Abstract
Introduction: Although single-suture craniosynostosis is diagnosed sporadically during pregnancy, timely referral is critical for its treatment. Additionally, craniosynostosis leads to increased maternofetal trauma during birth. In the Netherlands, 95% of pregnant women receive a standard ultrasound at around 20 weeks of gestation, potentially an ideal setting for detecting craniosynostosis prenatally. To enhance the prenatal detection of the metopic and the sagittal suture synostosis, we wished to identify new screening parameters.
Materials and methods: We retrospectively analyzed data of the 20-week anomaly scan in trigonocephaly patients (n = 41), scaphocephaly patients (n = 41), and matched controls (n = 82). We measured six different cranial dimensions, including head circumference, biparietal diameter, and occipito-frontal diameter, defining the cephalic index as the ratio between biparietal and occipito-frontal diameter.
Results: Prenatal biometric measurements did not differ significantly between trigonocephaly patients and controls. Although significantly lower in scaphocephaly patients (0.76 versus 0.79; p = .000), the cephalic index by itself is not appropriate for screening at 20 weeks of gestation. Longitudinal analysis suggests that a deflection in BPD curve is found in scaphocephaly patients, starting at 20 weeks of gestation.
Conclusions: Prenatal biometric measurements do not differ significantly between trigonocephaly patients and controls. The CI is lower in scaphocephaly patients. A deflection in BPD curve should be followed by 3 D imaging of the cranial sutures.
Introduction
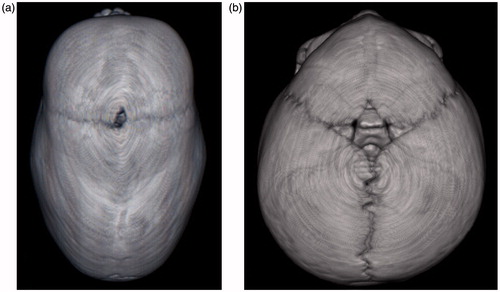
During normal development, skull development starts with the formation of bone centers within the membranous anlage, covering the brain. From these, calvarial bones enlarge by ongoing ossification at the edges and cranial suture development starts at the sites where the bones come in close proximity. While open, the cranial sutures are the major sites of calvarial growth [Citation1]. Normally, the metopic suture is completely fused at one year of age. The sagittal suture, however, remains open throughout the youth and eventually closes at 22–24 years of age [Citation2]. Premature fusion of a suture is known as craniosynostosis and results in an altered head shape and increased risk of elevated intracranial pressure (). The process of premature fusion is known to start at 15 weeks of gestation for trigonocephaly and at 18 weeks for scaphocephaly [Citation1].
Figure 1. Sagittal and metopic suture synostosis results in different skull shapes shown on a CT-scan before operation. (a) Sagittal suture synostosis, resulting in the typical boat-like shaped skull, scaphocephaly. (b) Metopic suture synostosis, resulting in trigonocephaly.
In 24% of patients, craniosynostosis is accompanied by additional anomalies (syndromic craniosynostosis). In the remaining 76% of patients, craniosynostosis is the only finding, referred to as the isolated form [Citation3]. The two commonest isolated forms are sagittal suture synostosis, called scaphocephaly for its resulting deformity, with an incidence of 1:3000 live births, and metopic suture synostosis, called trigonocephaly, with an incidence of 1:4500 live births [Citation4]. Neuropsychological development of children with single suture craniosynostosis is affected [Citation5]. Early surgery (before 6 months of age) may possibly prevent this to some extent [Citation6].
As routine part of free accessible antenatal care in the Netherlands, a 20-week anomaly scan is offered to all pregnant women and 95% chooses to participate [Citation7]. Apart from the syndromic forms, isolated craniosynostosis is detected only sporadically during prenatal ultrasound and, if so, in the third trimester [Citation8].
An important function of the cranial sutures is to enable molding of the skull when passing through the birth canal [Citation9]. Craniosynostosis may interfere with this natural adaptive process and preclude a normal birth. A higher rate of vaginal breech deliveries and secondary cesarean sections, compared to the general population, has been reported among neonates who were diagnosed with craniosynostosis after birth [Citation10]. Prenatal detection of craniosynostosis could anticipate delivery complications and would enable timely, well-anticipated and on average less invasive treatment and therefore less risk and subsequent complications.
