Abstract
Objective: To identify the level of amniotic fluid lactate (AFL), placental growth factor (PLGF), and vascular endothelial growth factor (VEGF) at second trimester amniocentesis, and to compare levels in normal pregnancies with pregnancies ending in a miscarriage, an intrauterine growth restricted fetus (IUGR) or decreased fetal movements.
Study design: A prospective cohort study. Amniotic fluid was consecutively collected at amniocentesis in 106 pregnancies. Fetal wellbeing at delivery was evaluated from medical files and compared with the levels of AFL, VEGF, and PLGF at the time of amniocentesis.
Results: The median level of AFL was 6.9 mmol/l, VEGF 0.088 pg/ml, and PLGF 0.208 pg/ml. The median levels of AFL in pregnancies ended in miscarriage were significantly higher (10.7 mmol/l) compared to those with a live new-born (6.9 mmol/L, p = .02). The levels of VEGF (p = .2) and PLGF (p = .7) were not affected. In pregnancies with an IUGR, the median level of AFL was higher compared to those with normal fetal growth (p = .003). No differences VEGF (p = .5), but significant lower PLGF were found in IUGR pregnancies (p = .03).
Conclusions: Pregnancies ending in a miscarriage or with IUGR had significantly higher median values of AFL but lower values of PLGF in the amniotic fluid at the time of second trimester amniocentesis compared to normal pregnancies.
Keywords:
Introduction
Four million children die annually at childbirth, one million of them due to asphyxia. A large proportion of these newborns have suffered from chronic hypoxia during pregnancy, and fatal fetal outcome does not always involve procedures during delivery. Intrauterine fetal death (IUFD) is a serious pregnancy complication with a strong correlation to chronic fetal hypoxia. Every year, more than 400 IUFDs are diagnosed in Sweden in pregnancies at gestational week 22 or more [Citation1]. Globally there are approximately 2.65 million intrauterine fetal deaths (with a gestational age >28 weeks) [Citation2]. Biochemical markers of hypoxia are circulating factors that when analyzed could potentially aid the diagnosis of chronic fetal hypoxia. The amniotic fluid (AF) is considered to mirror the intrauterine environment of the fetus and markers of interest could potentially be found there.
Amniocentesis (AC) is an invasive diagnostic tool in the assessment of fetal karyotype. Under ultrasound guidance, a narrow-gauge needle is inserted through the maternal abdominal wall into the uterus and gestational sac, followed by aspiration of AF.In Sweden; all pregnant women over the age 35 are offered a first trimester screening for chromosomal abnormalities. The result is presented as a weighted risk estimate [Citation3]. Patients with a high risk estimate are then offered a second trimester AC or chorionic villus sampling (CVB). Several studies have used AF for identifying metabolic or pregnancy problems [Citation4–9].
Regulation of angiogenesis within the placenta is critical for its development and function. Placental growth factor (PLGF) and vascular endothelial growth factor (VEGF) are both potent angiogenic factors that are expressed in a number of different cells, among them the trophoblasts [Citation10]. These angiogenic factors have earlier been used in the prediction of preeclampsia [Citation11], but so far not in predictive models for fetal complications during pregnancy. An increased anaerobic metabolism in the fetomaternal unit may induce an increased level of uterine lactate. The uterine produced lactate may then be transported to AF by lactate carriers in the myometrial cells [Citation12]. However, this link between different biomarkers in early pregnancy and fetal complications later in pregnancy has not been studied yet.
The aim of this study was to investigate the association between the levels of amniotic fluid lactate (AFL), PLGF and VEGF in AF in second trimester and fetal outcome during pregnancy and labor.
Materials and methods
The study was a prospective cohort study. It was performed at the maternity ward and ultrasound department of Sodersjukhuset, Stockholm. Ethical approval: the research related to human use has been complied with all the relevant national regulations, institutional policies, and in accordance the tenets of the Helsinki Declaration, and has been approved by the authors’ institutional review board (Karolinska institute, file record: 2012/322-31/1). All women scheduled for amniocentesis (AC) during the study time were eligible for participation and the women were consecutively included in the study after obtaining signed written informed consent.
