Abstract
Objective
To compare the neurodevelopmental outcome of monochorionic-diamniotic twins (MCDA) with type II selective intrauterine growth restriction (SIUGR-II) managed in utero either expectantly or with laser.
Materials and methods
Postnatal neurodevelopmental assessment was conducted on the children of patients that had been antenatally diagnosed with SIUGR-II between 16 and 26 weeks gestational age (GA) and that had been randomly assigned to expectant management (EM) versus laser therapy (LT). The assessment was conducted by trained specialists using the Battelle Developmental Inventory (BDI-2). BDI-2 total and domain (adaptive, personal–social, communication, motor, and cognitive) composite scores for the appropriately grown (AGA) and growth-restricted (IUGR) twins were compared by treatment arm.
Results
Twenty patients diagnosed with SIUGR had undergone block randomization between two centers to either expectant management (EM) (6) or laser therapy (LT) (14). The mean (SD) GA at diagnosis was no different between the EM and LT groups [21.5 (2.0) versus 21.1 (2.8) weeks, p = .7414, respectively]. However, GA at delivery was significantly lower in the EM versus LT groups [28.3 (1.8) versus 33.4 (3.8) weeks, p = .0039]. At 6 months, all 20 AGA babies were alive, whereas only 3/6 (50%) of the IUGR babies in the EM group and 4/14 (29%) in the LT group were alive (p = .6126). One family in the EM group and two families in the LT group declined BDI-2 assessment. The mean (SD) age at BDI-2 assessment was no different between the EM and LT groups [75.6 (14.4) versus 70.7 (18.2) months, p = .5618, respectively]. For the AGA children, there were no significant differences in total BDI-2 scores for the EM versus LT [97.4 (10.4) versus 98.0 (19.6), p = .8741], nor in any of the domain composite scores. For the IUGR children, no statistically significant differences were detected in total BDI-2 scores between the EM and LT [72.0 (31.1) versus 92.8 (22.1), p = .643], nor in any of the domain composite scores. The comparison of standardized scores between the AGA and IUGR pairs was significantly different, but within the normal range.
Conclusions
Neurodevelopmental outcomes for SIUGR-II MCDA twins were similarly favorable, whether managed expectantly or with laser treated. However, the significantly different GA at delivery (28.3 versus 33.4 weeks, p = .0039, expectant versus laser, respectively) may suggest improved outcomes in laser-treated patients in a larger cohort.
Introduction
Selective intrauterine growth retardation (SIUGR) [Citation1] occurs in approximately 12.5%–25% of all monochorionic pregnancies [Citation2,Citation3]. The condition is defined as an estimated fetal weight (EFW) of <10th percentile for one twin (SIUGR twin), while the other twin is appropriately grown for gestational age (AGA) (EFW between 10 and 90th percentile) [Citation1,Citation4]. Although SIUGR may occur from failure of the individual placental territory (IPT) [Citation4] of one twin to satisfy the nutritional aspects of that fetus [Citation4,Citation5], it may also result from the presence of placental vascular anastomoses, despite an adequate IPT for the SIUGR twin [Citation6,Citation7].
Expectant management of patients with SIUGR may be associated with an increased risk of adverse perinatal outcome, including prematurity and its attendant complications [Citation8]. Increased risk for spontaneous demise of the SIUGR twin may result in the concomitant demise of the AGA twin in up to 40% of cases or in neurologic damage of the AGA twin in up to 30% of cases [Citation9–21]. The adverse effects on the AGA twin resulting from the spontaneous demise of the SIUGR twin stem from postmortem fetofetal hemorrhage from the AGA twin to the demised SIUGR twin through placental vascular communications [Citation15,Citation22].
The risk of an adverse outcome may be higher depending on the umbilical artery Doppler waveform of the SIUGR fetus, such that fetuses with persistent absent end-diastolic velocity (AEDV) (SIUGR type II) are thought to be at a higher risk than SIUGR fetuses with umbilical artery forward diastolic flow (SIUGR type I) [Citation8,Citation23]. Fetuses with intermittent AEDV (SIUGR type III) are believed to have an unpredictable prognosis [Citation24]. To avoid the potential complications associated with expectant management, patients with SIUGR are often asked to consider either termination of pregnancy or cord occlusion of the SIUGR twin [Citation25].