This study aims to identify new ultrasound-based screening methods to enhance prenatal detection of sagittal and metopic suture synostosis. We retrospectively analyzed in a blinded fashion prenatal ultrasound scans performed between 18 and 22 weeks of gestation of 41 scaphocephaly patients, 41 trigonocephaly patients, and their matched controls. To our knowledge, this is the first study to investigate the prenatal ultrasound detection of the selected types of single-suture craniosynostosis in a large cohort with their matched controls.
Materials and methods
The Erasmus University Medical Center’s medical ethical board approved this study (MEC-2013–293). Patients who were treated for scaphocephaly or trigonocephaly from 2006 to 2013 in the Sophia Children’s Hospital in Rotterdam, the Netherlands, were identified. Exclusion criteria were twin pregnancy, a syndromic diagnosis and incomplete follow-up to surgery. Parents of all included patients were asked to provide permission to retrieve ultrasound data and images of the 20-week anomaly scan for the purpose of this study.
Matching
For all patients of whom ultrasound images were available of the 20-week anomaly scan a matched control was obtained. The control population was provided by the “Foundation Prenatal Screening, south-west region of the Netherlands”, which is responsible for the quality assurance and audit program of 20-week prenatal screening in the South-West region of the Netherlands (approx. 2.5 M inhabitants). Exclusion criteria for the control scans were presence of structural anomalies and twin pregnancy. Patients and controls were matched on gestational age (maximum discrepancy of 6 days) and fetal presentation at the 20-week anomaly scan, as breech position may have an effect on BPD [Citation11]. For each case, one control was obtained.
Biometrics
Biometric measurements performed during the 20-week anomaly ultrasound examination included head circumference (HC), biparietal diameter (BPD), occipito-frontal distance (OFD), trans-cerebellar diameter (TCD), inner and outer orbital distance (IOD and OOD), and cephalic index (CI). HC, BPD, and OFD were all measured in the standardized axial plane of the cranium [Citation12]. For HC, an ellipse was drawn around the outline of the skull, BPD was measured at the outer – outer diameter perpendicular to the midline and OFD was an anteroposterior measurement from outer skull to outer skull. For TCD, the distance between the outer lateral edges of the cerebellum was measured in the suboccipito – bregmatic plane [Citation9,Citation12–14]. The IOD was measured between the inner side of the medial margins of the orbitae and the OOD was measured between the inner sides of the lateral margins of the orbitae. All primary measurements were in the absence of any adjuvant information. The CI was calculated as the BPD/OFD ratio [Citation13]. Norm values were derived from Chitty et al. [Citation13]. One expert reviewer (I.A.), blinded for the diagnosis, reviewed all original measurements, substituting missing or manifest incorrect values by measurements using the standard Astraia Software for Women’s Health, Obstetric and Gynaecological Database, which allows importation and recalibration of images prior to making measurements.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics v21 (Armonk, NY). For the biometric measurements, we performed a paired samples t-test, as advocated for case-control studies, matched in a 1:1 fashion, by Niven et al. [Citation15]. The intention was an exploratory analysis. Threshold for testing was p = .05.
Bonferroni’s correction was applied to correct for multiple testing. To explore the potential for diagnostic use of the biometric measurements, in particular the cephalic index, we constructed a ROC curve. The yield of the cephalic index was established on various cutoff points; in particular, we explored whether above a particular threshold a substantial number of cases were included (screening purpose).
Results
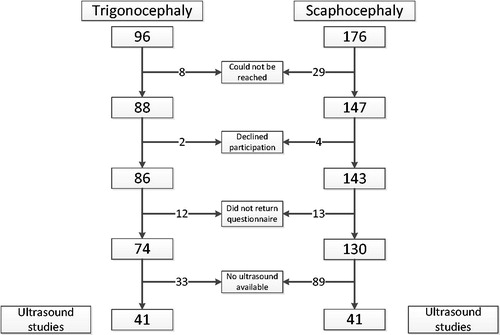
A total of 272 craniosynostosis patients were found eligible and were approached for participation. In total, 74 metopic suture synostosis patients and 130 sagittal suture synostosis patients returned the questionnaire ().
Of the participating patients, median (postnatal) age at referral to our center was significantly higher for trigonocephaly compared to scaphocephaly (5.1 versus 3.2 months, p = .003, ). None of the participating patients was diagnosed prenatally.
