Abstract
Objective: Umbilical cord blood offers a unique opportunity to study the basal level of immunoglobulin complexes. This study aims to determine the presence of immune complexes and complement deposition on erythrocytes from umbilical cord blood from normal, full-term pregnancies.
Methods: In vitro pre-formed IgA, IgG, and IgM complexes were used as positive control for flow cytometry detection, and for C3d deposition. Blood samples (34) of umbilical cord blood taken from vaginal and cesarean deliveries were tested for the presence of immunoglobulin complexes.
Results: Fourteen samples from vaginal deliveries and 20 samples from cesarean deliveries were assessed. IgG and IgM complexes were detected on erythrocytes, whereas no IgA complexes or complement deposition was observed. Interestingly, the percentage of IgG complexes was higher on erythrocytes from vaginal delivery samples compared to those from cesarean deliveries. No other associations between immune complexes and other maternal or newborn variables were found.
Conclusions: IgG and IgM complexes seem to be normally present on umbilical cord erythrocytes. Erythrocytes from vaginal deliveries have a higher percentage of IgG complexes present compared to that from cesarean deliveries. Since no C3d activity was detected, these complexes are non-pathological and should be part of the newborn’s initial innate immune response.
Introduction
Immune complexes are formed after the interaction between antibodies and antigens as part of a normal humoral immune response. However, immune complexes can also be formed with circulating immunoglobulins in the absence of their cognate antigens [Citation1]. These complexes are swept away from blood circulation with help from the complement system. The interaction between the FC fraction of antibodies and complement C1q allows further binding to the complement CR1 receptor that is present on the surface of different cells, including erythrocytes [Citation2]. Immune complexes are carried by erythrocytes and then cleared by the reticuloendothelial system, mainly by splenic macrophages [Citation3]. Nonetheless, immune complexes can also be pathological, particularly during the following events: self-antigen recognition, excessive immunoglobulin production, and deficiencies in the transportation system or altered clearance by erythrocytes [Citation4]. After endothelial deposition, immune complexes can trigger complement and neutrophil-activating tissue injury [Citation5]. In fetal and newborn hemolytic disease, antigen-specific maternal antibodies cross the fetal–placental barrier and then bind the surface of fetal erythrocytes, forming immunoglobulin complexes leading to complement-dependent hemolysis [Citation6,Citation7].
Several laboratory techniques have been used to detect immunoglobulin complexes, such as ELISA for soluble complexes [Citation8] and immunohistochemistry to trace their deposition in tissues [Citation9]. Recently, flow cytometry is applied to detect immune complexes on the surface of erythrocytes. This technique allows for the detection of immune complexes in patients with autoimmune hemolytic anemia, including some with a negative Coombs test [Citation10,Citation11,Citation12]. A direct Coombs test on umbilical cord blood has been used as a predictor of different clinical outcomes in neonatal hematological disorders [Citation7]. In general, the evaluation of immune complexes has focused primarily on their pathological role; however, little is known about the physiological properties of immune complexes that are transported by erythrocytes. Our group standardized a flow cytometry protocol that allows for the multiparametric and simultaneous detection of different isotyopes of immunoglobulin complexes along with C3d deposition on erythrocytes [Citation11].
The goal of this study was to determine if fetal erythrocytes from umbilical cord blood carry immune complexes on their membranes and whether these complexes can be detected by flow cytometry. Since this is a first approach to the biology of immune complexes in this context, the samples were studied in healthy, full-term, uncomplicated pregnancies in which deliveries occurred either by the vaginal route or cesarean section.
