ABSTRACT
Introduction
This paper summarizes the safety and immunogenicity data of Influvac Tetra across all age groups starting from 6 months of age, obtained during its clinical development program.
Areas covered
The article covers the clinical development program of Influvac Tetra based on five registration studies that included different age groups, different comparators, and participants from Europe and Asia. Safety and immunogenicity were assessed in all studies and in one study, the efficacy of Influvac Tetra was assessed.
Expert opinion
Seasonal influenza is a vaccine-preventable disease that can cause serious complications. Several types of influenza vaccines are available, including egg-based (standard dose, high dose, and adjuvanted), cell-based, and recombinant. The COVID-19 pandemic has stimulated innovation in the development such as mRNA vaccines. However, these vaccines are still in development and the true value still has to be proven. Regardless of the type of vaccine, it is also important to increase overall vaccination coverage. ECDC recommends that EU Member States implement action plans and policies aimed at reaching 75% coverage in at-risk groups and healthcare workers. Even so, vaccine coverage is still far from recommended.
1. Introduction
Influenza is a highly contagious respiratory disease. It remains a healthcare challenge as immunity acquired in one season may fail in another season due to changing viral antigenicity and waning immunity [Citation1]. Despite an increasing trend in testing and awareness, flu still represents a major burden; worldwide, annual seasonal influenza epidemics result in an estimated three to five million cases of severe illness and approximately 290,000 to 650,000 deaths [Citation2]. In the European Union (EU), up to 50 million symptomatic cases occur each year [Citation3], with more than 1.4 million hospitalizations and 23,000 deaths caused by influenza-related lower respiratory tract infections per year [Citation4]. The disease burden is greater in low- and middle-income countries than in high-income countries [Citation5].
Pneumonia is the most common complication, affecting approximately 30–40% of hospitalized patients with laboratory-confirmed influenza: these patients are more likely to be young children (<5 years old), older adults (≥65 years old), Caucasian, and nursing home residents; have chronic lung or heart disease and a history of smoking; and are more commonly immunocompromised [Citation6].
As seen during the 2022–2023 flu season in Europe, seasonal flu started earlier and was more prolonged than the previous four seasons [Citation7], highlighting that influenza should not be forgotten and urging effective actions to control it [Citation8].
Annual vaccination remains the most effective method for the prevention and control of influenza, for individual protection and beyond; for example, to safeguard health systems and reduce transmission [Citation1]. A 2012 study showed that influenza vaccination reduced flu-related hospitalizations by 71% among adults of all ages, 77% in those ≥50 years, and 62% in the pediatric population (aged six months to 17 years) [Citation9]. More recent data (2018) showed that vaccination among adults reduces the risk of ICU admission by 82% [Citation10], supporting the effectiveness of influenza vaccine programs.
In cases where the vaccination does not completely prevent infection, studies have shown that vaccination reduces the duration and severity of illness [Citation11–13]. Furthermore, vaccination directly protects the vaccinated subjects, reduces transmissibility in the overall population, and indirectly increases the protection of vulnerable subjects.
Parallelly, an increase in the uptake of influenza vaccination can improve public health concerns, such as reducing unnecessary antibiotic prescriptions, especially during flu peak, as shown by a US retrospective observational study that estimated that a 10% increase in influenza vaccination rates was associated with a 6.5% reduction in antibiotic prescription rates between January and March, equivalent to 14.2 fewer prescriptions per 1000 individuals, especially relevant in children and the elderly [Citation14].
The World Health Organization (WHO), along with various international guidelines, recommends annual influenza vaccination for all people over 6 months of age, especially those at higher risk for hospitalization and death, such as children, those affected by chronic medical conditions or immunodeficiencies, the elderly, pregnant women, or those in contact with them, such as healthcare workers, and nursing home residents, in which it is a priority to prevent the primary infection, mitigate the disease severity, or avoid viral transmission [Citation1,Citation15–17]. The WHO guidelines and the results of a recent survey have shown that there is a need to improve vaccine coverage [Citation1,Citation18]. Nonetheless, the influenza vaccination rates reported by the European countries are poor among high-risk groups, and extremely low in non-at-risk groups. By increasing the vaccination rates in Europe to 75% among high-risk populations, it was estimated that it was possible to prevent up to 1.7 million cases of seasonal flu, avoid more than 30,000 hospitalizations and 750,000 physician visits, and save more than one million lost days of work [Citation19].
With this background, it is important to have effective, safe, and accessible flu vaccines that reduce the clinical and public health burden of the disease.
The influenza vaccine brand Influvac (Abbott, Weesp, The Netherlands) has been available on the market since 1950. It is available in trivalent (trivalent influenza vaccine, TIV, branded Influvac®) and quadrivalent (quadrivalent influenza vaccine, QIV, branded Influvac® Tetra) formulations. TIVs contain two A lineages (H1N1 and H3N2) and only one B lineage, thus providing little or limited protection against diseases caused by the other B lineage. This is especially important during years of mismatch between the chosen and circulating strains because the two influenza virus B lineages are antigenically distinct and cross-protection is limited [Citation20,Citation21]. Indeed, in 6 out of 12 seasons between 2001 and 2012 in the United States [Citation21] and in 5 out of 10 seasons between 2001 and 2011 in Europe [Citation22], the chosen lineage was different from the circulating lineage, causing mismatch and suboptimal protection. Moreover, it was shown that the odds of mortality (both influenza-attributable and all-cause) are significantly greater with influenza B than with influenza A [Citation23]. Furthermore, average per-patient productivity-loss costs were greater in seasons with influenza B mismatches [Citation24].
To address the mismatch issue, in 2012, the World Health Organization (WHO) recommended the production of vaccines that include strains from both B lineage, B/Victoria, and B/Yamagata lineages, which led to the development of QIVs that have the potential to increase overall vaccine effectiveness (VE) in years of mismatch or when both lineages co-circulate. In 2013, the WHO issued its first guidance recommending that influenza vaccines include viruses of both B strains. Injectable QIVs have been available in the U.S.A. since 2012 [Citation20] and in some EU/EEA countries since the 2014/2015 season [Citation25]. Globally, Influvac Tetra is registered in 50 countries, and in many countriesQIVs are becoming increasingly available. In line with the WHO position paper, national influenza vaccination recommendations should not be limited to TIVs as QIVs may have a positive cost/benefit profile and may provide greater protection in seasons with a high circulation of influenza B [Citation1].
The 30-year clinical data Influvac were published by van de Witte et al 2012 [Citation26], concluding that the overall benefit-to-risk ratio was positive for all age groups from 6 months upwards, as evidenced by many clinical trials that had been performed. This paper summarizes the safety and immunogenicity data from the registrational trials of the tetravalent formulation of the subunit influenza vaccine (namely Influvac Tetra), and confirms the positive benefit-to-risk ratio of this vaccine in influenza prophylaxis.
2. Methodology
2.1. Overview of studies
The clinical development program for QIV started with two clinical immunogenicity studies, one in adults and elderly [Citation27] [study INFQ3001] and one in children from 3 years of age [Citation28] [study INFQ3002]. The objective of these studies was to confirm the comparability of the immunogenicity of the shared influenza strains contained in the QIV and TIV formulations and comparability of the overall safety profile of QIV and TIV. A clinical efficacy study in children 6–35 months of age [study INFQ3003] was conducted to demonstrate the efficacy of QIV in the younger pediatric population [Citation29].
Additionally, two studies were conducted for registration in India: a study by Kalappanavar et al 2022 [study INFQ3004] evaluated the immunogenicity and safety of QIV in Indian children aged 6–35 months and 3–17 years [Citation30], and the study by Basu et al [INFQ3005] compared the immunogenicity and safety of QIV with an India registered quadrivalent influenza vaccine in adults [Citation31].
The details of these studies () and their results are described herein.
Table 1. List of studies conducted with Influvac Tetra.