Table 1. Age at time of referral to our center.
Ultrasound images of the 20-week anomaly scan were digitally available in 41 out of the 74 trigonocephaly patients and in 41 out of the 130 scaphocephaly patients. Baseline and matching data are shown in . In both patient groups, 93% of the pairs were matched with a maximum difference of one day. Maximum difference in gestational age between case and control at time of prenatal ultrasound was six days.
Table 2. Baseline characteristics and matching data.
Biometrics
An example of both a trigonocephaly and scaphocephaly patient’s 20-week anomaly scan is shown in . shows the outcomes of the paired samples t-test for the biometric data. In the trigonocephaly group, no significant differences were found. In scaphocephaly patients, the cephalic index is significantly lower than in its matched control group (mean 0.76 versus 0.79; p < .001). Bonferroni’s correction implied that the threshold for significance was a p value of .007.
Table 3. Paired samples t-test on biometric data.
To assess the diagnostic value of the cephalic index in scaphocephaly patients, a ROC curve was plotted resulting in an area under the curve of 0.70, indicating a fair diagnostic test (. shows the different test characteristics for various cutoff values. The discriminative power, in terms of the positive likelihood ratio, is maximal (6.4) at CI =0.73.
Figure 3. Images of the 20-week anomaly scan of a trigonocephaly and scaphocephaly patient. Images A–D show ultrasound images of a trigonocephaly patient. Images E–H show the ultrasound study of a scaphocephaly patient.
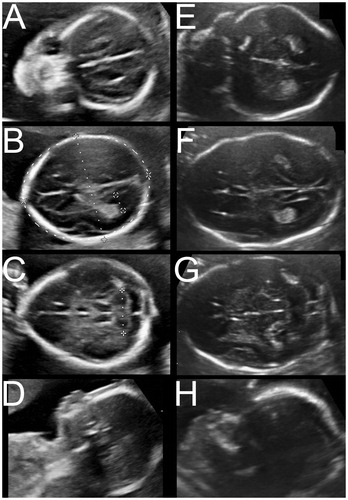
Table 4. Test characteristics of the cephalic index for scaphocephaly.
For eight scaphocephaly patients, additional ultrasound images at the late second and third trimester were available. HC, BPD, OFC, and CI were measured to explore the change of these parameters during fetal development (). A notable deflection in the BPD-curve, compared to the norm population, was noted. Two out of the eight patients were in breech position at the time of their last scan.
Figure 4. ROC curve ROC curve to assess the diagnostic value of the cephalic index in scaphocephaly patients (Area under the curve =0.702).
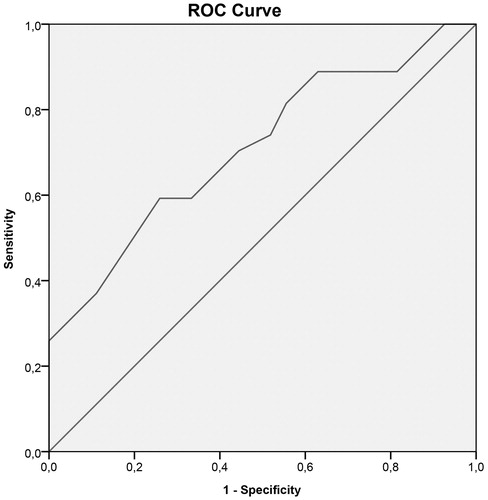
Figure 5. Longitudinal growth curves Growth curves of the HC, BPD, OFD, and CI of eight scaphocephaly patients in which additional ultrasounds were available. Norm curves are derived from Chitty et al. [Citation13]. None of the patients were diagnosed prenatally.
![Figure 5. Longitudinal growth curves Growth curves of the HC, BPD, OFD, and CI of eight scaphocephaly patients in which additional ultrasounds were available. Norm curves are derived from Chitty et al. [Citation13]. None of the patients were diagnosed prenatally.](/cms/asset/84d0c55f-0fcd-454a-a3ed-23f151b57623/ijmf_a_1335706_f0005_b.jpg)
Discussion
Scaphocephaly patients showed a significant lower cephalic index at 20 weeks gestation compared to controls. Theoretically, this morphological parameter can lead to earlier diagnosis of scaphocephaly. At the 20-week anomaly scan, biometric measurements in patients with metopic suture synostosis did not differ from controls, despite the difference in time of onset between metopic and sagittal suture synostosis (15 versus 18 weeks of gestation, respectively). Previous studies have shown an increased biparietal diameter in metopic suture synostosis patients before operation. In our data, this was not found statistically significant prenatally [Citation16,Citation17]. We hypothesize the following mechanism to be responsible: the increase of BPD in metopic suture synostosis patients is a secondary, compensatory, effect after metopic suture synostosis. In contrary to the decrease of BPD in scaphocephaly patients, which is a primary effect following sagittal suture craniosynostosis. Therefore, we would expect the enlarged BPD in trigonocephaly to become visible at a later stage.
