Abstract
Objectives
To evaluate the failure rate and performance of cell-free DNA (cfDNA) testing as a first-line screening method for major trisomies, performed by two laboratories using different analytical methods: a targeted chromosome-selective method (Harmony® prenatal Test) versus a home-brew genome-wide (GW) massively parallel sequencing method (HB-cfDNA test), and to evaluate the clinical value of incidental findings for the latter method.
Methods
CfDNA testing was performed in 3137 pregnancies with the Harmony® prenatal Test and in 3373 pregnancies with the HB-cfDNA test. Propensity score analysis was used to match women between both groups for maternal age, weight, gestational age at testing, in vitro fertilization, rate of twin pregnancies and that of aneuploidies. Detection rates for trisomy 21 were compared between the 2 laboratories. For the HB-cfDNA test, cases with rare incidental findings were reported, including their clinical follow-up.
Results
The Harmony® prenatal Test failed at the first attempt in 90 (2.9%) of 3114 women and the HB-cfDNA test in 413 (12.2%) of 3373 women. Postmatched comparisons of the women’s characteristics indicate a significantly lower failure rate in the Harmony® group (2.8%) than in the HB cfDNA group (12.4%; p < .001). Of the 90 women in whom the Harmony® prenatal Test failed, 61 had a repeat test, which still failed in 10, and of the 413 women in whom the HB-cfDNA test failed, 379 had a repeat test, which still failed in 110. The total failure rate after one or two attempts was therefore 1.3% (39/3114) for Harmony® and 4.3% (144/3373) for the HB cfDNA test. After the first or second Harmony® prenatal Test, a high-risk result was noted in 17 of the 17 cases with trisomy 21, in 5 of the seven cases with trisomy 18, and a no-call in two cases, and in the one case with trisomy 13. The respective numbers for the HB-cfDNA test are 17 of the 18 cases with trisomy 21, and a no-call in one case, 2 of the two cases with trisomy 18, and in 2 of the three cases with trisomy 13, and a no-call in one. Of the 3373 women with the HB-cfDNA test, a rare incidental finding was noted in 28 (0.8%) of the cases, of which only 2 were confirmed on amniocytes (one with microduplication 1q21.1q21.2 and one with a deletion Xp21.1), and in another case a deletion rather than a duplication of the long arm of chromosome 8 was found. In all 28 cases, there was normal clinical follow-up.
Conclusions
Comparison of cfDNA testing between these two laboratories showed a four-fold lower failure rate with the Harmony® prenatal Test, with a similar detection rate for trisomy 21. We showed no clinical relevance of disclosing additional findings beyond common trisomies with the GW HB-cfDNA test.
Introduction
Noninvasive prenatal testing (NIPT) is based on analysis of cell-free DNA (cfDNA) in maternal plasma and is becoming widely available as a method of screening for major trisomies [Citation1–9]. For singleton pregnancies, a recent meta-analysis of clinical validation studies including 1963 cases of trisomy 21 and 223,932 nontrisomy 21, reported a weighted pooled detection rate (DR) of 99.7% and false-positive rate (FPR) of 0.04%. The respective DR and FPR for trisomy 18 were 98.2 and 0.05% and for trisomy 13, 99.0 and 0.04% [Citation1]. In twin pregnancies, a recent meta-analysis including 56 trisomy 21 and 3718 non-trisomy 21 showed a weighted pooled DR and FPR of 100 and 0%, respectively [Citation2].
So far, four approaches to the analysis of cfDNA in maternal blood have been used in clinical studies: genome-wide massively parallel sequencing (GW-MPS) and targeted chromosome-selective methods based on analysis of non-polymorphic regions by massively parallel sequencing or array technology (CSS) or based on assessment of single nucleotide polymorphisms (SNPs) or based on four enzymatic steps that generate rolling circle products (RCPs) from chromosomal DNA targets. Data on cfDNA testing comparing various approaches remain limited. In a previous study, we reported that while the GW-MPS and the CSS targeted method may be equivalent in terms of performance in screening for major trisomies, they are not equivalent in terms of failure rate, the CSS targeted method showing a higher failure rate [Citation10–20]. The GW-MPS method reported in our study was a CE-in vitro Diagnostic Directive (CE-IVD) marked test. In Belgium, since 1 July 2017, cfDNA has been used as a first-line screening method for major trisomies in all pregnant women and the market is divided between those using a targeted technique or a home brew (HB) GW-MPS approach. To our knowledge, no previous studies have compared the CE-IVD targeted to the HB GW-MPS approach in populations where cfDNA is used as a first-line screening method for trisomies.