2.2. Influenza vaccine dosing and composition
Children between 6 months and 9 years of age, who had not been previously vaccinated with influenza vaccine (unprimed children), were to be vaccinated twice, about a month apart, with two 0.5 mL doses (either QIV or TIV). Children who had previously been vaccinated with a seasonal influenza vaccine (primed children), adults, and the elderly received a single 0.5 ml dose (either QIV or TIV).
A 0.5 ml dose of QIV contained 15 µg of each hemagglutinin (HA) of an A-H1N1 strain, an A-H3N2 strain, a B-strain of the Victoria lineage, and a B-strain of the Yamagata lineage, in line with the strains recommended by the WHO for the upcoming season.
2.3. Safety and efficacy data analysis
In the five studies immunogenicity was assessed by analyzing post-vaccination Geometric Mean Titers (GMT) for Hemagglutination Inhibition (HI), seroconversion rates (SCRs), Geometric Mean Fold Increases (GMFIs), and Seroprotection Rates (SPRs). In some studies, Virus Neutralization assays (VN) as well as Neuraminidase Inhibition (NI) assays were performed. In one study efficacy was assessed by measuring the incidence of RT-PCR in the prevention of symptomatic influenza infection due to any circulating seasonal influenza strain/antigenically matching influenza strains. Cell-mediated immunity (CMI) was assessed in the global studies by distinguishing between CD4+ and CD8+ cells and was further characterized by production of the cytokines TNF alpha and IFN gamma and interleukins IL2+ and IL6+ upon stimulation with influenza virus.
In the current paper, MFIs and SCRs were presented consistently across studies to describe the immunogenicity of QIV and other parameters were highlighted in case of relevant details.
Safety was assessed based on the unsolicited adverse events (AEs) and solicited adverse reactions (ADRs) via home diaries (assessing both local and systemic ADRs during the first 7 days after vaccination), and additional post-market safety data for Influvac Tetra from 2018 to 2022 are reported.
3. Key results from individual studies
3.1. Children
3.1.1. Study INFQ3003 (Esposito et al, 2022)
This vaccine efficacy study, conducted in Europe and Asia, included children aged 6–35 months. A total of 1005 children were randomized to receive QIV and 995 received a non-influenza childhood control vaccine (NIV). Children received either two 0.5 mL doses of QIV or two doses of the control vaccine and were vaccinated twice, on Day 1 and Day 29. This study was conducted over three influenza seasons (NH 2017/2018, NH 2018/2019, and SH 2019).
3.1.1.1. Demographics
Overall, 50.6% of the participants were female and 49.4% were male, and the majority (98.0%) were either White (73.9%) or Asian (24.1%).
3.1.1.2. Efficacy
Compared to the NIV group, the QIV group had a lower incidence of RT-PCR-confirmed influenza due to any circulating seasonal influenza strain. The vaccine efficacy of QIV, referring to the proportion of influenza cases prevented, was 54% overall, with a 95% confidence interval of 37% to 66%. Further analysis confirmed the absolute vaccine efficacy of QIV in the prevention of symptomatic influenza infection throughout the surveillance period ().
Figure 1. Kaplan-Meier plot of time since 28 days post-second vaccination to RTPCR confirmed influenza A and/or B infection.
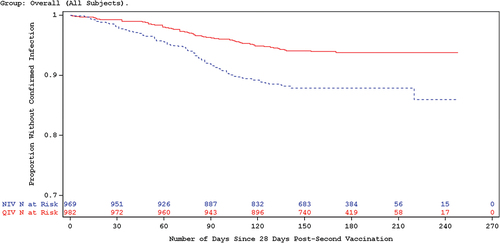
The incidence of RT-PCR confirmed influenza due to antigenically matching influenza strains was 68%, with a 95% confidence interval of 45–81%, further demonstrating the absolute vaccine efficacy of QIV in the prevention of symptomatic influenza due to matching strains. Further, a sub-group analyses estimating vaccine efficacy among 4 age ranges showed very similar results (), indicating that Influvac Tetra is a priming vaccine in children under the age of 2 years.
Figure 2. Influenza vaccine efficacy estimates due to antigenically matching influenza strains by age group.
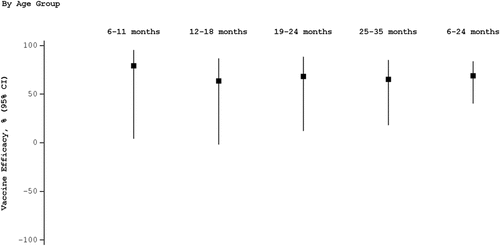
Although the study reported a high degree of matching to A(H1N1) and B-lineage strains, there was near complete mismatch to A(H3N2)-strains. Nonetheless, the overall vaccine efficacy estimate for the A(H3N2)-strains was comparable with that of the other strains, indicating that QIV confers a degree of crossprotection against mismatched influenza strains.
3.1.1.3. Immunogenicity
The geometric mean HI antibody titers increased from pre-vaccination to post-vaccination for all strains in the QIV group, while the increase was minimal in the NIV group. The QIV group showed a varying GMFIs ranging from 7.7 to 67.1 for the A-strains, and 1.8 to 5.4 for the B-lineage strains across the cohorts evaluated.
About 12 months after primary vaccination with QIV, post-vaccination geometric mean antibody HI titers were still moderately higher than pre-vaccination titers for both A strains and the B/Yamagata lineage strain, indicating the persistence of the immune response in subjects who were revaccinated, even if there was a waning trend over the long term. Additionally, participants who were revaccinated with QIV after 12 months showed an antibody response against all four strains, at least as strong as for the primary vaccination, indicating the boosting effect of revaccination.
SCRs were higher in the QIV group than in the NIV group for all strains across the cohorts evaluated. In the QIV group, SCRs were notably higher for the A-strains (>65%) than the B-strains ().
Table 2. Comparison of post-vaccination seroconversion rates (%) across all the studies.
In addition, the results for the VN and NI assays in participants that received the QIV were consistent with those for the HI assay. Post-vaccination increase in mean VN and NI titers were observed for all strains consistent among the age groups evaluated.
3.1.1.4. Safety
During the primary immunization period, a similar proportion of treatment-emergent adverse events (TEAEs) was reported for subjects in the QIV group (62.8%) and the NIV group (65.8%); the most commonly reported TEAEs included Influenza-like illness (41.3% and 44.9% of subjects in the QIV and NIV groups, respectively), upper respiratory tract infection (6.5% and 7.4%, respectively), and rhinitis (5.7% and 7.4%, respectively). Most cases were unrelated to the vaccine. A low and similar proportion of treatment-emergent serious adverse events (TESAEs) was reported for subjects in the QIV group (3.7%) and the NIV group (5.4%), with only 1 TESAE considered to be possibly related to the study vaccine (an event of moderate febrile convulsion reported in the NIV group). No clinically meaningful differences were noted in the incidence of medically attended adverse events (MAEs), adverse events of special interest (AESIs), or new chronic illnesses (NCIs) between the two vaccination groups.
During the revaccination immunization period, the proportion of reported TEAEs (47.0%) was lower than that during the primary immunization period. Overall, there were no clinically meaningful differences in the incidence of TEAEs, vaccine related TEAEs, TESAEs, or MAEs between the QIV and NIV groups.
No deaths due to AE were reported during the study period.
3.1.1.4.1. Local reactions
The incidence of overall local reactions was lower in the QIV group (30.4%) than in the NIV group (38.1%) during the primary immunization period (). Among the re-vaccinated participants, the proportion of local reactions in the QIV group was 26.9%, similar to the primary immunization rates. Most of these reactions were mild, and no more than two subjects (0.6%) experienced a severe local reaction for any individual type.
Table 3. Comparison of reactogenicity data (%) across all the studies.
The reactions lasted for 1–3 days in the majority of the subjects, in both the primary and revaccination immunization periods. However, in more than 10% of subjects, few symptoms lasted ≥7 days in either vaccination group or immunization period: vaccination site induration (6.8% and 11.8% in the QIV and NIV groups, respectively) and vaccination site ecchymosis (25.0% and 12.8%, respectively) in the primary immunization period; vaccination site swelling (11.1%), and vaccination site ecchymosis (16.7%) in the revaccination immunization period.