The main indications for AC were maternal age (≥ 35 years), high-risk result in CUB (Combined Ultrasound and Biochemical test) or the women’s wish. Two women were included after failed chorionic villus sampling (CVB) and were converted to AC. Twenty-two women were excluded during the study time due to failed consent or unsuccessful sampling.
Study population
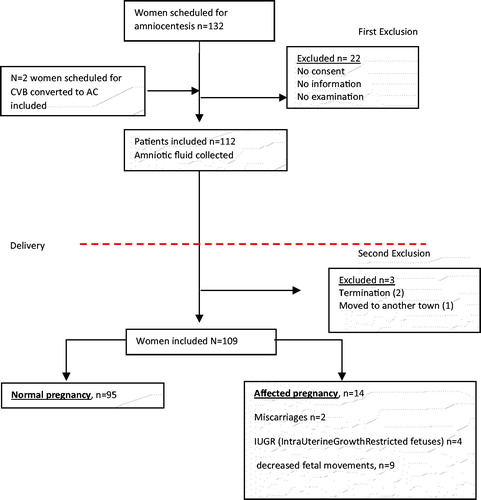
One hundred thirty-two eligible women were identified, who were scheduled for AC during 2014 (). Inclusion criteria for the study were (1) AC performed at gestational age between 15th and 20th weeks, (2) written consent from the woman, (3) at least 1-ml amniotic fluid obtained for testing, (4) singleton pregnancy. After delivery, the maternal medical records were studied and a composite fetal morbidity was made of factors closely identified with a risk of intrauterine asphyxia.
Intrauterine growth restriction (IUGR) was according to Swedish guidelines defined as a fetus with a weight below the 10th percentile for the gestational age. Miscarriage was defined as death of a fetus before it is able to survive independently, and according to Swedish guidelines the cut off “before 22 weeks of gestation” was used.
The constructed composite morbidity was defined as “adverse neonatal pregnancy outcome” and pregnancies with (1) miscarriages, (2) IUGR, (3) less fetal movements during pregnancy were included. Some of the neonates who were classified as having adverse neonatal outcome had more than one of the factors identified.
Women seeking help for reduced fetal movements were analyzed as a subgroup. In Sweden, pregnant women are encouraged to notice intrauterine fetal movements. In the absence of or different fetal movement patterns, women are asked to seek care for control.
Sample collection
All amniocentesis samples in this study were collected by six senior medical doctors with prior experience of amniocentesis. During examination, the first 2 ml of amniotic fluid obtained after insertion into the gestational sac was collected as sample for the study, of which a minimum of 1 ml was required for the study analyses.
Lactate concentration analysis
Lactate concentration analysis was performed immediately after amniocentesis using a device (LMU061, ObsteCare AB, Sweden) which measures lactate concentration in amniotic fluid between 0.5 and 25 mmol/L. The lactate concentration data was stored in the device’s software.
After the analysis of lactate concentrations, the remaining 1 ml of amniotic fluid (AF) was immediately frozen and transferred to a −70 °C freezer. These samples were later used for analyzing VEGF and PLGF. The samples were stored at the biobank of Sodersjukhuset with the registration number 407.
Enzyme-linked immunosorbent assay
The analysis of VEGF and PLGF were performed using a quantitative sandwich enzyme-linked immunosorbent assay (ELISA) technique. In this study, Quantikine ELISA VEGF and Quantikine ELISA PLGF (cat. No DVE00 and DPG00, respectively, both from R & D Systems Inc, Bio-Techne®, Minneapolis, MN) were used and performed according to the protocol for each kit. The samples were run in singles and data analyses were performed group-wise.
Statistical methods
Nonparametric Mann–Whitney U-test was used to assess significant differences between the subgroups, and median values were presented with ranges. Chi-squared test was used for categorical variables. In expected frequencies <5, Fisher’s exact test was used. p value of less than .05 was considered as statistically significant. All calculations were made using the statistical software Statistica 13 by developer Stat Soft and SPSS 23.0 (SPSS Inc, Chicago, IL.).