The objective of this study was to evaluate the failure rate and performance of cfDNA testing, performed by two laboratories using different analytical methods, namely the targeted approach and the HB GW-MPS. Our second objective was to evaluate whether the reported incidental findings at NIPT (such as rare autosomal trisomies or RATs, and partial imbalances or PIs) with the GW-MPS approach are clinically relevant.
Materials and methods
This was a retrospective observational study of cfDNA tests performed in pregnancies between July 2017 and April 2019, in the two largest maternities in Brussels, Belgium: the Departments of Obstetrics and Gynecology, University Hospital Brugmann and University Hospital St.-Pierre. Women from the University Hospital Brugmann had a CE-IVD Harmony® prenatal Test (Labocita, Citadelle Hospital in Liège, Belgium) in a Roche Sequencing Solutions technology transfer laboratory and women from the University Hospital St.-Pierre had an HB GW-MPS cfDNA test using the Illumina HiSeq platform, further referred to as HB-cfDNA test (BRIGHTcore, Center of Medical Genetics, UZ Brussel in Brussels, Belgium). Inclusion criteria were singleton and twin pregnancies with cfDNA testing performed after 10 weeks of gestational age from 1 July 2017 onwards. In fact, the Belgium Ministry of Public Health announced in May 2017 that NIPT in screening for trisomy 21 would be subsidized by the government for all pregnant women irrespective of the a priori risk and the decision took effect on 1 July 2017.
Women underwent cfDNA testing as a first-line screening regardless of a previous screening for fetal aneuploidy by conventional strategies. However, the 11–13 weeks scan is still subsidized by the government and therefore nearly all pregnant women in Belgium still get the first-trimester nuchal scan. Even if the nuchal translucency is increased above 3.5 mm, both cfDNA testing and a karyotype are offered.
For both laboratories a nonreportable result can occur due to either a low fetal fraction (FF) < 4% or to quality metrics issues including unusually high variation in cfDNA counts, failed sequencing, and atypical Z-scores, depending on the technique of each laboratory. All data from the participating centers were centralized in a single database at the Department of Obstetrics and Gynecology, University Hospital Brugmann. The study was approved by the appropriate Ethics Committees (EC 2015/27 and CE/19-05-10).
For the Harmony® prenatal Test, NIPT was performed by targeted cfDNA testing as described previously [Citation21,Citation22]. Harmony® uses DANSR assays targeting sequences on chromosomes 21, 18, and 13 for chromosome quantitation and single nucleotide polymorphisms on chromosomes 1–12 for FF measurement. After providing written informed consent, approximately 20 mL of blood was collected from each woman in Roche cfDNA collection tubes (Ariosa Diagnostics, Roche Sequencing Solutions, San Jose, CA, USA). Samples were sent via courier directly to the laboratory without processing and were received on the day of collection at room temperature. The data were analyzed with the FF optimized FORTE algorithm, which calculates probability scores for fetal trisomy, with greater than 1% considered to be high probability. In cases where the cfDNA test did not provide results, the parents were offered repeat testing. In cases with a high-risk result from the cfDNA test, the parents were advised to consider having invasive fetal karyotyping before deciding on the further management of their pregnancy.
For the HB-cfDNA test, circulating cfDNA analysis was performed by GW-MPS as previously described [Citation23,Citation24]. Approximately 10 mL of blood was collected from each subject in a Cell-free™ DNA BCT tube (Streck, Omaha, NE, USA) and sent the same day at room temperature to the genetic laboratory where the sample was processed. Data analyses were performed according to a previously published method [Citation23,Citation24]. The results were expressed as “positive” or “negative” once the experiments fulfilled the metric criteria.