Overall, there was no notable difference in reactogenicity between the first and second vaccinations for both vaccination groups in terms of severity and duration, with the exception of vaccination site pain, which was more severe and lasted longer for a higher proportion of subjects after the first vaccination. The low incidence of AEs after revaccination supports a good safety profile of QIV.
3.1.1.4.2. Systemic reactions
Subjects in the QIV (51.4%) and NIV (52.5%) groups experienced a similar proportion of systemic reactions () within 7 days after vaccination in the primary immunization period. In the QIV group, this proportion further reduced to 27.2% during the revaccination immunization period. The severity reporting of individual systemic reactions of mild, moderate, and severe was comparable between the QIV and NIV groups for the overall population and was consistently lower during the revaccination immunization period.
Overall, reactogenicity after the second vaccination was lower than that the first vaccination in both the QIV and NIV groups in terms of severity/duration for most individual systemic reactions. During the revaccination immunization period, most systemic reactions were mild to moderate in severity.
3.1.2. Study 3004 (Kalappanavar et al, 2022)
3.1.2.1. Demographics
This baseline-controlled immunogenicity study, conducted in India, included two age groups of children: 6–35 months and 3–17 years. The children received either two doses (if unprimed) or one dose (if primed) of QIV (0.5 mL).
A total of 236 children were vaccinated, with 118 children in each age group. More males (54.7%) than females were enrolled in this study. Six children (2.5%) were primed: four in the 6–35 months group and two in the 3–17 years group. All 236 subjects received at least one dose of the study medication, and 232 completed the study.
3.1.2.2. Immunogenicity
The pre-vaccination mean titers were low for all four strains in the 6–35 months group, low for the two B strains, and moderate for the two A strains in the 3–17 years group. Good immune responses were observed in both age groups for the A strains and were less pronounced for the B strains. Post-vaccination SPRs were > 70% for both A strains in the 6–35 months group and for the A and B strains in the 3–17 years group. SCRs were > 60% for both A and B strains in both groups, except for the B-Victoria strain in the 6–35 months group (41.2%) ().
3.1.2.3. Safety
Local reactions were reported in 16 (13.6%) children in the 6–35 months group and 20 (16.9%) children in the 3–17 years group. Vaccination site pain of mild to moderate severity was the most common local reaction reported in both age groups (12.7% in 6–35 months group; 16.9% in 3–17 years group) which lasted for 3 to 4 days. Systemic reactions occurred in 20 (16.9%) children in the 6–35 months group and in 9 (7.6%) children in the 3–17 years group (). Fever was the most common systemic reaction in both the groups (7.8% in 6–35 months group; 5.9% in 3–17 years) and all the events were mild to moderate except one. All the events resolved within 5 days except two reactions in 6–35 months age group that resolved in 7 days.
3.1.3. Study INFQ3002 (Vesikari et al, 2020)
This randomized, double-blind, active-controlled, immunogenicity study was conducted in children and adolescents aged 3–17 years. Participants received either two 0.5 mL doses if unprimed or a single 0.5 mL dose if primed. The primary objective of this study was to demonstrate that post-vaccination HI antibody responses were non-inferior for each of the shared strains in QIV against TIV, and to compare the reactogenicity and safety of QIV with that of the TIV treatment arms.
3.1.3.1. Demographics
Children vaccinated with QIV had a mean (standard deviation [SD]) age of approximately 7.4 (4.0) years, and children vaccinated with TIV(Vic) and TIV(Yam) had a mean (SD) age of 7.7 (3.9) and 7.6 (3.9) years, respectively. Approximately 52% of the subjects vaccinated with QIV and about 49% and 55% with TIV(Vic) and TIV(Yam), respectively, were male, and the majority were white (98.5% [QIV]) and 99.0 [TIV]).
The number of subjects per age category and priming status (3–8 years unprimed, 3–8 years primed and 9–17 years) was nearly equally distributed between the QIV, TIV(Vic), and TIV(Yam).
In general, the demographics of the subjects were similar in all vaccination groups.
3.1.3.2. Immunogenicity
The non-inferiority of QIV to TIV was evaluated by comparing the post-vaccination geometric means of HI titers between the two vaccines ().
Table 4. Non-inferiority of QIV versus TIV against shared strains based on post-vaccination geometric mean HI titers – per-protocol sample – study INFQ3002.
For all four strains, the upper limit of the 95% confidence interval for the geometric mean ratio (GMR; TIV versus QIV) fell below the predefined non-inferiority margin of 1.5, demonstrating the non-inferiority of QIV to TIV.
The post-vaccination HI titers were comparable between the QIV and TIV groups, which were independent of age or priming status, showing that priming status does not influence the post-vaccination immune response in children of all age groups within the 3–17 years of age range.
For all four strains in the QIV arm, the post-vaccination GMFIs in VN and NI titers was high and in line with HI titers; SCRs were over 60% across all vaccination groups and similar for HI and VN titers (60%) but lower for NI titers (>30%) ().
For both Bstrain lineages, the GMT of the TIV group was about one-third and less than one-seventh of the GMT in the QIV group: 104.5 vs 306.7 (B-Victoria lineage) and 38.3 vs 280.8 (B-Yamagata lineage). Both differences were statistically significant (P < 0.0001 for both comparisons). Thus, the HI antibody responses elicited by the Bstrain antigens were superior to those elicited by cross-reactivity antigens of the alternate B-strain lineages.
3.1.3.3. Safety
During the immunization period, up to and including Day 29 (primed subjects)/Day 57 (unprimed subjects), the proportion of subjects reporting any TEAEs was low and similar between the QIV (18.9%) and TIV groups (22.6%). The most commonly reported TEAEs included upper respiratory tract infection (4.7% and 4.6% of subjects in the QIV and TIV groups, respectively), cough (1.7% and 3.0%, respectively), pyrexia (2.5% and 2.4%, respectively), and nasopharyngitis (1.7% and 2.5%, respectively). Most of the TEAEs were considered unrelated. Only a small number of SAEs (6 subjects) were reported, which were unrelated. No deaths, AESIs, or TEAEs leading to study termination were reported during this period.
In the 6-month safety follow-up period, the incidence of SAEs, NCIs, and AESIs were low and none of these events were considered to have a reasonable possibility for a causal relationship with the study vaccine. None of the SAEs were reported by more than one subject during the safety follow-up period, except for gastroenteritis, which was reported by three subjects (0.4%) in the TIV group.
3.1.3.3.1. Local reactions
The reporting rates for any local reaction in pediatric subjects were 62.7% and 57.9% for the QIV and TIV groups, respectively (). While a higher proportion of subjects aged 3 to 8 years reported local reactions in the QIV group, a higher proportion aged 9 to 17 years reported local reactions in the TIV group (62.8%).
Overall, the incidence of local reactions was less than 20%, except for vaccination site pain, which was reported for 237 subjects (59.0%) in the QIV group and 417 subjects (52.5%) in the TIV group. In subjects aged 3–8 years, the difference between QIV and TIV for vaccination site pain was most pronounced for primed subjects (60.9% and 48.5%, respectively), and for unprimed subjects (61.8% and 50.2%, respectively). In contrast, subjects aged 9–17 years had a slightly lower reporting for the QIV group than for the TIV group (54.1% and 58.7%, respectively).
Most local reactions were not severe. A similar proportion of subjects with severe local reactions of each type was reported in the QIV and TIV groups.
Overall, for the majority, local reaction symptoms lasted for 1–3 days, with the exception of vaccination site ecchymosis lasting 1–7 days.
3.1.3.3.2. Systemic reactions
The reporting rates for any systemic reaction in pediatric subjects were similar between the vaccination groups (38.1% for both QIV and TIV) (). However, in unprimed subjects aged 3–8 years (41.9% vs. 38.2%) and 9–17 years (47.4% vs. 44.6%), the proportion of subjects with systemic reactions was higher in the QIV than in the TIV group, whereas in primed subjects aged 3–8 years, the proportion was lower in the QIV than in the TIV group (24.8% vs. 31.5%).