In 2001, Quintero et al. reported on the feasibility of performing laser therapy for SIUGR [Citation1] by ablating the placental vascular anastomoses, similar to the laser technique described for the treatment of twin–twin transfusion syndrome (TTTS), namely selective laser photocoagulation of communicating vessels (SLPCV) [Citation26]. In that study, 17 patients with SIUGR were managed expectantly and 11 patients were managed with SLPCV. Both perinatal survival (14/17, 82.4% versus 8/11, 72.7%, p = .6) and neurological morbidity (3/22, 13.6% versus 0/12, 0%, p=.5) were comparable between the groups. However, neurological morbidity was limited to the expectantly managed group [Citation1].
To examine the potential merit of SLPCV in the management of SIUGR, our group previously conducted a randomized clinical trial comparing expectant management versus laser therapy in a population of type II SIUGR patients. The primary aim of the study was to compare the neurodevelopmental outcome of the AGA twin in the two groups using the Battelle Developmental Inventory-2 (BDI-2).
Materials and methods
The study population consisted of children born to mothers that had been antenatally diagnosed with type II SIUGR between 16 and 24 0/7 weeks of gestation who had been randomized to either expectant management (EM) or laser therapy (LT). Inclusion criteria comprised the following: surviving children diagnosed in utero as being members of a monochorionic diamniotic twin gestation with type II SIUGR, balanced karyotype, no major congenital anomalies, at least 24 months corrected age (+ or −6 weeks) and less than 7 years and 11 months. Exclusion criteria included families who refused the neurodevelopmental assessment and examination of their children, families unable to complete the measures in English or Spanish, and families who the research team was unable to contact after making reasonable efforts to contact them. The study was approved by the Institutional Review Board of Sidra Medical and Research Center in Doha, Qatar (IRB Protocol 1505000971), Children’s Mercy Hospital in Kansas City, MO (IRB Protocol 16020132), and the University of Southern California, Los Angeles, CA (IRB Protocol HS-06–00194).
Parents were contacted by a member of the research team who explained the nature of the study. Information about the study and consent form to participate in the study was mailed or emailed to the parents. The parents were then contacted by phone to determine whether they wished to participate in the study. If the family wanted to participate in the study, they were asked if they were willing to travel to a central location for the evaluation, or if they preferred a member of the research team to visit their home. The parents signed the informed consent at the time of the visit.
The children were evaluated with the Battelle Developmental Inventory, Second Edition (BDI-2). This tool was selected on the basis of availability of normative data, targeted age range, and the availability of standardized versions in both English and Spanish languages. The BDI-2 involves direct individual assessment and parental interview to measure key developmental skills in children from birth to 95 months (7 years, 11 months) [Citation27]. Based on widely accepted developmental milestones for children, the BDI-2 assesses five developmental domains (personal–social, adaptive, motor, communication, and cognitive) comprising overall development. The total BDI-2 developmental quotient score is computed as a sum of the 5 BDI-2 subdomains, and it has a mean of 100 with a standard deviation of 15. Individual item scores range from 0 to 2 points, with scores based on 1 of 3 predetermined criteria that include observation, parent interview, and/or performance on a structured task. Neurodevelopmental impairment (NDI) was defined as having bilateral blindness (unable to fix on or track an object), bilateral deafness (requiring amplification), cerebral palsy (based on physical examination), and/or a BDI-2 total developmental quotient of <70. For the assessment, the parents were asked to bring their child(ren) to the clinic. If the family was unable or unwilling to travel, arrangements were made for the assessor to travel to the home of the family to assess the children at their home. The BDI-2 was administered by a clinical psychologist, developmental-behavioral pediatrician, and/or occupational therapist (OT) with experience in the administration and interpretation of the tests.