For both hospitals, in cases where the cfDNA test did not provide results after repeat testing, the parents were advised to consider having an invasive test since the combined test screening is no longer available in Belgium or alternatively to rely on the results of the second-trimester scan. Amniocentesis was favored as a control test following a positive cfDNA test.
Statistical analysis
The difference between the various characteristic variables of the two populations (Harmony® prenatal Test versus HB-cfDNA test) was tested using the Pearson Chi-Squared test for discrete data. For continuous variables, we checked the two assumptions of the T-test (normality of the residuals with the Shapiro-Wilks test and homogeneity of the variances with Bartlett’s test), and if both were met we performed a T-test and report means and standard deviations by group and if at least one was not met we performed a Wilcoxon signed rank test and report the medians and interquartile ranges by group.
We undertook a propensity score analysis to match women between both groups. The CBPS R package was used to determine the propensity score, estimating an Average Treatment Effect (ATE), using covariate balancing and requesting an exact match, which has been shown to be superior to traditional logistic regression approaches and boosted classification and regression trees [Citation25]. An absolute standardized difference (ASD) <10–15% was considered to support the assumption of balance between the groups because it is not affected by sample size, unlike p-values, and it may be used to compare the relative balance of variables measured in different units [Citation26]. The mean and standard deviation obtained after matching are presented for continuous variables, and the percentage is presented for categorical variables. After the propensity score, the survey R package was used to perform logistic regressions for binary outcome, which includes the treatment group effect, the weight resulting from the matching, and variables present in the propensity score in order to obtain a doubly robust estimator which corrects the last remaining possible imbalance between the covariates and produces an unbiased treatment effect [Citation27].
The failure rate at first attempt was considered for the comparison of the results between the two laboratories. The total failure rate after redraw if available was also calculated for both laboratories. Similarly, the proportion of women with turn-around-time (TAT) results <7 calendar days as recommended by the Belgian Ministry of Public Health was compared between both laboratories.
For the comparison of test performance, we excluded women with unknown pregnancy outcome and reported the detection rates and false-positive rates for the three major trisomies: 21, 18 and 13. Detection rates for trisomy 21 at the two laboratories were compared using the chi-square test. However, given the small numbers, only qualitative comparisons were done for trisomy 18 and 13.
Finally, for the HB-cfDNA test performed at the University Hospital St.-Pierre, we counted all cases with rare incidental findings at NIPT other than the three major trisomies, including their complete pregnancy outcomes.
Data were analyzed with the statistical software packages R version 3.4.3 (R Core Team, 2017), and Excel version 15.0 (Microsoft, Redmond, WA, USA). A two-sided p < .05 was considered to be statistically significant.
Results
Study population
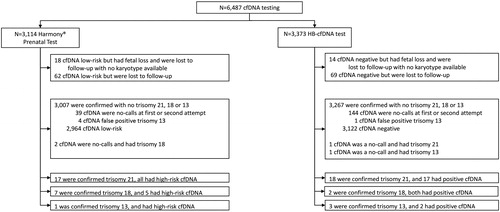
During the study period, cfDNA testing was performed in 6487 pregnancies: 3114 with a Harmony® prenatal Test and 3373 with a HB-cfDNA test. Among the 6487 pregnancies, 6322 (97.5%) had a known outcome. The flowchart of the study data is presented in .
Study population characteristics comparing women undergoing the two tests are summarized in . Maternal age, weight, the proportion twin pregnancies and the proportion of pregnancies with major trisomies did not differ between the two populations, whereas gestational age at testing, IVF status were significantly different.
Table 1. Summary of study population characteristics.
Failure of cfDNA testing with propensity score analysis
The Harmony® prenatal Test failed in 90 (2.9%) of 3114 women and the HB-cfDNA test in 413 (12.2%) of 3373 women. Postmatched comparisons of the women’s characteristics are presented in . After matching, data indicate a higher failure rate in the HB-cfDNA group (12.4%) than in the Harmony® group (2.8%; p < .001).