Results
gives the details of the populations of women in each study group. Some women were lost to the trial for the following reasons; one woman moved to another town, and her data were not available for analysis. Two pregnancies included in the project had an early termination due to abnormal karyotype found in the sample of AF at the time of amniocentesis (osteogenesis imperfecta and trisomy 21). Two women had late miscarriages in 19th and 18th gestational week, respectively. One newborn died after delivery due to abnormal karyotype (unbalanced translocation). In the group of two miscarriages the median level of AFL was significantly higher (10.7 versus 6.90 mmol/l, p = .02). No differences were found in the levels of PLGF (0.208 versus 0.191 pg/ml, p = .65) or VEGF (0.086 versus 0.097 pg/ml, p = .20, ). After these study losses, 109 women gave birth to a live fetus and the maternal and fetal background data are presented in on group level.
Table 1. Baseline levels of the three measured markers of hypoxia (AFL, PLGF, and VEGF) at the time of amniocentesis (n = 109).
Table 2. Maternal and fetal background data, among women with a normal or adverse neonatal pregnancy outcome.
AFL levels
A baseline median AFL concentration of 6.90 mmol/l (4.10–11.10 mmol/l) was found at the time of amniocentesis. AFL levels in the group of “adverse neonatal pregnancy outcome” were significantly higher than the normal outcome group (8.0 versus 6.8 mmol/l, p = .002, ). The highest individual values were seen among the two pregnancies that ended up in late miscarriages compared to the normal ones (10.2 and 11.1 mmol/l, p = .02). The AFL levels in pregnancies with IUGR (n = 4) had a median AFL value of 7.5 mmol/l (5.2–9.1 mmol/l) and were statistically significantly higher (p = .003) than the normal ones. In pregnancies where the woman attended the clinic for experience of reduced fetal movements the AFL level did not differ from the normal levels (n = 9, 7.5 versus 6.9 mmol/l, p = .6).
Table 3. AFL, PLGF, and VEGF values in the different groups of adverse neonatal pregnancy outcomeTable Footnotea (n = 106).
PLGF and VEGF levels in AF
A baseline median PLGF concentration of 0.208 pg/mL (0.131–0.299 pg/mL) was found in AF at the time of amniocentesis (). A significant difference in the concentration of PLGF was found when normal and the “adverse neonatal pregnancy outcome group” were compared (0.230 versus 0.170 pg/mL, p = .001) (). No difference was seen in the group of late miscarriages (0.190 versus 0.210 pg/mL, p = .7), but a reduction of the concentration was seen in the group with IUGR (0.160 versus 0.220 pg/mL, p = .03) and in the group of less fetal movements (0.170 versus 0.220 pg/mL, p = .04). A baseline median VEGF concentration of 0.088 pg/mL (0.062–0.127 pg/mL) was found at the time of amniocentesis (). No difference in the concentration of VEGF was observed when normal versus “adverse neonatal pregnancy outcome” were studied (0.08–0.09 pg/mL, p = .5, ) nor between the different subgroups.
Reduced fetal movements
The group with reduced fetal movements were found to be heterogeneous: 5/9 (56%) has an AFL over baseline but no significant difference in AFL values was found (6.9 versus 7.5 mmol/l, p = .6) 8/9 (89%) has a significant decreased of PLGF (0.22 versus 0.17 pg/mL, p = .04), but only 1/9 (11%) had a VEGF over the baseline value (0.08 versus 0.09, p = .1).
Discussion
The result of this pilot study showed that the AFL, PLGF values in AF, at the time of second trimester amniocentesis, are changed in the group with later fetal complications compared to the group with normal pregnancies. The values of VEGF seem to be unaffected by the course of pregnancy in this study.