Methods
Study and population
This study was conducted with a total of 34 umbilical cord blood samples from vaginal (n = 14) or cesarean deliveries (n = 20). All births took place at the Hospital Universitario Fundación Santa Fe de Bogotá, in Bogotá D.C., Colombia, during 2015. Donors were selected from mothers (>18 years) with healthy, full-term pregnancies between 37 and 39 weeks of gestation. Both parents acknowledged participation in the study by signing an informed consent. Pregnancies with a history of severe disease, including hypertensive disorders, congenital diseases, autoimmune diseases, metabolic alterations, or any other major complication were excluded. Samples from multiple gestations or gestations conceived through in vitro methods were also excluded. The Ethical Committees of Universidad de los Andes (261/2014) and the Hospital Universitario Fundación Santa Fe de Bogota (CCEI-993-2014) approved this work according to national regulations and the Declaration of Helsinki.
Cord blood sampling
Samples were obtained by directly collecting blood from umbilical cord in a sterile, dry test-tube. The sample was taken from the cord segment proximal to the placenta, before the placenta was delivered and after extensively tissue washing with saline solution. From each blood sample, 16 μl were immediately diluted in 2 ml of phosphate buffer saline at 0.01 M pH 7.4 (1× phosphate-buffered saline [PBS]). This process was performed in order to prevent coagulation or agglutination. The diluted samples were preserved at 4 °C and transported to the laboratory for analysis by flow cytometry within the next 8 h.
In vitro immune complex formation
Immunoglobulin complexes were formed in vitro based on a previously described protocol by Alzate et al. [Citation11], with some variations, such as the use of umbilical erythrocytes and the titration of sera for the complement assays. For complement deposition and detection, pre-formed IgA, IgG and IgM complexes were exposed to different dilutions of normal serum or complement-depleted serum (56 °C for 30 min), starting at a 1:2 dilution ratio in 1× PBS. Briefly, IgG complex formation was based on the use of anti-Rh antibodies that were taken from previously sensitized Rh(−) mothers, at a 1:256 dilution added to Rh (+) erythrocytes. IgM complex formation was achieved by adding natural IgM antibodies against the A and B factors from O-type blood that was added to AB erythrocytes at a 1:256 dilution. IgA complexes were generated using purified human IgA (Sigma-Aldrich, St. Louis, MO) on erythrocytes previously treated with tannic acid.
Detection of complement deposition on immunoglobulin complexes
C3d complement deposition was initially assessed on IgM complexes: (a) alone, (b) plus complemented-depleted serum or (c) plus untreated serum. After incubation, a primary anti-human C3d antibody (ab17453, Abcam Cambridge, MA) was used, followed by a secondary goat anti-mouse IgG antibody conjugated with Alexa Fluor 647 (115-605-164, Jackson ImmunoResearch, West Grove, PA). Controls were prepared by adding the secondary antibody in the absence of the primary anti-C3d antibody. All samples were checked visually for hemolysis after serum was added (data not shown), and then the samples were run in a FACsCanto II flow cytometer (BD Biosciences, San Jose, CA).
Flow cytometry identification of immune complexes on umbilical cord blood
Detection of in vitro-formed complexes (positive controls) and those in vivo from umbilical cords were revealed using isotype-specific secondary antibodies (all from Jackson ImmunoResearch). Goat anti-human IgA conjugated with PerCP (109-125-011), goat anti-human IgG conjugated with FITC (109-095-098) and donkey anti-human IgM conjugated with PE (709-116-073) secondary antibodies were used at 1:200 dilutions. Isotype-matched antibodies were used as negative controls: goat anti-rabbit IgA conjugated with PerCP (Jackson ImmunoResearch 111-126-144), goat anti-rabbit IgG conjugated with FITC (Sigma-Aldrich F-0382) and donkey anti-rabbit IgM conjugated with PE (Jackson ImmunoResearch 711-116-152). Samples were run in a BD FACSCanto II flow cytometer and analyzed with FACSDIVA version 6.1 software (BD Biosciences). The results are shown as flow cytometry dot plots, identifying the populations of interest and determining the percentage of erythrocytes carrying complexes on their membranes. For each sample analyzed, an isotype control was always run. Based on these results, the quadrants (cutoff) in the dot plot figures were established.