At the assessment, the neurobehavioral specialist administered the BDI-2 using structured administration and parent interview. For Spanish-speaking families, the BDI-2 was administered by a bilingual assessor. The data were recorded in standard BDI-2 forms and transcribed to an electronic database. The principal investigator was blinded to the status of the subjects (growth restricted or appropriately in utero grown twin, or management group: expectant management or laser).
The mothers of the children had been diagnosed with type II SIUGR and had been randomized to either expectant management or laser therapy. Randomization had been conducted blindly with the use of randomization tables. Mothers randomized to expectant management were followed by their own obstetricians and delivered for standard obstetrical indications. Mothers that had been randomized to laser therapy had undergone selective laser photocoagulation of communicating vessels [Citation26] and had been referred back to their obstetricians for follow-up. Follow-up recommendations to either group included weekly ultrasounds to assess cervical length, amniotic fluid volume, and Doppler studies of the umbilical artery, as well as serial fetal growth assessments every 3–4 weeks. Weekly antenatal testing with either nonstress tests or biophysical profiles was recommended starting at 28 weeks. Indication for delivery was left to the discretion of the referring obstetrician.
Statistical analysis
Analyses were conducted using IBM SPSS for Windows, version 24.0. Armonk, NY, USA: IBM Corp. Categorical data were analyzed by the chi-square test and Fisher’s exact test as appropriate. Interval data were assessed for normality and analyzed with Student’s t-test, ANOVA, or Mann–Whitney U test as appropriate. A p values of <.05 was considered statistically significant.
Partial funding for this study was obtained through a charitable grant from the Brianna-Marie Foundation (“The Foundation”), a nonprofit, tax-exempt 501-(c) three organization. (http://www.briannamariefoundation.com/home.html.)
Results
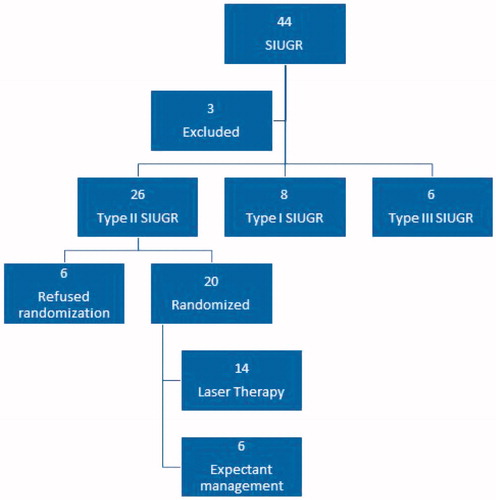
shows the study population. Of a total of 44 maternal patients referred with a diagnosis of SIUGR, three patients were excluded at presentation: One had a double fetal demise, one had the demise of the SIUGR fetus, and in the third, the mother was less than 18 years of age. Of the remaining 41 maternal patients assessed, 26 patients (63%) had type II SIUGR and were eligible for randomization. Six patients in the eligible group refused randomization: four of them elected to continue with expectant management, and the other two requested umbilical cord occlusion of the SIUGR twin. Of the remaining 20 type II SIUGR patients, block randomization yielded six patients to expectant management and 14 to laser therapy. Although the group sizes seemed unequal, the distribution was no different than 1:1 (p=.11 binomial test). The study was discontinued due to poor recruitment. Of the 15 patients ineligible for randomization, 11 patients had type I SIUGR, and four patients had type III SIUGR. Only type II SIUGR infants are the subject of this study.
shows maternal demographics of the 20 randomized patients. The mean (SD) GA at diagnosis was no different between the EM and LT groups [21.5 (2.0) versus 21.1 (2.8) weeks, p=.7414, respectively]. However, GA at delivery was significantly lower in the EM versus LT groups [28.3 (1.8) versus 33.4 (3.8) weeks, p=.0039]. shows the indications for delivery. Three of the 14 (21%) patients in the LT group were delivered due to concerns regarding the IUGR (two patients) or abruption (one patient), compared to 6/6 (100%) patients in the EM group (p=.006, Fisher’s exact test). shows the perinatal outcomes. At 6 months, all 20 AGA babies were alive, whereas 3/6 (50%) of the IUGR babies in the EM group and 4/14 (28.6%) in the LT group were alive (p=.6126). Although there was no overall difference in the survival rates of the IUGR babies between the two groups, the timing of demise was different. In the EM group, all the demises occurred after delivery (two within 1 month, one within 6 months). In the LT group, all the demises were in the fetal period.