Fatigue/tiredness was reported by 23.6% and 22.1% of subjects in the QIV and TIV group, respectively. Headache was reported by 24.0% and 20.9% of subjects in the QIV and TIV groups, respectively. All other systemic reactions have been reported with an incidence rate of less than 20%. Most of the systemic reactions reported in both groups were mild or moderate in severity. Overall, all systemic reaction symptoms lasted for 1–2 days in most subjects in both vaccination groups.
3.2. Adults and elderly
3.2.1. Study INFQ3001 (van de Witte S et al, 2018)
This was a randomized, double-blind, active-controlled immunogenicity study in adults and elderly individuals. In total, 1980 participants received a single 0.5 mL dose of either QIV or TIV (with a B-strain of either the Victoria lineage or the Yamagata lineage). The objectives of this study were to demonstrate the non-inferiority of QIV to TIV with respect to antibody response to the shared strains, the superiority of QIV to TIV with respect to antibody response to the alternate lineage B strain and to compare the reactogenicity and safety of QIV with that of the TIV treatment arms.
3.2.1.1. Demographics
Adults vaccinated with QIV had a mean (SD) age of 41 (12.5) years, and adults vaccinated with TIV had a mean (SD) age of 41 (SD: 12.9 with TIV(Vic) and 12.3 with TIV(Yam)). Approximately 44% of those vaccinated with QIV and 46% and 39% with TIV(Vic) and TIV(Yam), respectively, were male. The majority of the subjects were white in both groups (QIV, 99.1%; TIV, 100%).
Elderly adults vaccinated with QIV had a mean (SD) age of 70 (6.4) years, and those vaccinated with TIV(Vic) had a mean (SD) age of 70 (6.6) and 70 (6.5) with TIV(Yam). Approximately 42% of the subjects vaccinated with QIV were male and approximately 44% and 46% with TIV(Vic) and TIV(Yam), respectively. The majority of the subjects were white in both groups (QIV, 99.7%; TIV, 100%).
A subgroup analysis of at-risk vs. not-at-risk (the definition of at-risk is as per the WHO and ECDC recommendations) for the complications of influenza due to underlying chronic conditions was conducted to determine if there was any difference in the immune response between the two groups. In the QIV group, the number of subjects at-risk/not at-risk for influenza complications was 106 (13.8%)/662 (86.2%) among the adults and 347 (45.2%)/420 (54.8%) among the elderly. In the TIV group, there were 21 (9.5%)/201 (90.5%) and 88 (40.2%)/131 (59.8%). Thus, the majority of the at-risk subjects were in the elderly group.
3.2.1.2. Immunogenicity
The non-inferiority of QIV to TIV was tested by comparing the post-vaccination geometric mean HI titers of the two formulations.
For all four strains, the upper limit of the 95% confidence interval for the geometric mean ratio (GMR; TIV versus QIV) fell below the predefined noninferiority margin of 1.5, demonstrating the noninferiority of QIV to TIV ().
Table 5. Non-inferiority of QIV versus TIV against shared strains based on post-vaccination geometric mean HI titers – per-protocol sample – study INFQ3001.
In the subgroup analysis of at-risk vs. not at-risk for complications of influenza, the post-vaccination anti-HA antibody levels were not affected by at-risk status and were comparable between QIV and TIV.
SCRs based on HI titers were found in more than 51.0% of adult subjects and more than 39.0% of elderly subjects in the QIV group for all four strains ().
For both Bstrain lineages, the GMT of the TIV group was less than half of the GMT in the QIV group: 64.1 vs 153.1 (B-Victoria lineage) and 47.2 vs 101.9 (B-Yamagata lineage; P < 0.0001, both comparisons). Thus, the antibody responses elicited by the Bstrain antigens were superior to those elicited by cross-reactivity antigens of the alternate B-strain lineages.
3.2.1.3. Safety
For both adults and the elderly, most local and systemic reactions were mild or moderate in severity. Overall, all local reaction symptoms lasted for 1–3 days for the majority of the subjects in both vaccination groups.
3.2.1.3.1. Local reactions
The incidence of overall local reactions was 24.8% in adults and 9.1% in elderly in the QIV group and 21.7% in adults and 6.4% in elderly in the NIV group (). Reporting rates in adult subjects were generally low (<10%), except for vaccination site pain, which occurred in 24.9% of the subjects in the QIV group and 8.5% in the TIV group. The reporting rates were also low (<5%) in elderly subjects, except for vaccination site pain, which was reported by 7.6% in the QIV group and 5.9% in the TIV group.
3.2.1.3.2. Systemic reactions
Adults: The reporting rates of systemic reactions were comparable between the QIV and the TIV groups, although the QIV group reported less (). A total of 12.4% of the subjects in the QIV group and 13.1% in the TIV group reported headache, 11.9% in the QIV group, and 12.6% in the TIV group reported fatigue/tiredness.
Elderly: Headache (8.1% in the QIV group vs. 7.3% in the TIV group) and fatigue/tiredness (10.6% in the QIV group vs. 6.8% in the TIV group) were the most frequent systemic reactions reported by the elderly within 7 days after vaccination in both vaccination groups. Most of the systemic reactions were mild or moderate in severity. The differences in rates between QIV and TIV were relatively small, although one reaction (arthralgia/joint pain, 5.8% [QIV] vs. 2.3% [TIV]) reached statistical significance; thus, it was flagged as having a potentially higher reporting rate for elderly subjects in the QIV group.
The frequency of unsolicited AEs was low and comparable between the QIV and TIV groups, with reporting rates of 4.8% and 3.6% in adults and 3.8% and 2.7% in the elderly, in QIV and TIV, respectively. None of the reported SAEs or NCIs during the long-term follow-up period were related to the study vaccine, demonstrating that the additional B lineage in QIV did not compromise the safety.
3.2.2. Study 3005 (Basu et al, 2022)
This was a randomized, observer-blind, parallel-group study to compare the antibody response and safety of QIV and VaxiFlu-4 (split virion inactivated influenza vaccine, Zydus Cadila Healthcare) in 480 healthy Indian adults and elderly subjects. The subjects were randomized in a 1:1 ratio and received one dose of the study vaccine (0.5 mL).
3.2.2.1. Demographics
The mean age of the participants was 51.6 (±18.1) years, with 51.0% male and 49.0% female.
3.2.2.2. Immunogenicity
Pre- and post-vaccination HI antibody titers was comparable between the two vaccine groups for both age groups. The two A-strains showed higher average pre- and post-vaccination titers than the two B-strains.
Post-vaccination SPRs were comparable between the test and reference vaccine groups, and were, on average, higher in adults than in elderly individuals. The rates were > 90% for the A strains and between 43% and 60% for the B strains.
The GMFIs was between 4.3–22.7 in the test and 3.7–21.6 in the reference vaccine group. SCRs varied between 41.4%- 78.8% and were comparable between both vaccine groups ().
3.2.2.3. Safety
The frequency of TEAEs was 0.8% and 0.4% in QIV and in the test groups, respectively. None of these was related to the study vaccine. No deaths, TESAEs, severe TEAE, or TEAEs leading to the study termination were reported.
The rates of local and systemic reactions occurring within 7 days of vaccination were low (<5%) and similar between the test and reference vaccines. Local reactions were observed in 2.9% (n = 7) of participants in the test group and 4.2% (n = 10) in the reference group. Systemic reactions were observed in 4.6% (n = 11) of participants in the test group and 3.3% (n = 8) of participants in the reference group. The incidence of systemic and local reactions was slightly higher in adult participants than in the elderly participants ().