Although single-suture craniosynostosis is diagnosed most often in the first year of life, its pathology starts in the early second trimester, with fusion of the sutures, resulting in bone-center displacement [Citation1,Citation18]. Our study shows the effect of the developing single-suture craniosynostosis on biometric measurements of the skull, particularly the CI, during the second trimester of pregnancy.
The CI was introduced in 1987 to detect fetuses with Down’s syndrome who were often more brachycephalic than normal infants [Citation19]. Numerous follow-up studies, however, showed CI to be insufficiently reliable to screen for Down’s syndrome prenatally, hence its use in prenatal screening was discarded [Citation20]. However, this study suggests that CI may have a role in prenatal screening for scaphocephaly.
To assess the diagnostic value of the CI in screening for scaphocephaly, we constructed a ROC curve. The positive likelihood ratio was maximal (6.4) at CI =0.73. This implies a more than six-fold risk increase for scaphocephaly if CI is less than or equal to 0.73. While a positive likelihood ratio of more than six in general terms is a promising figure in screening, the clinical importance in scaphocephaly is mainly decided by the prevalence of the condition (1:1600 live births) [Citation4]. The rarity of the condition implies that even among the selected screen positives, scaphocephaly would be uncommon. A “watchful waiting” policy after a positive screen would imply a stressful period for the parents. It is clear that the discriminative power of the CI at 20 weeks of gestation is too limited to solely rely on this early single measure.
Several strategies for improvement can be considered. An obvious strategy is to take advantage of the fact that craniosynostosis is an ongoing process. As numerous studies reported a pre-operative cephalic index of scaphocephaly infants of approximately 0.67 at the age of 5–6 months suggesting continued CI decrease over time, we expect a substantial gain in discrimination with a repeated measurement [Citation21–23]. The repeat scans, executed for growth assessment, in eight fetuses with postnatally diagnosed scaphocephaly confirm this hypothesized continued decrease of BPD and CI (. As only two out of the eight fetuses were in breech position at the time of the scan, we assess this has not played a role in these curves.
A second strategy is to combine biometric information with independent other diagnostic information, either scan related or otherwise. Recently, the “brain shadowing sign” has been suggested as an independent novel marker of craniosynostosis [Citation24]. If truly independent as marker, it could improve discriminatory power if it is added to CI information, like the 1st trimester combination test where the combination of several parameters increase the discriminative power of the test [Citation25]. The presence of the brain shadowing sign can, however, easily be missed in a routine clinical setting, hence field testing must show its additive value.
A third strategy is to add routine measurement of cranial sutures with 3 D-ultrasound [Citation26–28]. However, 3 D-imaging of the cranial sutures for screening purposes is not feasible as it is time-consuming and highly demanding in terms of expertise. While a defined deflection of BPD or CI justifies further diagnostic imaging, such as 3 D imaging of the cranial sutures, this so far does not seem a feasible part of screening.
Third trimester ultrasound is becoming more common to assess fetal growth and provides a chance for the above-mentioned strategy concerning repeated measurements. When the third trimester ultrasound shows a deflection in BPD or CI curve it should be followed by 3 D-imaging of the cranial sutures [Citation26]. However, before advocating the routine assessment of skull parameters at the third trimester ultrasound in order to detect craniosynostosis, the findings of the present study should first be validated in a larger population.