Table 1. Antenatal demographics.
Table 2. Indication for delivery.
Table 3. Perinatal outcome.
shows the gross neurological outcomes. There were no significant differences in the incidence of Grade III–IV intraventricular hemorrhage or cerebral palsy. There was no difference in neurodevelopmental impairment (cerebral palsy, BDI-2 less than 70, blindness, and/or deafness) between the two groups.
Table 4. Gross neurological outcome.
shows the neurodevelopmental outcomes using the BDI-2 tool for the AGA babies. One family in the EM group and two families in the LT group declined BDI-2 assessment, leaving a total of 17 AGA children (5 AGA children in the EM group and 12 AGA children in the LT group) available for comparison. The mean (SD) age at BDI-2 assessment was no different between the EM and LT groups [75.6 (14.4) versus 70.7 (18.2) months, p=.5618, respectively]. There were no significant differences in total BDI-2 scores for the EM versus LT AGA children [97.4 (10.4) versus 98.0 (19.6), p=.8741], nor in any of the domain scores. Only one AGA child (1/12, 8.3%) in the LT group had a standardized BDI-2 score < 70, compared to none (0/5) in the EM group, but this difference was not significant (p = 1.0, Fisher’s exact test). shows the neurodevelopmental outcomes for the IUGR twins (two IUGR children in the EM group and four IUGR children in the LT group). One of the two children in the EM group had a BDI-2 score < 70, compared to none of the four IUGR children in the LT group (p=.33). No statistically significant differences were detected in total BDI-2 scores between the EM and LT [72.0 (31.1) versus 92.8 (22.1), p=.643], nor in any of the domain scores, but the small number of children limits the value of this conclusion. However, there was a tendency for the motor development scores to be significantly lower in the EM group than in the LT group (69.5 versus 89.75, p=.062, ().
Figure 2. Motor developmental quotient in the IUGR children. There was a tendency for lower scores in the expectantly managed group (p=.062). IUGR: growth restricted twin.
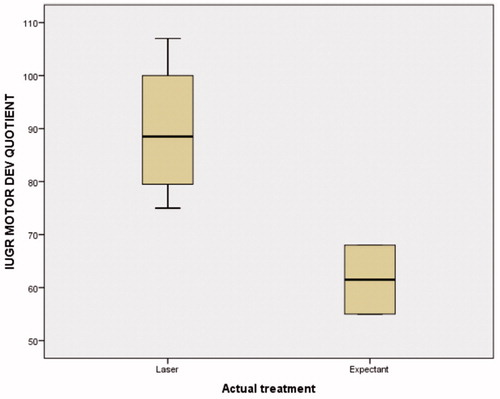
Table 5. Neurodevelopmental outcomes: AGA baby.
Table 6. Neurodevelopmental outcomes: IUGR baby.
Six patients had both AGA and SIUGR babies alive at the time of BDI-2 assessment (two in the EM group and four in the LT group). The standardized scores between AGA and IUGR pairs were significantly different for combined EM and LT groups, with higher AGA scores, but still within the normal range (). Within each group, there was a tendency for higher AGA scores in the LT group but not in the EM group ().
Table 7. Total developmental quotient AGA versus IUGR in paired children.
Discussion
To the best of our knowledge, this is the first study of a US cohort of children antenatally diagnosed as having type II SIUGR who were randomly assigned to expectant management or laser therapy and subsequently underwent formal neurodevelopmental testing. Although the small sample size and the resulting low power of the study (5%) in detecting a true difference in the neurodevelopmental outcome may limit our conclusions, there are enough data to suggest that patients treated with laser therapy are likely to have an improved outcome over those managed expectantly. Indeed, given the statistically significant difference in gestational age at delivery of the two groups (28.3 versus 33.4, EM versus LT, respectively, p=.0039), an important clinical difference in the neurodevelopmental outcome of the AGA twin was to be expected [Citation28].