Data presentation and statistical analysis
Population characteristics are shown as means, standar deviations (SDs) and percentages. The data sets were submitted to a distribution analysis using the Shapiro–Wilk test. When a normal distribution was obtained, the data are shown as the means and SDs, and comparisons were carried out using ANOVA with differences between two groups confirmed by a t-test or chi square test. In case of a non-parametric distribution, data are shown as the median and interquartile ranges (IQR), and comparisons were performed by the Kruskal–Wallis test with differences corroborated by the Mann–Whitney U test. Values were considered significant at p > 0.05.
Results
Detection of preformed immunoglobulin complexes and complement deposition on umbilical cord erythrocytes
Umbilical cord erythrocytes are suitable for in vitro formation of IgA, IgG and IgM complexes, and those complexes can fix and activate complement, as detected by C3d deposition. Normal sera dilutions (with complement) were necessary to avoid erythrocyte lysis. shows an example of a flow cytometry dot plot with in vitro-formed immune complexes on umbilical cord erythrocytes. Negative isotype controls are shown in . The simultaneous detection of IgG and IgM complexes is depicted in , and simultaneous detection of IgA and IgG is depicted in . Complement-dependent lysis of erythrocytes was observed in serum dilutions until the level of a 1:8 dilution. For this reason, serum was used at a 1:16 concentration. Complement-depleted sera did not show any C3d staining (). C3d deposition was detected on erythrocytes with IgM and IgG preformed complexes, , respectively. No C3d deposition was detected on IgA in vitro-formed complexes (data not shown).
Figure 1. Flow cytometry dot plots of in vitro-formed immune complexes. Immunoglobulin complex detection is shown from (A) to (D), and C3d complement deposition is shown from (E) to (H) on umbilical cord erythrocytes. Left panels correspond to isotype controls and right panels with human secondary antibodies. (A) and (B) dot plots depict simultaneous detection of IgG and IgM complexes. (C) and (D) dot plots simultaneous detection of IgA and IgG complexes. (F) and (H) dot plots show C3d deposition on independent IgM and IgG complexes, respectively; (E) and (G) controls with decomplemented serum. IgG complexes were formed with anti-Rh serum containing antibodies on Rh+, IgM complexes were formed with anti-AB serum on AB and IgA complexes were formed with human IgA added to acid tannic-treated erythrocytes.
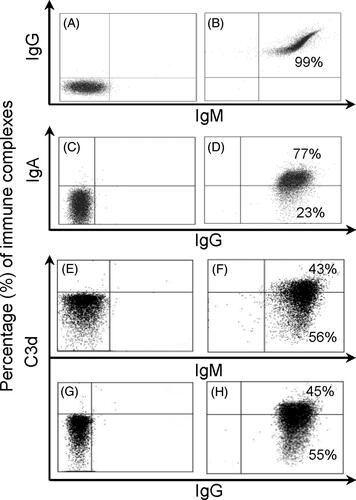
Detection of naturally formed immune complexes on umbilical cord erythrocytes
A total of 34 cord blood samples were collected, with 41.2% (14) obtained from vaginal deliveries and 58.8% (20) obtained from cesarean sections. The mean maternal age was 33.6 ± 4.1 years, and the mean gestational age was 38.5 ± 0.71 weeks. The mean neonatal weight was 3026.0 ± 383.3 g. A 35.3% of neonates were males, while 64.7% were females. All neonates achieved an APGAR score of at least 9 points at 5 min, as scored by a neonatologist. The data from mothers and newborns are summarized in .
Table 1. Characteristics of the population studied.