3.3. Post marketing safety of Influvac and Influvac Tetra
The estimated cumulative post-marketing patient exposure to the trivalent influenza vaccine worldwide from the available sales records from 1 July 1998 until 15 March 2023, is 643 million patient-treatments (vaccinations) and two hundred million doses administered for Influvac Tetra. The most frequently reported ADRs are local and/or systemic reactions, which are in line with the Summary of Product Characteristics (SPC) and are known risks for inactivated influenza vaccines and commonly reported (>1/100 and < 1/10 cases). The safety data generated during the above-mentioned reporting periods continue to support the favorable benefit-risk profile of the influenza vaccine for approved indications [data on file (INFL INFQ PSUR 42; 16MAR2022-15MAR2023)].
Furthermore, enhanced passive safety surveillance (EPSS) was implemented for Influvac starting in the season 2014/15 and continued for Influvac Tetra from the 2018/19 season. The AE reporting rate was nearly 30% for both vaccines. The most frequently reported local and systemic AEIs across all age groups were injection-site pain and myalgia/arthralgia. The reporting rates were higher in adults than in the elderly, and in females than in males. Overall, the reactogenicity data for Influvac Tetra were consistent across all seasons and were in line with Influvac. This confirms the favorable benefit risk ratio for Influvac Tetra across all age groups [Citation32].
4. Discussion
Seasonal flu vaccination and adequate vaccine coverage rates have been highlighted by the WHO as effective preventive strategies to mitigate the clinical and social burdens of influenza.
The clinical development of Influvac Tetra aimed to support an indication for use identical to the existing approved Influvac (TIV), that is prophylaxis of influenza, especially in those at increased risk of associated complications. It was assumed that the only clinically relevant change between the QIV and TIV formulations was the presence of a second (alternate-lineage) B-strain; thus, similar immunogenicity and reactogenicity were expected for Influvac Tetra. Moreover, Influvac Tetra includes both HA and NA, the major surface glycoproteins of influenza viruses, which play a crucial role in the early stage of virus infection, for the four selected strains.
Hemagglutinin is responsible for binding the virus to cell surface receptors and mediates the liberation of the viral genome into the cytoplasm through membrane fusion. It is the primary target of the immune response to influenza infection or vaccination. In contrast, NA is involved in the release of virions and their subsequent spread from infected cells. In addition to the NA’s impact on viral shedding and on symptoms, NA has demonstrated various advantages over HA, i.e. reduced and independent antigenic drift to HA, and the ability of many NA-specific antibodies to bind to domains that are well conserved within a subtype, protecting against heterologous viruses. NA-specific antibodies do not have neutralizing capacity but would significantly reduce viral replication, virus shedding, and infection severity. Therefore, the presence of NA in vaccines in addition to HA mimics the immune response to natural infection more closely, improves homologous immunization against influenza and provides heterovariant immunity when an epidemic virus emerges with unexpected antigenic changes, a relevant feature, especially for children that lack or have limited memory of previous exposure to antigens and thus needed additional protection. Moreover, it is known that unprimed children had a higher response rate to NA than HA and high levels of pre-outbreak anti-NA antibodies were protective and correlated better with protection compared to high anti-HA antibodies, as highlighted in a study conducted in Japanese school children [Citation33]. Two studies with Influvac Tetra in children showed a post-vaccination increase in NI titers against all the strains [Citation28,Citation29]. Unlike the recombinant, cell-based vaccines, and current mRNA vaccines in the most advanced development stage, only containing HA antigen, the presence of NA in Influvac Tetra® may translate into additive protection [Citation34].
In addition, the CMI assessed across the entire age range demonstrated increased post-vaccination CD4+ T cells producing TNF alpha, IFN gamma, and IL2+, suggesting that vaccination with QIV activates T helper cells and confirms the role of Influvac Tetra in inducing cell-mediated immunity. The increase in post-vaccination CD8+ T cells was minimal or undetectable.
The results of five clinical studies with Influvac Tetra showed consistent immunogenicity across age groups [Citation27–31]. The QIV demonstrated non-inferiority to the TIVs for shared strains and was superior to the TIV for the alternate lineage influenza B strains. This confirmed that the additional B strain did not interfere with antibody response to the three TIV antigens. Derived serological parameters (seroprotection, seroconversion, and MFI) according to the former CHMP criteria were consistent with the findings on antibody titers. This is in line with several other studies demonstrating the immunogenicity of QIVs in several population groups, including adults and elderly subjects [Citation35–38].
This review confirms that in adults and the elderly population, post-vaccination anti-HA antibody levels were not affected by at-risk status, and there was no difference in immune response between the at-risk and not-at-risk groups. However, relative to the baseline, the increase in post-vaccination anti-HA antibody levels tended to be lower in at-risk subjects. These differences could be explained by higher pre-vaccination antibody levels in at-risk as compared to subjects not at-risk.
The study by Esposito et al. [Citation29] confirmed the efficacy of QIV in the prevention of symptomatic influenza infection by antigenically matching influenza strains and/any circulating strains. This is consistent with results from previous studies demonstrating the efficacy of split QIVs in the prevention of vaccine-matched and vaccine-mismatched influenza among children aged 6–35 months [Citation39, Citation40]. This indicates that sub-unit vaccines demonstrate clinical efficacy similar to split vaccines in comparable population. Further, Esposito et al. demonstrated consistent vaccine efficacy estimates for the antigenically matching strains among all age groups, including 6–11 months, making a case that Influvac Tetra is a priming vaccine in very young children. Vaccine efficacy persisted despite a degree of strain mismatch, indicating excellent cross-protection offered by the Influvac Tetra [Citation29].
The review also showed that Influvac Tetra has a positive benefit risk ratio in children (over 6 months of age), adolescents, adults, and the elderly, which is comparable to that of trivalent vaccinations [Citation41]. The overall safety was further confirmed by the favorable post-marketing data obtained from the EPSS program.
In one study in adults, Influvac Tetra demonstrated a better safety profile than the comparator, a split influenza vaccine. Indeed, as a subunit vaccine, Influvac Tetra undergoes additional purification steps during the manufacturing process, which is reflected in a lower frequency of local and systemic reactions than split and whole vaccines. This finding is in accordance with a 2010 study comparing a subunit vaccine with a split influenza vaccine that found the two vaccines to be immune-equivalent. However, the split vaccine was associated with higher rates of reported muscle pain and swelling following vaccination in adults when compared to the subunit counterpart [Citation42,Citation43].
Additionally, the reactogenicity and safety of other available QIV formulations have been shown to be consistent with the established profiles of the respective TIV formulations in both adult and pediatric populations [Citation35,Citation44–48].
In general, inactivated influenza vaccines have demonstrated an excellent safety profile and are well tolerated by recipients of all ages, including individuals with underlying conditions and pregnant women. Inactivated influenza vaccines have been administered to pregnant women for the past 50 years. Clinical and observational studies in pregnant women, have found no evidence of any inactivated influenza vaccine associated AEs in either the women or their newborn babies [Citation1], while showing the clinical benefit for the the pregnant women and the newborn (reduction of incidence of preterm, stillbirth birth and infant mortality, and infants passive protection) [Citation49–52]
Recent evidence has highlighted the risk of under-reporting when influenza infections are identified based on symptoms rather than serology [Citation53]. This data poses major relevance to the implementation and improvement of adequate preventive strategies against influenza, such as broad vaccination campaigns and the use of all available and age-appropriate vaccines to reach the WHO-recommended vaccine coverage target of 75% [Citation54].
The protection from the flu could be further increased by overcoming different psychological, contextual, sociodemographic, and physical barriers that have been deeply analyzed in recent literature and today prevent reaching the target coverage [Citation55–57].
In addition to public health benefits, a recent model evaluated the impact of different influenza vaccination strategies on the public health budget, highlighting that the increase in vaccine coverage in the at-risk population using QIV would result in lower costs and fewer infections than those of enhanced and more expensive vaccines, at the actual vaccination rate [Citation58].