According to our previous work on neurodevelopmental outcomes after laser surgery for TTTS by Vanderbilt et al., prematurity is one of the biggest risk factors for low BDI-2 scores [Citation27]. A recent study in France showed an incidence of cerebral palsy of 4% in babies born between 27 and 31 weeks compared to 1% of those born between 32 and 34 weeks [Citation29]. Using such figures, a total of approximately 852–978 cases of type II SIUGR would be needed to show a 3% difference in cerebral palsy between expectantly managed and laser-treated type II SIUGR patients.
There is a paucity of data regarding neurodevelopmental outcomes in patients diagnosed with type II SIUGR. When counseling patients diagnosed with Type II SIUGR, a clear understanding of perinatal outcomes would be ideal. In our study, there are no differences between expectant management and laser therapy in the overall BDI-2 scores, nor in the individual domains, neither for the AGA nor for the SIUGR infants. One AGA child in the LT group (1/12, 8.3%) had a standardized score <70, whereas none of the AGA children (0/5). This difference was not significant. We surmise that, in a larger cohort, it is possible that neurodevelopmental differences could become apparent, in favor of the laser-treated group, given the significantly higher gestational age at delivery of this group. Indeed, despite the small number of children assessed, there was a tendency for the motor development in the IUGR twin to be significantly better in the LT group, compared to the EM group (p=.062) ().
The management of SIUGR remains controversial [Citation30]. Our original pilot study showed that laser ablation of the placental vascular anastomoses in SIUGR patients was possible [Citation1]. The goal of such proposed treatment was to render these pregnancies as “functionally dichorionic,” with the original purpose of averting the adverse consequences that could result from the demise of the SIUGR twin. Our current study confirms such hypothesis, as none of the patients managed with laser experienced demise of the AGA twin after fetal demise of the SIUGR twin and the neurodevelopmental findings of the AGA twin were within normal range. While laser therapy indeed protects the AGA twin from the adverse consequences that could result from the fetal demise of the SIUGR twin, we have found in previous studies that it has two additional clinical benefits: First, laser therapy may result in improved growth of the SIUGR twin [Citation6]. Indeed, in a population of 211 patients with twin–twin transfusion syndrome, growth restriction of the donor twin was present in 136 patients before laser, but only in 61 patients (44.8%) after laser [Citation7]. This suggests that laser therapy can result in improvement of fetal growth in over 50% of patients affected by growth restriction of a monochorionic twin. Parenthetically, this observation suggests the role of placental vascular anastomoses in the etiology of SIUGR itself. Second, the resulting “functionally dichorionic” pregnancy eliminates the need to deliver the patient for deterioration in the status of the SIUGR twin. Indeed, if demise of the SIUGR twin was to occur before a desired gestational age, the AGA twin would not be affected, and the pregnancy could be allowed to continue. In our experience, however, many patients choose to be delivered on behalf of the SIUGR twin, particularly if they have reached 27 weeks or more.
Laser therapy for SIUGR is associated with its own set of risks and complications. In our current study, in utero survival of the SIUGR twin was only noted in close to 30% of SIUGR twins. This is similar to that reported by Ishii et al. [Citation31]. Although in our study, as well as in that of Ishii et al. [Citation31], 100% of the AGA twins survived, demise of the AGA twin after laser therapy may indeed occur and thus should be kept as a potential complication of the surgery. Laser surgery for SIUGR may be technically more challenging than that for twin–twin transfusion syndrome (TTTS), given the lack of polyhydramnios in the entry sac and the presence of fluid in the SIUGR sac. Both of these factors, lack of polyhydramnios in the AGA twins’ sac and the presence of fluid in the SIUGR twin’s sac, may hinder proper identification and occlusion of the vascular anastomoses.