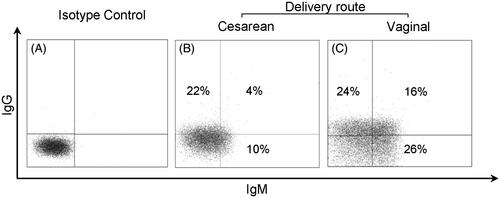
The median percentages of erythrocytes carrying immune complexes were as follows: 9.5% for IgG (IQR: 13.4%), 11.7% for IgM (IQR: 15.1%) and 0.6% for IgA (IQR: 0.2%), and 0.2% for C3d (IQR: 0.2%), as shown in . Cord blood erythrocytes obtained from vaginal deliveries had a median percentage of IgG complexes detected at 15.9%, which was significantly higher than the 5.7% detected on samples from a cesarean section (Mann–Whitney U test, p = 0.0043, ), whereas IgM complexes were similar (see . Examples of flow cytometry dot plots from two different cord blood donors, cesarean and vaginal delivery, are shown in , respectively. No other correlations were found when comparing the presence of immunoglobulin complexes on cord blood erythrocytes with the weight and height of the newborn, the maternal age, or the gestational age (data not shown). The time between birth (sampling) and lab processing did not have a statistical impact on the results. IgA complexes were barely detected on samples from either vaginal or cesarean section deliveries. Complement deposition was not observed in any of the immunoglobulin complexes, as denoted by the lack of C3d staining.
Figure 2. Detection of immune complexes in umbilical cord erythrocytes. (A) Median and interquartile range of immune complexes percentages from all samples. (B) Median and interquartile ranges of erythrocytes cells carrying IgG complexes according to the delivery route and (C) IgM complexes. Mann–Whitney U test was used to obtain p values.
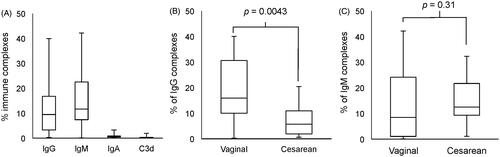
Figure 3. Flow cytometry dot plot examples. Dot plot flow cytometry analysis of Isotype control (A), sample from cesarean delivery (B) and (C) vaginal delivery sample. As mentioned, comparing vaginal and cesarean delivery, there was a difference for IgG complexes (p = 0.0043), but not in IgM complexes (p = 0.31).
Discussion
Umbilical cord erythrocytes can form and transport immunoglobulin complexes both in vitro and naturally. Complexes formed in vitro also activate a complement cascade, as demonstrated by positive C3d staining. Natural complexes present on umbilical cord are primarily of the IgG and IgM isotypes. However, neither of these naturally formed immune complexes detected in cord blood showed C3d staining. In fact, all donors did not present with any type of fetal or maternal pathology; the samples that were processed came from “healthy” pregnancies. Therefore, it seems that these immunoglobulin complexes do not have a pathological role, and on the contrary, they have a physiological one.
Interestingly, the samples that were derived from vaginal deliveries had a higher percentage of IgG-isotype immune complexes detected compared to those from cesarean section deliveries. This finding may facilitate hypotheses pertinent to the neonatal immune response. Fetal immunoglobulin production starts in utero as early as the 20th gestational week [Citation13,Citation14]. There is also evidence supporting the presence of fetal-derived immunoglobulins of the IgM, IgG and IgA isotypes before the end of the second trimester of gestation [Citation15]. Antibody transfer from the mother to the fetus is restricted, and only maternal IgG crosses the fetal–placental barrier [Citation13,Citation15,Citation16]. This process occurs primarily through active transport mechanisms that involve Fcγ receptors that are widely present in the placental tissue [Citation13]. Hence, it could be hypothesized that labor, but not cesarean delivery, potentiates the mechanisms responsible for the active transport of IgG. Because other immunoglobulin isotypes are not transported across the placental barrier, their concentration in fetal circulation is unaffected by the route of delivery. Consequently, the low levels of IgM complexes that were identified in both vaginal- and cesarean-derived samples probably have a fetal origin. In this case, complexes are formed by naturally occurring antibodies that are typically identified as the IgM isotype [Citation17] or antibodies that are produced due to direct antigenic stimuli, as in the case of subclinical infection [Citation15,Citation18].