We finally discuss the potential benefits of early diagnosis. In our view, at least there are three. The first was already mentioned: adequate risk management of delivery, as complicated births may be expected [Citation10]. Secondly, prenatal diagnosis enables psychological anticipation. In a recent paper parents, after the first shock, were shown to value the possibility of anticipation and precise treatment planning [Citation29]. Thirdly, scaphocephaly infants are at non-trivial risk for developing increased intracranial pressure and timely treatment is of undisputed benefit. Early referred infants in our center are treated with a spring-assisted cranioplasty between 4.5 and 6 months of age to prevent this complication. At a later age, treatment consists of a total-vault remodeling procedure, which entails a slightly poorer neurodevelopmental outcome, a prolonged hospital stay and greater blood loss [Citation23]. To be able to perform surgery within the preferred period, a referral at a minimum of 6 weeks before maximum age at operation is required. This implies that scaphocephaly patients should be referred before 4.5 months of age. Our results show that 24% of scaphocephaly patients is referred too late to be operated on in time according to our center’s treatment protocol. In principle, prenatal detection of single-suture craniosynostosis allows early referral to a craniofacial team and would prevent late surgery.
This study has a number of limitations. Some of these are intrinsic: craniosynostosis is a rare disease and even with national centralization in two centers in the Netherlands, study numbers are limited. Also, the retrospective design clearly contributes to further limitation of numbers. Twenty-five percent of patients (68 out of the 263) could not be reached, refused to participate or did not return the questionnaire in time. Additionally, although the 20-week anomaly scan was officially introduced in 2007 and required the storage of images, ultrasound images could only be retrieved for 82 out of the 204 participating patients. We note that the number of retrievable ultrasound studies was less prior to 2009, as also reported by our audit of the prenatal screening program in our region [Citation30]. The same protocol was followed in both patient groups, therefore we do not have a logical explanation for the difference in number of retrievable ultrasounds between scaphocephaly (41/130) and trigonocephaly (41/74). As all trigonocephaly and scaphocephaly patients were approached for inclusion and all cases were detected postnatally, selection bias most likely did not affect our results.
In conclusion, this study presents a first step towards the prenatal diagnosis of single-suture craniosynostosis. At this stage, the cephalic index is not suitable for screening on scaphocephaly at 20 weeks of gestation. A repeat measurement at a third trimester ultrasound is promising and subject to future research. A deflection of the BPD or CI curve at third trimester ultrasound should be followed by 3 D-imaging of the cranial sutures.
Acknowledgements
The authors thank the ultrasonographers for providing the ultrasound images. The authors would also like to thank Sorg-Saem B.V. Amsterdam, the Netherlands, for providing Astraia Software for Women’s Health, Obstetric and Gynaecological Database.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Mathijssen IM, van Splunder J, Vermeij-Keers C, et al. Tracing craniosynostosis to its developmental stage through bone center displacement. J Craniofac Genet Dev Biol. 1999;19:57–63.
- Vu HL, Panchal J, Parker EE, et al. The timing of physiologic closure of the metopic suture: a review of 159 patients using reconstructed 3D CT scans of the craniofacial region. J Craniofac Surg. 2001;12:527–532.
- Sharma VP, Fenwick AL, Brockop MS, et al. Mutations in TCF12, encoding a basic helix-loop-helix partner of TWIST1, are a frequent cause of coronal craniosynostosis. Nat Genet. 2013;45:304–307.
- Cornelissen M, Ottelander BD, Rizopoulos D, et al. Increase of prevalence of craniosynostosis. J Craniomaxillofac Surg. 2016;44:1273–1279.
- Speltz ML, Collett BR, Wallace ER, et al. Intellectual and academic functioning of school-age children with single-suture craniosynostosis. Pediatrics. 2015;135:e615–e623.
- Patel A, Yang JF, Hashim PW, et al. The impact of age at surgery on long-term neuropsychological outcomes in sagittal craniosynostosis. Plast Reconstr Surg. 2014;134:608e–617e.
- Foundation Prenatal Screening SWRTN. Annual report; 2012. Available from: http://www.prenatale-screening.nl/files/documents/Jaarverslag_2012.pdf.
- Delahaye S, Bernard JP, Renier D, et al. Prenatal ultrasound diagnosis of fetal craniosynostosis. Ultrasound Obstet Gynecol. 2003;21:347–353.
- Lapeer RJ, Prager RW. Fetal head moulding: finite element analysis of a fetal skull subjected to uterine pressures during the first stage of labour. J Biomech. 2001;34:1125–1133.
- Weber B, Schwabegger AH, Oberaigner W, et al. Incidence of perinatal complications in children with premature craniosynostosis. J Perinat Med. 2010;38:319–325.