On 27 September 2023, the WHO influenza vaccine composition advisory committee expressed the opinion that the inclusion of B/Yamagata antigen as a component of influenza vaccines is no longer warranted and recommended excluding it as soon as possible [Citation59]. The decision was backed by the very low risk of future epidemics driven by lack of confirmed detection of naturally occurring B/Yamagata lineage viruses by the GRSRS network since March 2020 [Citation60]. Ongoing testing in sentinel samples for B/Yamagata lineage, as well as active surveillance on new emergent strains, are crucial to protect vulnerable populations from influenza virus infection and sequelae. In case of future outbreaks of new strain subtypes or lineages, the inclusion of more than one strain subtype or lineage in the flu vaccine composition is the appropriate approach to provide adequate protection. Indeed, the safety and efficacy of QIVs have been previously demonstrated in multiple trials and are historically recognized by WHO as potentially offering wider protection against flu disease.
5. Conclusions
The clinical and economic burden of influenza is high worldwide, with greater impact in low- and middle-income countries. Despite this, vaccine coverage remains below the target, also in many high-income countries.
QIVs may provide greater protection than TIVs in influenza seasons with a high circulation of influenza B, depending on the match between the influenza B lineage in the TIV and the circulating B viruses, and have been shown to effectively reduce the risk of influenza in both adults and high-risk populations such as the elderly. Crucially, the addition of another B strain lineage does not interfere with the antibody responses to the other three strains.
The results of the clinical program and post marketing surveillance in various countries and regions in Europe and Asia show that Influvac Tetra is an efficacious vaccine for the prophylaxis of influenza in subjects starting from 6 months of age, regardless the priming or risk status. Additionally, it shows that it has a safety profile similar to well-established safety of TIV (Influvac).
Increased Influvac Tetra uptake is therefore an effective and equitable option to prevent seasonal influenza in any region and, in particular, it provides increased protection against influenza B strains and elicit a wide humoral immune response against HA and NA.
6. Expert opinion
Every year, seasonal influenza affects a significant number of individuals worldwide, but it is a disease that can be prevented through vaccination. While older individuals, younger children, and those with chronic conditions are more susceptible to severe outcomes, everyone is at risk of experiencing serious complications such as pneumonia, myocarditis, and encephalitis, which can even be fatal.
Several types of influenza vaccines are available today to cater to this need, such as standard dose egg-based, high dose egg-based, adjuvanted egg-based, cell-based, and recombinant. Influvac Tetra is a standard dose egg-based vaccine that has demonstrated its immunogenicity and safety since 2018, making it a suitable option to combat the annually recurring medical and economic burden of influenza epidemics.
The COVID-19 pandemic has stimulated innovation in the development of vaccines, and mRNA technology has emerged as a crucial intervention in public health.
Applying mRNA technology to influenza vaccines would enable the creation of vaccines with mRNA sequences tailored to multiple influenza strains, allowing for a rapid response to virus shift and drift. The mRNA seasonal flu vaccines are currently in the development stages and confirmatory phase 3 trials are needed to demonstrate their immunogenicity data across all strains and target groups. A careful evaluation of safety is also essential because it is crucial to determine if the side effects associated with COVID-19 mRNA, such as myocarditis, are specific to the mRNA platform or unique to the COVID-19 vaccines. More evidence is needed to show the risk-benefits of the mRNA flu vaccine over the standard platform.
The other direction for modern research is the development of universal influenza vaccines that can protect against more strains of the virus. The broad spectrum of antibodies produced by these universal influenza vaccines could be advantageous in the event of an influenza pandemic arising from a new strain. However, the development of these vaccines is still in the early stages.
While a large focus is currently being given to improving vaccine efficacy, very little effort is provided to improve vaccination coverage. A series of systematic reviews published by the ECDC group has also shown that the evidence base of the newer vaccines against the standard dose egg-based vaccine is limited at present. The percentage of seasonal influenza vaccination coverage in high-risk groups shows significant variation across countries. For older individuals, who are the primary target group with the most available data, the coverage ranges from less than 1% to more than 75%. However, vaccination coverage among people with chronic diseases and healthcare workers is below 40% in most of countries. Higher influenza vaccine coverage rates contribute to the development of herd immunity. When a significant portion of the population is vaccinated, it creates a protective barrier that limits the spread of the virus. This indirectly protects vulnerable individuals who may not be able to receive the vaccine due to medical reasons or those who have a diminished immune response to the vaccine. Thus, it is crucial to prioritize and promote strategies that increase influenza vaccine uptake to maximize the positive impact on public health.
Besides the efforts to develop improved vaccines, countries should focus on increasing vaccine coverage by using of all available vaccines. Annual vaccination against influenza is crucial, irrespective of the vaccine chosen.
Article highlights
Trivalent influenza vaccines (TIVs) contain two A-strains (H1N1 and H3N2) and only one B-strain lineage, thus providing little or limited protection against disease caused by the other B-strain lineage.
Quadrivalent influenza vaccines (QIVs) contain two A-strains (H1N1 and H3N2) and two B-strain lineages (B/Victoria and B/Yamagata) and therefore can increase overall VE in seasons when both lineages co-circulate.
The clinical development of Influvac Tetra, a QIV, aimed to support an indication for use similar to the existing approved TIV (Influvac), i.e. prophylaxis of influenza, especially in those at increased risk of associated complications.
The results of two clinical studies with Influvac Tetra showed consistent immunogenicity across age groups, demonstrating non-inferiority for shared strains and superiority to TIV for the alternate lineage influenza B strains.
At-risk status did not affect post-vaccination anti-HA antibody levels with Influvac Tetra.
The absolute vaccine efficacy of Influvac Tetra in the prevention of symptomatic influenza against the matching strains in children aged 6-35 months was 68%, and against any circulating seasonal influenza strain was 54%. Subgroup analyses further demonstrated consistent vaccine efficacy estimates among all age groups, including children under the age of 2 years, making a case that Influvac Tetra is a priming vaccine in young children. Vaccine efficacy persisted throughout the season.
Besides the hemagglutination inhibition (HI) antibody titers, Influvac Tetra also elicits an increase in neuraminidase inhibition (NI) antibody titers and Virus Neutralization titers (VN).
Cell-mediated immunity assessed across the entire age range demonstrated increased postvaccination CD4+ T cells producing TNF alpha, IFN gamma, and IL2+, suggesting that vaccination with Influvac Tetra activates T helper cells and confirms its role in inducing cell-mediated immunity.
Influvac Tetra has a positive benefit-risk balance in children (over 6 months of age), adolescents, adults, and the elderly, and its safety profile is in line with the well-established safety profile of Influvac (TIV).
Declaration of interests
J Rogoll, J Nauta, S van de Witte, and A Kondratenko, are employed by Abbott, the company that manufactures Influvac and Influvac Tetra. S Hadigal and L Colombo are employees of Viatris. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or material discussed in the manuscript apart from those disclosed.
Reviewer disclosures
A reviewer on this manuscript discloses that they are an owner of intellectual property pertaining to the molecular clamp vaccine platform. They are also a scientific advisor to Vicebio Ltd. A second reviewer has disclosed that they receive honoraria from Abbott. Peer reviewers on this manuscript have no other relevant financial or other relationships to disclose.