In this work, no complement deposition was associated with the immunoglobulin complexes that were detected; consequently, these complexes do not induce erythrocyte lysis. However, their presence can mediate phagocytosis by splenic macrophages that interact with complexes bound to the erythrocyte surface via CR1 receptors [Citation19]. This complement receptor is presented in newborn with a higher density per erythrocyte as compared to adult cells [Citation20]. Similarly, these complexes can activate the neonatal innate immune response, particularly after vaginal delivery. IgG immune complexes crosslink with the FcγRII receptor on macrophages [Citation21]. This process triggers intracellular signaling pathways in macrophages that result in the activation and translocation of nuclear transcription factors, leading to cytokine secretion or production of molecules from the oxidative burst [Citation22]. Indeed, vaginal delivery increased the level of IL-8, and the expression of IL-8 receptor on neutrophils, as well as enhanced the in vitro spontaneous and IL-8-dependent neutrophil transmigration. Additionally, there is an increase of the surface expression and secretion of soluble adhesion molecules such as E-Selectin compared to newborns from a cesarean section [Citation23,Citation24]. TNF-α, an inflammatory cytokine associated with the expression of adhesion molecules in neutrophils and endothelium, is increased in the neonatal circulation following vaginal deliveries [Citation25]. Finally, cord blood of newborns from cesarean sections has significantly lower counts of leukocytes [Citation26,Citation27].
In the last few decades, there has been a dramatic increase in the rate of cesarean section deliveries [Citation28]. This phenomenon has been associated with several adverse outcomes, including immune system dysfunction. As a result, cesarean sections are associated with a higher risk of autoimmune, respiratory, and gastrointestinal diseases in the newborn [Citation28,Citation29]. It is necessary to have a larger population size that would allow to confirm the association between the presence of immunoglobulin complexes in normal pregnancies, and also to include samples from maternal–fetal diseases such as blood type incompatibility to identify “pathological complexes”. Despite these limitations, evidence suggests that the delivery route has a direct effect on the development and activation of the newborn’s immune system. This study proposes that IgG immune complexes can trigger the neonatal immune response in a sterile environment.
Acknowledgements
We extend our sincere thanks to the Gynecology and Obstetrics Department from Fundación Santa Fe de Bogota, Colombia for the support of this project. A.d.L. was partially supported by the program “Semillero de Investigación” 619, COLCIENCIAS, Colombia.
Disclosure statement
The authors do not report any conflicts of interests.
References
- Steensgaard J, Johansen AS. Biochemical aspects of immune complex formation and immune complex diseases. Allergy 1980;35:457–72.
- Schifferli JA, Ng YC, Paccaud JP, et al. The role of hypocomplementaemia and low erythrocyte complement receptor type 1 numbers in determining abnormal immune complex clearance in humans. Clin Exp Immunol 1989;75:329–35.
- Lutz HU. Naturally occurring autoantibodies in mediating clearance of senescent red blood cells. Adv Exp Med Biol 2012;750:76–90.
- Toong C, Adelstein S, Phan TG. Clearing the complexity: immune complexes and their treatment in lupus nephritis. Int J Nephrol Renovasc Dis 2011;4:17–28.
- Podolska MJ, Biermann MH, Maueröder C, et al. Inflammatory etiopathogenesis of systemic lupus erythematosus: an update. J Inflamm Res 2015;8:161–71.
- Zwingerman R, Jain V, Hannon J, et al. Alloimmune red blood cell antibodies: prevalence and pathogenicity in a Canadian prenatal population. J Obstet Gynaecol Can 2015;37:784–90.
- Dinesh D. Review of positive direct antiglobulin tests found on cord blood sampling. J Paediatr Child Health 2005;41:504–7.
- Stanilova SA, Slavov ES. Comparative study of circulating immune complexes quantity detection by three assays-CIF-ELISA, C1q-ELISA and anti-C3 ELISA. J Immunol Methods 2001;253:13–21.