Table 2. Women’s characteristics and cell-free DNA testing failure rates obtained before and after matching of populations from both laboratories, using the propensity score.
Among the 90 women in whom the Harmony® prenatal Test failed, failure was due to a low (<4%) FF in 40 (44.4%) and to quality issue in 50 (55.6%), whereas for the 413 women in whom the HB-cfDNA test failed, failure was due to a low FF in 193 (46.7%) and to atypical laboratory results in 220 (53.3%).
Of the 90 women in whom the Harmony® prenatal Test failed, 61 had a repeat test at a median of 3.7 weeks (range; 0.3–13.7) after the first attempt, which still failed in 10. The total failure rate after 1 or 2 attempts was therefore 1.3% (39/3114). Of the 413 women in whom the HB-cfDNA test failed, 379 had a repeat test at a median of 2.3 weeks (range; 0.6–12.6) after the first attempt, which still failed in 110. The total failure rate after 1 or 2 attempts was therefore 4.3% (144/3373).
TAT of cfDNA testing in both laboratories
For the Harmony® prenatal Test at the University Hospital Brugmann, 3059 (98.2%) of the 3114 women had a result in < 7 calendar days whereas the corresponding numbers for the HB-cfDNA test at the University Hospital St.-Pierre was 1958 (58.0%) of the 3137 women.
Performance of the cfDNA test in both laboratories
After the first or second Harmony® prenatal Test, a high-risk result was noted in 17 of the 17 cases with trisomy 21, in 5 of the seven cases with trisomy 18, and a no-call in two cases, and the one case with trisomy 13 (). Conversely, the Harmony® prenatal Test gave a high-risk result in 4 of the 3089 fetuses with no trisomy 21, 18, or 13 (4 trisomy 13; 0.1% FPR).
Table 3. Performance of the cfDNA test in the two different laboratories.
After the first or second HB-cfDNA test, a high-risk result was noted in 17 of the 18 cases with trisomy 21, and a no-call in one case, in 2 of the two cases with trisomy 18, and in 2 of the three cases with trisomy 13, and a no-call in one case (). Conversely, the HB-cfDNA test gave a high-risk result in 1 of the 3350 fetuses with no trisomy 21, 18, or 13 (1 trisomy 13 and 1 trisomy18; 0.06% FPR).
The trisomy 21 detection rate did not differ between the two laboratories.
Results and clinical outcome of additional findings beyond common trisomies on NIPT performed with the HB-cfDNA test
Of the 3373 women with HB-cfDNA test, an additional finding beyond common trisomies (sex chromosome aneuploidy, SCA; RAT; rare autosomal monosomy, RAM; PI) on NIPT was found in 28 (0.8%) of the cases.
Eleven were aneuploidies (6 RATs, 1 RAM and 4 SCAs); 17 were large or submicroscopic PIs. After genetic counseling, 9 out of the 28 women agreed to undergo amniocentesis and the remaining had postnatal karyotyping at birth. In cases of PIs or SCAs nonconfirmed in the fetus, a maternal chromosomal microarray was always performed.
None of the 11 RATs, RAM and SCAs positive results were confirmed in the fetus/newborn. Three out of the four positive cases for SCAs showed the presence of a constitutional mosaic SCA in the maternal DNA.
The positive case for autosomal monosomy showed a normal microarray on amniocytes and maternal DNA. No further investigations for maternal cancer workup were performed.
The finding at NIPT was confirmed in the newborn in 3 out of 17 cases with PI (one with microduplication 1q21.1q21.2 and one with a deletion Xp21.1) and in another case a deletion rather than duplication of the long arm of chromosome 8 was found. Of the 14 PIs not confirmed in fetus/newborn, 8 were retrieved to be of maternal origin.
All but two pregnancies with positive results had strictly normal ultrasound follow-up. In one case there was borderline unilateral ventriculomegaly and in the other one moderate hydronephrosis. Both had a microdeletion that was confirmed to be of maternal origin and not fetal. In all 28 cases, there was normal clinical follow-up ().