Author contributions
Laura Colombo, Sanjay Hadigal, Jutta Rogoll and Serge van de Witte contributed to the design and implementation, data interpretation, drafting and editing of this review paper. Jos Nauta was the statistical lead and was primarily involved in the clinical study design, statistical analyses, and data interpretation. Alona Kondratenko revised the manuscript critically for important intellectual content. All authors contributed to the data interpretation, development, and review of this manuscript and confirm that they have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. The sponsor also provided a formal review of this manuscript. All authors met ICMJE criteria and all those who fulfilled those criteria are listed as authors. All authors had access to the study data and made the final decision about where to publish these data and approved submission to this journal.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- World Health Organization. Vaccines against influenza: WHO position paper – May 2022: World Health Organization; 2022 [cited 2023 Apr 6]. Available from: https://www.who.int/publications/i/item/who-wer9719
- World Health Organization. Influenza (Seasonal) 2023 [cited 2023 2 Jun]. Available from: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal)
- European Centre for Disease Prevention and Control. Factsheet about seasonal influenza. 2022 [cited 2022 Jul 26]. Available from: https://www.ecdc.europa.eu/en/seasonal-influenza/facts/factsheet
- Collaborators GBDI. Mortality, morbidity, and hospitalisations due to influenza lower respiratory tract infections, 2017: an analysis for the Global burden of disease study 2017. Lancet Respir Med. 2019 Jan;7(1):69–89. doi: 10.1016/S2213-2600(18)30496-X
- Fischer WA, Gong M, Bhagwanjee S, et al. Global burden of influenza: contributions from resource limited and low-income settings. Global Heart. 2014;9(3):325. doi: 10.1016/j.gheart.2014.08.004
- Uyeki TM, Hui DS, Zambon M, et al. Influenza. Lancet. 2022 Aug 27;400(10353):693–706. doi: 10.1016/S0140-6736(22)00982-5
- World Health Organization. Flu news Europe: 2022-2023 season overview 2023 [cited 2023 Apr 5]. Available from: https://flunewseurope.org/SeasonOverview
- Li T, Asakawa T, Liu H, et al. The role of influenza in the era of COVID-19: can we forget it? Biosci Trends. 2022 Nov 20;16(5):374–376. doi: 10.5582/bst.2022.01297
- Talbot HK, Zhu Y, Chen Q, et al. Effectiveness of influenza vaccine for preventing laboratory-confirmed influenza hospitalizations in adults, 2011-2012 influenza season. Clin Infect Dis. 2013 Jun;56(12):1774–1777. doi: 10.1093/cid/cit124
- Thompson MG, Pierse N, Sue Huang Q, et al. Influenza vaccine effectiveness in preventing influenza-associated intensive care admissions and attenuating severe disease among adults in New Zealand 2012-2015. Vaccine. 2018 Sep 18;36(39):5916–5925. doi: 10.1016/j.vaccine.2018.07.028
- Casado I, Dominguez A, Toledo D, et al. Effect of influenza vaccination on the prognosis of hospitalized influenza patients. Expert Rev Vaccines. 2016;15(3):425–432. doi: 10.1586/14760584.2016.1134328
- Ferdinands JM, Thompson MG, Blanton L, et al. Does influenza vaccination attenuate the severity of breakthrough infections? A narrative review and recommendations for further research. Vaccine. 2021 Jun 23;39(28):3678–3695. doi: 10.1016/j.vaccine.2021.05.011
- Godoy P, Romero A, Soldevila N, et al. Influenza vaccine effectiveness in reducing severe outcomes over six influenza seasons, a case-case analysis, Spain, 2010/11 to 2015/16. Euro Surveill. 2018 Oct;23(43). doi: 10.2807/1560-7917.ES.2018.23.43.1700732
- Klein EY, Schueller E, Tseng KK, et al. The impact of influenza vaccination on antibiotic use in the United States, 2010-2017. Open Forum Infect Dis. 2020 Jul;7(7):ofaa223. doi: 10.1093/ofid/ofaa223
- Vora A, Di Pasquale A, Kolhapure S, et al. The need for vaccination in adults with chronic (noncommunicable) diseases in India - lessons from around the world. Hum Vaccin Immunother. 2022 Nov 30;18(5):2052544. doi: 10.1080/21645515.2022.2052544
- Bhugra P, Grandhi GR, Mszar R, et al. Determinants of influenza vaccine uptake in patients with cardiovascular disease and strategies for improvement. J Am Heart Assoc. 2021 Aug 3;10(15):e019671. doi: 10.1161/JAHA.120.019671
- World Health Organization. Seasonal influenza vaccines: an overview for decision-makers. 2020.
- European Centre for Disease Prevention and Control. Seasonal influenza vaccination and antiviral use in EU/EEA member States. 2018 [cited 2022 May 10]. Available from: https://www.ecdc.europa.eu/sites/default/files/documents/seasonal-influenza-antiviral-use-2018.pdf
- Preaud E, Durand L, Macabeo B, et al. Annual public health and economic benefits of seasonal influenza vaccination: a European estimate. BMC Public Health. 2014 Aug 7;14(1):813. doi: 10.1186/1471-2458-14-813
- Tisa V, Barberis I, Faccio V, et al. Quadrivalent influenza vaccine: a new opportunity to reduce the influenza burden. J Prev Med Hyg. 2016;57(1):E28.
- Dolin R. The quadrivalent approach to influenza vaccination. J Infect Dis. 2013 Aug 15;208(4):539–540. doi: 10.1093/infdis/jit264
- Ambrose CS, Levin MJ. The rationale for quadrivalent influenza vaccines. Hum Vaccin Immunother. 2012 Jan;8(1):81–88. doi: 10.4161/hv.8.1.17623
- Tran D, Vaudry W, Moore D, et al. Hospitalization for influenza a versus B. Pediatrics. 2016 Sep;138(3). doi: 10.1542/peds.2015-4643
- Karve S, Meier G, Davis KL, et al. Influenza-related health care utilization and productivity losses during seasons with and without a match between the seasonal and vaccine virus B lineage. Vaccine. 2013 Jul 18;31(33):3370–3388. doi: 10.1016/j.vaccine.2013.04.081
- European Centre for Disease Prevention and Control. Types of seasonal influenza vaccine 2023 [cited 2023 Jul 27]. Available from: https://www.ecdc.europa.eu/en/seasonal-influenza/prevention-and-control/vaccines/types-of-seasonal-influenza-vaccine
- van de Witte SV, Nauta J, Giezeman-Smits KM, et al. Trivalent inactivated subunit influenza vaccine Influvac®: 30-year experience of safety and immunogenicity. Trials Vaccinol. 2012;1:42–48. doi: 10.1016/j.trivac.2012.10.001
- van de Witte S, Nauta J, Montomoli E, et al. A phase III randomised trial of the immunogenicity and safety of quadrivalent versus trivalent inactivated subunit influenza vaccine in adult and elderly subjects, assessing both anti-haemagglutinin and virus neutralisation antibody responses. Vaccine. 2018 Sep 25;36(40):6030–6038. doi: 10.1016/j.vaccine.2018.04.043
- Vesikari T, Nauta J, Lapini G, et al. Immunogenicity and safety of quadrivalent versus trivalent inactivated subunit influenza vaccine in children and adolescents: A phase III randomized study. Int J Infect Dis. 2020 Mar;92:29–37. doi: 10.1016/j.ijid.2019.12.010
- Esposito S, Nauta J, Lapini G, et al. Efficacy and safety of a quadrivalent influenza vaccine in children aged 6-35 months: a global, multiseasonal, controlled, randomized phase III study. Vaccine. 2022 Apr 20;40(18):2626–2634. doi: 10.1016/j.vaccine.2022.02.088
- Kalappanavar NK, Ghosh A, Sharma M, et al. Immunogenicity, reactogenicity and safety of an inactivated quadrivalent influenza vaccine in children and adolescents 6 months through 17 years of age in India. Human Vaccines Immunother. 2022;18(6). doi: 10.1080/21645515.2022.2104527
- Basu I, Agarwal M, Shah V, et al. Immunogenicity and safety of two quadrivalent influenza vaccines in healthy adult and elderly participants in India - a phase III, active-controlled, randomized clinical study. Hum Vaccin Immunother. 2022 Dec 31;18(1):1–10. doi: 10.1080/21645515.2021.1885278
- Moeller-Arendt M, van de Witte S, Nauta J, et al. Enhanced passive safety surveillance of Influvac(R) and Influvac(R) Tetra: results from seven consecutive seasons. Vaccine. 2023 Jan 9;41(2):606–613. doi: 10.1016/j.vaccine.2022.12.001
- Yamane N, Odagiri T, Arikawa J, et al. Effect of specific immunity to viral neuraminidase on subsequent influenza virus infection in man. Microbiol Immunol. 1979;23(6):565–567. doi: 10.1111/j.1348-0421.1979.tb00497.x
- Giurgea LT, Morens DM, Taubenberger JK, et al. Influenza Neuraminidase: A Neglected Protein and Its Potential for a Better Influenza Vaccine. Vaccines (Basel). 2020 Jul 23;8(3):409. doi: 10.3390/vaccines8030409
- Domachowske JB, Pankow-Culot H, Bautista M, et al. A randomized trial of candidate inactivated quadrivalent influenza vaccine versus trivalent influenza vaccines in children aged 3-17 years. J Infect Dis. 2013 Jun 15;207(12):1878–87. doi: 10.1093/infdis/jit091
- Hartvickson R, Cruz M, Ervin J, et al. Non-inferiority of mammalian cell-derived quadrivalent subunit influenza virus vaccines compared to trivalent subunit influenza virus vaccines in healthy children: a phase III randomized, multicenter, double-blind clinical trial. Int J Infect Dis. 2015 Dec;41:65–72.
