage malaria antigens using exogenous plasmid DNA. Infect Immun. 2013;81(10):3709–3720. doi: 10.1128/IAI.00180-13
- Vijayachari P, Vedhagiri K, Mallilankaraman K, et al. Immunogenicity of a novel enhanced consensus DNA vaccine encoding the leptospiral protein LipL45. Hum Vaccin Immunother. 2015;11(8):1945–1953. doi: 10.1080/21645515.2015.1047117
- Liang Y, Cui L, Xiao L, et al. Immunotherapeutic effects of different doses of Mycobacterium tuberculosis ag85a/b DNA vaccine delivered by Electroporation. Front Immunol. 2022;13: doi: 10.3389/fimmu.2022.876579
- Datta D, Bansal GP, Gerloff DL, et al. Immunogenicity and malaria transmission reducing potency of Pfs48/45 and Pfs25 encoded by DNA vaccines administered by intramuscular electroporation. Vaccine. 2017;35(2):264–272. doi: 10.1016/j.vaccine.2016.11.072
- Datta D, Bansal GP, Kumar R, et al. Evaluation of the impact of codon optimization and N-linked glycosylation on functional immunogenicity of Pfs25 DNA vaccines delivered by in vivo electroporation in preclinical studies in mice. Clin Vaccin Immunol. 2015;22(9):1013–1019. doi: 10.1128/CVI.00185-15
- Kumar R, Nyakundi R, Kariuki T, et al. Functional evaluation of malaria Pfs25 DNA vaccine by in vivo electroporation in olive baboons. Vaccine. 2013;31(31):3140–3147. doi: 10.1016/j.vaccine.2013.05.006
- Donate A, Heller R. Assessment of delivery parameters with the multi-electrode array for development of a DNA vaccine against Bacillus anthracis. Bioelectrochemistry. 2013;94:1–6. doi: 10.1016/j.bioelechem.2013.04.004
- Broderick KE, Humeau LM. Electroporation-enhanced delivery of nucleic acid vaccines. Expert Rev Vaccines. 2015;14(2):195–204. doi: 10.1586/14760584.2015.990890