Previous studies from our research group have shown that during labor the myometrium is producing lactate which is transferred to and can be detected in AF. During labor the level of lactate in myometrial capillary blood and AF is increased as a sign of an exhausted/hypoxic uterus [Citation12–14]. A new and important finding in this study is that the level of lactate in AF in early pregnancy is comparable with levels of lactate in AF at labor, and that high levels seem to associate with later fetal complications in pregnancy. In 1997, Genbacev et al. reported that in a healthy pregnancy, embryo implantation takes place in a low oxygen environment (2–3% of O2) [Citation15]. Before cytotrophoblast invasion of maternal vessels is established the uteroplacental circulation will be in this hypoxic situation. During this period, the mass of the placenta increases much more rapidly than that of the embryo caused by an increased production of angiogenic factors, such as PLGF and VEGF. The level of oxygen will then rise to 6–8% of O2 between 10 and 12 weeks of gestation [Citation16]. Therefore, our normal finding of levels of lactic acid and angiogenic factors in AF in the second trimester, at the time of amniocentesis, can be explained by these physiological events.
However, unlike pathological angiogenesis in other situations, the placental angiogenesis is a normal physiological process that must be tightly regulated during the beginning of pregnancy. Deranged placental vascularization is the most common placental pathology that has been identified in numerous pregnancy complications both in animals and humans. VEGF is critically required for all steps of placental vascular formation and development because of the importance of placental angiogenesis during pregnancy. PLGF together with VEGF play a big role in the formation of the vascular network with the development of the villous tree [Citation17]. Few earlier studies have been made measuring these two biochemical markers in AF and no one have ever measured the level of lactate before. A case-control study of 15 preeclamptic women and 15 controls was conducted by Tranquilli et al. in 2004, and showed that the level of PLGF in AF in the second trimester was significantly lower in the group that later developed preeclampsia compared to normal pregnancies [Citation18]. This is consistent with our finding of lower PLGF in growth restricted fetuses, suggesting similar vascular etiologies may apply.
Inappropriate maternal blood flow to the intervillous space during labor may underlie pathologies and has been proposed to be one of the pathways in recurrent miscarriages. In this study, we had two late miscarriages; both of them were found to have significantly elevated levels of AFL at the time of amniocenteses, which can be interpreted as a sign of increased anaerobic metabolism associated with an inappropriate maternal blood flow. It can be suggested that the degree of inappropriate maternal blood flow may lead to different degrees of disturbances in the fetomaternal unit, resulting in an abnormal development of the fetus. In this situation, the fetus will not reach its full growth potential. All four IUGR fetuses had a very close and decreased level of PLGF combined with an increased AFL.
A special group we analyzed was women seeking help due to reduced fetal movements during pregnancy. It is well-known that most of these pregnancies are completely normal, but it is also known that in this group, pregnancies with an increased risk of subsequent intrauterine fetal death can be hidden [Citation19]. In this study, 9/109 (8.3%) deliveries had the diagnosis of reduced fetal movements, which means that the problem was considered so serious that the woman was induced to labor. Our conclusion was that pregnancies with reduced fetal movements are a heterogeneous group, and probably there are subgroups that are currently unknown. Most of these pregnancies are normal, but for example, the number of growth-inhibited fetuses is thought to be overrepresented.
Strengths and limitations
The number of pregnancies included in this trial is relatively small, and hence the number of affected pregnancies is limited. Anyhow, important information has been obtained by these new tests, and the method can hopefully be retained for certain selected pregnancies such as IUGR in the future. The strength of this work is that it gives insight in factors linked to very early human fetal environment and whether these factors are antecedent to poor pregnancy outcomes, they have now been studied for the first time. Importantly we showed this can be a new way to identify risk pregnancies, as well as obtaining crucial data for the understanding of the etiology of pregnancies complications.
Conclusions
Pregnancies with defined adverse fetal outcome later in pregnancy have high levels of AFL and low levels of PLGF in amniotic fluid at the time of amniocentesis. This can be a new way of identifying risk pregnancies in the future.