- Mubarak M, Kazi JI, Kulsoom U, et al. Detection of immunoglobulins and complement components in formalin fixed and paraffin embedded renal biopsy material by immunoflourescence technique. J Nephropathol 2012;1:91–100.
- Thedsawad A, Taka O, Wanachiwanawin W. Development of flow cytometry for detection and quantitation of red cell bound immunoglobulin G in autoimmune hemolytic anemia with negative direct Coombs test. Asian Pac J Allergy Immunol 2011;29:364–7.
- Alzate MA, Manrique LG, Bolaños NI, et al. Simultaneous detection of IgG, IgM, IgA complexes and C3d attached to erythrocytes by flow cytometry. Int J Lab Hematol 2015;37:382–9.
- Chaudhary R, Das SS, Gupta R, et al. Application of flow cytometry in detection of red-cell-bound IgG in Coombs-negative AIHA. Hematology 2006;11:295–300.
- Saji F, Samejima Y, Kamiura S, et al. Dynamics of immunoglobulins at the feto-maternal interface. Rev Reprod 1999;4:81–9.
- Van Furth R, Schuit HR, Hijmans W. The immunological development of the human fetus. J Exp Med 1965;122:1173–88.
- Alkan Ozdemir S, Ozer EA, Kose S, et al. Reference values of serum IgG and IgM levels in preterm and term newborns. J Matern Fetal Neonatal Med 2016;29:972–6.
- Madi A, Bransburg-Zabary S, Kenett DY, et al. The natural autoantibody repertoire in newborns and adults: a current overview. Adv Exp Med Biol 2012;750:198–212.
- Ailus K, Palosuo T. IgM class autoantibodies in human cord serum. J Reprod Immunol 1995;29:61–7.
- Blankenship WJ, Cassady G, Schaefer J, et al. Serum gamma-M globulin responses in acute neonatal infections and their diagnostic significance. J Pediatr 1969;75:1271–81.
- De Back DZ, Kostova EB, van Kraaij M, et al. Of macrophages and red blood cells; a complex love story. Front Physiol 2014;5:9. doi: 10.3389/fphys.2014.00009.
- Buffone GJ, Leatherwood CM, Person DA, et al. An immunoradiometric assay for erythrocyte complement (C3b) receptor activity applied to a pediatric population with connective tissue disease. Clin Chem 1983;29:1720–3.
- Mellman I, Plutner H. Internalization and degradation of macrophage Fc receptors bound to polyvalent immune complexes. J Cell Biol 1984;98:1170–7.
- Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol 2008;8:34–47.
- Yektaei-Karin E, Moshfegh A, Lundahl J, et al. The stress of birth enhances in vitro spontaneous and IL-8-induced neutrophil chemotaxis in the human newborn. Pediatr Allergy Immunol 2007;18:643–51.
- Gessler P, Dahinden C. Increased respiratory burst and increased expression of complement receptor-3 (CD11b/CD18) and of IL-8 receptor-A in neutrophil granulocytes from newborns after vaginal delivery. Biol Neonate 2003;83:107–12.
- Malamitsi-Puchner A, Protonotariou E, Boutsikou T, et al. The influence of the mode of delivery on circulating cytokine concentrations in the perinatal period. Early Hum Dev 2005;81:387–92.
- Nikischin W, Peter M, Oldigs HD. The influence of mode of delivery on hematologic values in the umbilical vein. Gynecol Obstet Invest 1997;43:104–7.
- Thilaganathan B, Meher-Homji N, Nicolaides KH. Labor: an immunologically beneficial process for the neonate. Am J Obstet Gynecol 1994;171:1271–2.
- Cho CE, Norman M. Cesarean section and development of the immune system in the offspring. Am J Obstet Gynecol 2013;208:249–54.
- Kristensen K, Henriksen L. Cesarean section and disease associated with immune function. J Allergy Clin Immunol 2016;137:587–90.