Our study has important strengths. First, we were able to assess a group of infants with type II SIUGR managed randomly antenatally to either expectant management or laser therapy at a time when there was equipoise in the antenatal management of these pregnancies. In all pregnancies, both fetuses were given a chance to survive and 100% of the AGA children did. Although the survival rate of the SIUGR twin was approximately 30% in the LT group, this is still significantly better, both medically and statistically, than if selective reduction of this fetus via cord occlusion would have been undertaken (5/14 versus 0/14, p=.04), as it is commonly offered to these pregnancies. Second, the clinical outcomes reflect actual current practice, as providers were allowed to make clinical decisions as needed after randomization. Third, although the actual neurodevelopmental outcomes did not differ between the groups, overall they reflect a good prognosis for these extremely complicated monochorionic twin pregnancies. Given the significantly different gestational ages at delivery between the groups, we would expect clinical differences to become apparent with a larger cohort, in favor of laser-treated patients. Lastly, the age at which the infants were tested, with a mean of 70–75 months, allowed time for any potential neurodevelopmental abnormality to become manifest. Our study’s biggest limitation is the small cohort size, which is not uncommon in our field.
In conclusion, no neurodevelopmental differences were observed in children who were antenatally managed either expectantly or with laser therapy after being diagnosed with type II SIUGR. However, we would suspect that such differences may become apparent in larger cohorts, in favor of laser-treated patients, given the significantly higher gestational age of delivery of the laser group.
Acknowledgments
We are indebted to Dr. Robert Sokol, Dr. M. Jeremiah Mahoney, Dr. Frank Chervenak and Dr. Giuseppe Rizzo for their leadership and assistance in conducting the randomized clinical trial. We thank Dr. Lisa Korst for providing statistical support for this study. We thank Dr. Carolina Pena-Ricardo for conducting the Battelle Developmental Inventory, second edition, instrument on the Spanish-speaking subjects from the Los Angeles site. We thank our research nurse, Arlyn Llanes RN, BSN, for her invaluable assistance in the conduction of this study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Quintero RA, Bornick PW, Morales WJ. Selective photocoagulation of communicating vessels in the treatment of monochorionic twins with selective growth retardation. Am J Obstet Gynecol. 2001;185:689–696.
- Alexander GR, Kogan M, Martin J, et al. What are the fetal growth patterns of singletons, twins, and triplets in the United States? Clin Obstet Gynaecol. 1998;41:114–125.
- Wenstrom KD, Gall SA. Incidence, morbidity and mortality, and diagnosis of twin gestations. Clin Perinatol. 1988;15:1–11.
- Quintero RA, Martínez JM, López J, et al. Individual placental territories after selective laser photocoagulation of communicating vessels in twin–twin transfusion syndrome. Am J Obstet Gynecol. 2005;192:1112–1118.
- Gou C, Li M, Zhang X, et al. Placental characteristics in monochorionic twins with selective intrauterine growth restriction assessed by gradient angiography and three-dimensional reconstruction. J Matern Fetal Neonatal Med. 2017;30:2590–2595.
- Chmait RH, Chon AH, Schrager SM, et al. Donor catch-up growth after laser surgery for twin–twin transfusion syndrome. Early Hum Dev. 2015;91:751–754.
- Chmait RH, Korst LM, Bornick PW, et al. Fetal growth after laser therapy for twin–twin transfusion syndrome. Am J Obstet Gynecol. 2008;199:47.e1–47.e6.
- Ishii K, Murakoshi T, Takahashi Y, et al. Perinatal outcome of monochorionic twins with selective intrauterine growth restriction and different types of umbilical artery Doppler under expectant management. Fetal Diagn Ther. 2009;26:157–161.
- Dudley DK, D'Alton ME. Single fetal death in twin gestation. Semin Perinatol. 1986;10:65–72.
- Fusi L, Gordon H. Twin pregnancy complicated by single intrauterine death. Problems and outcome with conservative management. BJOG. 1990;97:511–516.
- Lin IJ, Chen CH, Wang TM, et al. Infants of twin pregnancies with one twin demise in the uterus: a retrospective study. Acta Paediatr Taiwan 1999;40:92–96.
- Axt R, Hippach M, Mink D, et al. Maternal and neonatal outcome in a monochorionic twin pregnancy complicated by single intrauterine demise. Clin Exp Obstet Gynecol. 1999;26:155–157.