Acknowledgments
We acknowledge all the women who consented to participate in this trial and the large number of doctors and assistant nurses at the Ultrasound Department of Sodersjukhuset, Stockholm, who practically performed this study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Socialstyrelsen. Available from: http://www.socialstyrelsen.se/statistik/statistikdatabas/graviditeter-forlossningarochnyfodda
- WHO; Maternal, newborn, child and adolescent health. Available from: http://www.who.int/maternal_child_adolescent/epidemiology/stillbirth/en/
- Spencer K, Souter V, Tul N, et al. A screening program for trisomy 21 at 10–14 weeks using fetal nuchal translucency, maternal serum free beta-human chorionic gonadotropin and pregnancy-associated plasma protein-A. Ultrasound Obstet Gynecol. 1999;13(4):231–237.
- Virgiliou C, Gika HG, Witting M, et al. Amniotic fluid and maternal serum metabolic signatures in the second trimester associated with preterm delivery. J Proteome Res. 2017;16(2):898–910.
- Liu Y, Liu Y, Zhang R, et al. Early- or mid-trimester amniocentesis biomarkers for predicting preterm delivery: a meta-analysis. Ann Med. 2017;49(1):1–10.
- Scioscia M, Vimercati A, Selvaggi LE, et al. Inositol phosphoglycan putative insulin mediator in human amniotic fluid. J Matern Fetal Neonatal Med. 2006;19(1):9–12.
- Boué A, Muller F, Nezelof C, et al. Prenatal diagnosis in 200 pregnancies with a 1-in-4 risk of cystic fibrosis. Hum Genet. 1986;74(3):288–297.
- Giannubilo SR, Tiano L, Ciavattini A, et al. Amniotic coenzyme Q10: is it related to pregnancy outcomes? Antioxid Redox Signal. 2014;21(11):1582–1586.
- Quenby S, Pierce SJ, Brigham S, et al. Dysfunctional labour and myometrial lactic acidosis. Obstet Gynecol. 2004;103(6):1344.
- Lash GE, Cartwright JE, Whitley GS, et al. The effects of angiogenic growth factors on extra villous trophoblast invasion and motility. Placenta. 1999;20(8):661–667.
- Kleinrouweler CE, Wiegerinck MM, Ris-Stalpers C, et al. Accuracy of circulating placental growth factor, vascular endothelial growth factor, soluble fms-like tyrosine kinase 1 and soluble endoglin in the prediction of pre-eclampsia: a systematic review and meta-analysis. BJOG. 2012;119(7):778–787.
- Akerud H, Ronquist G, Wiberg-Itzel E. Lactate distribution in culture medium of human myometrial biopsies incubated under different conditions. Am J Physiol Endocrinol Metab. E1414. 2009;297(6):E1414–E1419.
- Wiberg-Itzel E, Pettersson H, Cnattingius S, et al. Association between lactate concentration in amniotic fluid and dysfunctional labor. Acta Obstet Gynecol Scand. 2008;87(9):924–928.
- Wiberg-Itzel E, Pettersson H, Andolf E, et al. Lactate concentration in amniotic fluid: a good predictor of labor outcome. Eur J Obstet Gynecol Reprod Biol. 2010;152(1):34–38.
- Genbacev O, Zhou Y, Ludlow JW, et al. Regulation of human placental development by oxygen tension. Science. 1997;277(5332):1669–1672.
- Caniggia I, Winter J, Lye SJ, et al. Oxygen and placental development during the first trimester: implications for the pathophysiology of pre-eclampsia. Placenta. 2000;21(Suppl A):S25–SS30.
- Chen D, Zheng J. Regulation of placental angiogenesis. Microcirculation. 2014;21(1):15–25.
- Tranquilli AL, Bezzeccheri V, Giannubilo SR, et al. Amniotic vascular endothelial growth factor (VEGF) and nitric oxide (NO) in women with subsequent preeclampsia. Eur J Obstet Gynecol Reprod Biol. 2004;113(1):17–20.
- Hofmeyr GJ, Novikova N. Management of reported decreased fetal movements for improving pregnancy outcomes. Cochrane Database Syst Rev. 2012;4(4):CD009148.