- Kieninger D, Sheldon E, Lin WY, et al. Immunogenicity, reactogenicity and safety of an inactivated quadrivalent influenza vaccine candidate versus inactivated trivalent influenza vaccine: a phase III, randomized trial in adults aged ≥18 years. BMC Infect Dis. 2013 Jul 24;13(1):343. doi: 10.1186/1471-2334-13-343
- Cadorna-Carlos JB, Nolan T, Borja-Tabora CF, et al. Safety, immunogenicity, and lot-to-lot consistency of a quadrivalent inactivated influenza vaccine in children, adolescents, and adults: A randomized, controlled, phase III trial. Vaccine. 2015 May 15;33(21):2485–2492. doi: 10.1016/j.vaccine.2015.03.065
- Claeys C, Zaman K, Dbaibo G, et al. Prevention of vaccine-matched and mismatched influenza in children aged 6–35 months: a multinational randomised trial across five influenza seasons. The Lancet Child Adol Health. 2018;2(5):338–349. doi: 10.1016/S2352-4642(18)30062-2
- Pepin S, Dupuy M, Borja-Tabora CFC, et al. Efficacy, immunogenicity, and safety of a quadrivalent inactivated influenza vaccine in children aged 6-35 months: a multi-season randomised placebo-controlled trial in the northern and southern hemispheres. Vaccine. 2019 Mar 22;37(13):1876–1884. doi: 10.1016/j.vaccine.2018.11.074
- Keech M, Beardsworth P. The impact of influenza on working days lost: a review of the literature. PharmacoEconomics. 2008;26(11):911–924. doi: 10.2165/00019053-200826110-00004
- Leeb A, Carcione D, Richmond PC, et al. Reactogenicity of two 2010 trivalent inactivated influenza vaccine formulations in adults. Vaccine. 2011 Oct 19;29(45):7920–7924. doi: 10.1016/j.vaccine.2011.08.073
- Beyer WE, Palache AM, Osterhaus AD. Comparison of serology and reactogenicity between influenza subunit vaccines and whole virus or split vaccines: a review and meta-analysis of the literature. Clin Drug Investig. 1998;15(1):1–12. doi: 10.2165/00044011-199815010-00001
- Greenberg DP, Robertson CA, Noss MJ, et al. Safety and immunogenicity of a quadrivalent inactivated influenza vaccine compared to licensed trivalent inactivated influenza vaccines in adults. Vaccine. 2013 Jan 21;31(5):770–776. doi: 10.1016/j.vaccine.2012.11.074
- Airey J, Albano FR, Sawlwin DC, et al. Immunogenicity and safety of a quadrivalent inactivated influenza virus vaccine compared with a comparator quadrivalent inactivated influenza vaccine in a pediatric population: A phase 3, randomized noninferiority study. Vaccine. 2017;35(20):2745–2752. doi: 10.1016/j.vaccine.2017.03.028
- Langley JM, Wang L, Aggarwal N, et al. Immunogenicity and reactogenicity of an inactivated quadrivalent influenza vaccine administered intramuscularly to children 6 to 35 months of age in 2012-2013: a randomized, double-blind, controlled, multicenter, multicountry, clinical trial. J Pediatric Infect Dis Soc. 2015 Sep;4(3):242–51. doi: 10.1093/jpids/piu098
- Pepin S, Szymanski H, Rochin Kobashi IA, et al. Safety and immunogenicity of an intramuscular quadrivalent influenza vaccine in children 3 to 8 y of age: a phase III randomized controlled study. Hum Vaccin Immunother. 2016 Dec;12(12):3072–3078. doi: 10.1080/21645515.2016.1212143
- Beran J, Peeters M, Dewe W, et al. Immunogenicity and safety of quadrivalent versus trivalent inactivated influenza vaccine: a randomized, controlled trial in adults. BMC Infect Dis. 2013 May 20;13(1):224. doi: 10.1186/1471-2334-13-224
- Sheffield JS, Greer LG, Rogers VL, et al. Effect of influenza vaccination in the first trimester of pregnancy. Obstet Gynecol. 2012 Sep;120(3):532–537. doi: 10.1097/AOG.0b013e318263a278
- Thompson MG, Kwong JC, Regan AK, et al. Influenza Vaccine Effectiveness in Preventing Influenza-associated Hospitalizations During Pregnancy: A Multi-country Retrospective Test Negative Design Study, 2010-2016. Clin Infect Dis. 2019 Apr 24;68(9):1444–1453. doi: 10.1093/cid/ciy737
- Lu QC, Zhang TY, Bundhun PK, et al. One “misunderstood” health issue: demonstrating and communicating the safety of influenza a vaccination in pregnancy: a systematic review and meta-analysis. BMC Public Health. 2021 Apr 9;21(1):703. doi: 10.1186/s12889-021-10740-w
- Mehrabadi A, Dodds L, MacDonald NE, et al. Association of Maternal influenza vaccination during pregnancy with early childhood health outcomes. JAMA. 2021 Jun 8;325(22):2285–2293. doi: 10.1001/jama.2021.6778
- Wagenhauser I, Mees J, Reusch J, et al. Determinants of Influenza A infection rate in post-COVID-19 era. J Infect. 2023 Oct;87(4):361–364. doi: 10.1016/j.jinf.2023.08.003
- Van Ranst M, Zollner Y, Schelling J, et al. The burden of seasonal influenza: improving vaccination coverage to mitigate morbidity and its impact on healthcare systems. Expert Rev Vaccines. 2023 Jan;22(1):518–519. doi: 10.1080/14760584.2023.2221345
- Welch VL, Metcalf T, Macey R, et al. Understanding the Barriers and Attitudes toward Influenza Vaccine Uptake in the Adult General Population: A Rapid Review. Vaccines (Basel). 2023 Jan 13;11(1):180. doi: 10.3390/vaccines11010180
- Schmid P, Rauber D, Betsch C, et al. Barriers of Influenza Vaccination Intention and Behavior - A Systematic Review of Influenza Vaccine Hesitancy, 2005 - 2016. PLoS One. 2017;12(1):e0170550. doi: 10.1371/journal.pone.0170550
- Chen C, Liu X, Yan D, et al. Global influenza vaccination rates and factors associated with influenza vaccination. Int J Infect Dis. 2022 Dec;125:153–163.
- Pahmeier K, Speckemeier C, Neusser S, et al. Vaccinating the German population aged 60 years and over with a quadrivalent high-dose inactivated influenza vaccine compared to standard-dose vaccines: a transmission and budget impact model. PharmacoEconomics. 2023 Nov;41(11):1539–1550. doi: 10.1007/s40273-023-01299-y
- World Health Organization. Recommended composition of influenza virus vaccines for use in the 2024 southern hemisphere influenza season 2023 [cited 2023 Nov 7]. Available from: https://www.who.int/publications/m/item/recommended-composition-of-influenza-virus-vaccines-for-use-in-the-2024-southern-hemisphere-influenza-season
- Caini S, Meijer A, Nunes MC, et al. Is influenza B/Yamagata extinct and what public health implications could this have? An updated literature review and comprehensive assessment of global surveillance databases. 2023.