- Petersen IR, Nyholm HC. Multiple pregnancies with single intrauterine demise. Description of twenty-eight pregnancies. Acta Obstet Gynecol Scand. 1999;78:202–206.
- Saito K, Ohtsu Y, Amano K, et al. Perinatal outcome and management of single fetal death in twin pregnancy: a case series and review. J Perinat Med. 1999;27:473–477.
- Nicolini U, Poblete A. Single intrauterine death in monochorionic twin pregnancies. Ultrasound Obstet Gynecol. 1999;14:297–301.
- Kilby MD, Govind A, O’Brien PM. Outcome of twin pregnancies complicated by a single intrauterine death: a comparison with viable twin pregnancies. Obstet Gynecol. 1994;84:107–109.
- Conte G, Righini A, Griffiths PD, et al. Brain-injured survivors of monochorionic twin pregnancies complicated by single intrauterine death: MR findings in a multicenter study. Radiology. 2018;288:582–590.
- Mackie FL, Morris RK, Kilby MD. Fetal brain injury in survivors of twin pregnancies complicated by demise of one twin: a review. Twin Res Hum Genet. 2016;19:262–267.
- Mackie FL, Rigby A, Morris RK, et al. Prognosis of the co-twin following spontaneous single intrauterine fetal death in twin pregnancies: a systematic review and meta-analysis. BJOG. 2019;126:569–578.
- Cherouny PH, Hoskins IA, Johnson TR, et al. Multiple pregnancy with late death of one fetus. Obstet Gynecol. 1989;74:318–320.
- Hillman SC, Morris RK, Kilby MD. Co-twin prognosis after single fetal death: a systematic review and meta-analysis. Obstet Gynecol. 2011;118:928–940.
- Okamura K, Murotsuki J, Tanigawara S, et al. Funipuncture for evaluation of hematologic and coagulation indices in the surviving twin following co-twin’s death. Obstet Gynecol. 1994;83:975–978.
- Buca D, Pagani G, Rizzo G, et al. Outcome of monochorionic twin pregnancy with selective intrauterine growth restriction according to umbilical artery Doppler flow pattern of smaller twin: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2017;50:559–568.
- Gratacós E, Lewi L, Muñoz B, et al. A classification system for selective intrauterine growth restriction in monochorionic pregnancies according to umbilical artery Doppler flow in the smaller twin. Ultrasound Obstet Gynecol. 2007;30:28–34.
- Parra-Cordero M, Bennasar M, Martínez JM, et al. Cord occlusion in monochorionic twins with early selective intrauterine growth restriction and abnormal umbilical artery Doppler: a consecutive series of 90 cases. Fetal Diagn Ther. 2016;39:186–191.
- Quintero RA, Morales WJ, Mendoza G, et al. Selective photocoagulation of placental vessels in twin–twin transfusion syndrome: evolution of a surgical technique. Obstet Gynecol Surv. 1998;53:S97–S103.
- Vanderbilt DL, Schrager SM, Llanes A, et al. Predictors of 2-year cognitive performance after laser surgery for twin–twin transfusion syndrome. Am J Obstet Gynecol. 2014;211:388.e1–388.e7.
- Briana DD, Malamitsi-Puchner A. Twins and neurodevelopmental outcomes: the effect of IVF, fetal growth restriction, and preterm birth. J Matern Fetal Neonatal Med. 2019;32:2256–2261.
- Pierrat V, Marchand-Martin L, Arnaud C, et al. Neurodevelopmental outcome at 2 years for preterm children born at 22 to 34 weeks’ gestation in France in 2011: EPIPAGE-2 cohort study. BMJ. 2017;358:j3448.
- Townsend R, D'Antonio F, Sileo FG, et al. Perinatal outcome of monochorionic twin pregnancy complicated by selective fetal growth restriction according to management: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2019;53:36–46.
- Ishii K, Nakata M, Wada S, et al. Feasibility and preliminary outcomes of fetoscopic laser photocoagulation for monochorionic twin gestation with selective intrauterine growth restriction accompanied by severe oligohydramnios. J Obstet Gynaecol Res. 2015;41:1732–1737.