- Johnsen SL, Rasmussen S, Sollien R, et al. Fetal age assessment based on ultrasound head biometry and the effect of maternal and fetal factors. Acta Obstet Gynecol Scand. 2004;83:716–723.
- International Society of Ultrasound in O, Gynecology Education C. Sonographic examination of the fetal central nervous system: guidelines for performing the ‘basic examination’ and the ‘fetal neurosonogram’. Ultrasound Obstet Gynecol. 2007;29:109–116.
- Chitty LS, Altman DG, Henderson A, et al. Charts of fetal size: 2. Head measurements. Br J Obstet Gynaecol. 1994;101:35–43.
- van der Ham LI, Cohen-Overbeek TE, Paz y, et al. The ultrasonic detection of an isolated craniosynostosis. Prenat Diagn. 1995;15:1189–1192.
- Niven DJ, Berthiaume LR, Fick GH, et al. Matched case-control studies: a review of reported statistical methodology. Clin Epidemiol. 2012;4:99–110.
- Maltese G, Tarnow P, Wikberg E, et al. Intracranial volume before and after surgical treatment for isolated metopic synostosis. J Craniofac Surg. 2014;25:262–266.
- Ruiz-Correa S, Starr JR, Lin HJ, et al. New severity indices for quantifying single-suture metopic craniosynostosis. Neurosurgery. 2008;63:318–324. discussion 24–25.
- Mathijssen IM, Vaandrager JM, van der Meulen JC, et al. The role of bone centers in the pathogenesis of craniosynostosis: an embryologic approach using CT measurements in isolated craniosynostosis and Apert and Crouzon syndromes. Plast Reconstr Surg. 1996;98:17–26.
- Lockwood C, Benacerraf B, Krinsky A, et al. A sonographic screening method for Down syndrome. Am J Obstet Gynecol. 1987;157:803–808.
- Shah YG, Eckl CJ, Stinson SK, et al. Biparietal diameter/femur length ratio, cephalic index, and femur length measurements: not reliable screening techniques for Down syndrome. Obstet Gynecol. 1990;75:186–188.
- Agrawal D, Steinbok P, Cochrane DD. Long-term anthropometric outcomes following surgery for isolated sagittal craniosynostosis. J Neurosurg. 2006;105:357–360.
- Sakamoto Y, Nakajima H, Tamada I. Outcome analysis of morcellation craniotomy with distraction osteogenesis for scaphocephaly. Pediatr Neurosurg. 2013;49:248–253.
- van Veelen ML, Mathijssen IM. Spring-assisted correction of sagittal suture synostosis. Childs Nerv Syst. 2012;28:1347–1351.
- Krajden Haratz K, Leibovitz Z, Svirsky R, et al. The ‘Brain Shadowing Sign’: a novel marker of fetal craniosynostosis. Fetal Diagn Ther. 2016;40:277–284.
- Snijders RJ, Noble P, Sebire N, et al. UK multicentre project on assessment of risk of trisomy 21 by maternal age and fetal nuchal-translucency thickness at 10–14 weeks of gestation. Fetal Medicine Foundation First Trimester Screening Group. Lancet. 1998;352:343–346.
- Dikkeboom CM, Roelfsema NM, Van Adrichem LN, et al. The role of three-dimensional ultrasound in visualizing the fetal cranial sutures and fontanels during the second half of pregnancy. Ultrasound Obstet Gynecol. 2004;24:412–416.
- Faro C, Benoit B, Wegrzyn P, et al. Three-dimensional sonographic description of the fetal frontal bones and metopic suture. Ultrasound Obstet Gynecol. 2005;26:618–621.
- Chaoui R, Levaillant JM, Benoit B, et al. Three-dimensional sonographic description of abnormal metopic suture in second- and third-trimester fetuses. Ultrasound Obstet Gynecol. 2005;26:761–764.
- van der Steen SL, Riedijk SR, Verhagen-Visser J, et al. The psychological impact of prenatal diagnosis and disclosure of susceptibility loci: first impressions of parents’ experiences. J Genet Couns. 2016;25:1227–1234.
- Ursem NTC, Peters IA, van der Est MN, et al. An audit of second-trimester fetal anomaly scans based on a novel image-scoring method in the Southwest region of the Netherlands. J Ultrasound Med. 2017;36:1171–1179.