Table 4. Details of the all cases with rare incidental findings on NIPT in this study.
Discussion
Principal findings of the study
Our data demonstrate that the failure rate of the HB-cfDNA test is 12.2% and is about four times that of the Harmony® prenatal Test (2.9%). Even after repeating the test, the failure rate remains high for the HB-cfDNA test at 4.3% as compared to the Harmony® prenatal Test at 1.3%. As a consequence this had an impact on the TAT since for the HB-cfDNA test a bit more than half of the patients had their results within a week while nearly all did for the Harmony® prenatal Test. When a woman had her results, the performance in screening for major trisomy 21 is comparable between both approaches. Finally, our study has shown no clinical relevance of disclosing or reporting additional findings beyond common trisomies on NIPT with the GW HB-cfDNA test.
Comparison with results of previous studies
Failure rate
In a recent prospective study of 23,495 singleton and 928 twin pregnancies undergoing screening for fetal trisomy by targeted cfDNA testing, namely the Harmony® prenatal Test as used in our study, the authors examined factors affecting the rate of failure to obtain a result from cfDNA testing and found no result from cfDNA testing after first sampling in 3.4% of singletons, 11.3% of dichorionic twins and 4.9% of monochorionic twins [Citation28]. Maternal age, weight, racial origin, parity, gestational age, dichorionicity, method of conception and serum levels of free β-hCG and PAPP‐A were found to be independent predictors of cfDNA test failure. Our failure rate for the Harmony® prenatal Test was comparable to this large study. It is interesting to note that main reason for failure for both tests was slightly more following quality issue or atypical laboratory results rather than for low FF.
Data on differences in estimates between laboratories with different methodologies for cfDNA analysis are scarce. We have recently compared the “Cerba test”, a CE-IVD MPS method, to a targeted method, the Harmony® prenatal Test and showed that the former had a lower no-result rate than the CSS-based approach. Yet both methods had relatively low failure rates: <2% at the first attempt and <1% at the second attempt [Citation20].
Data on failure rates with the HB-cfDNA test are even more scarce. In the study by Malan et al. and where the results were given only for trisomy 21, failure rate at first attempt was quite high, 4.3%, and the mean time interval between sample receipt and result availability was 13 days [Citation29]. In the present study, the failure rate at first attempt was even higher than in the study by Malan et al. since it reached 12.2% at first attempt and 4.3% after redraw. To our knowledge, such high failure rates have not previously been reported. One explanation could be related to overrepresentation of patients with high weight or samples taken at early gestational age, known to correlate with high failure rates. However, this was not the case since the population with the HB-cfDNA test was quite comparable to the one with the Harmony® prenatal Test: they had similar maternal weight and differed only by 2 days for gestational age at sampling. Surprisingly, failure to give a result was due to a low FF, i.e., <4%, in more than 5% of the cases, which is also strange. One explanation is that the method used for FF measurement actually underestimates the true value [Citation20], so a larger part of the population with the same characteristics are classified as <4% [Citation30].
Performance in screening for major trisomies
When cfDNA was successful, test performance was similar in the two laboratories and was comparable to previous reports and a meta-analysis on performance in screening for major trisomies.
Additional findings beyond common trisomies during NIPT
The rate of other findings including SCAs was 0.8%, while not including SCAs is 0.7% (24/3373), which is comparable with the recent study of Benn et al. who projected the type and the incidence RATs and PIs starting from cytogenetic results of CVS cytotrophoblast [Citation31]. RAT frequency in our study is 0.18% (6/3373) which is comparable to the RAT frequency found in a recent retrospective analysis of 10 recently published cfDNA studies [Citation32]. The RAT frequency in cfDNA varied more than eight-fold between the studies considered, ranging from 0.12% to 1.03%, and the most commonly represented abnormalities were trisomy 7, 15, 16 and 22 [Citation32]. For most chromosomes, the authors could not draw any conclusion about risk for fetal abnormality or pregnancy outcome due to an insufficient number of cases with a clinical follow up. However, in 151 RAT positive cases in which the clinical outcome was available, the most likely pregnancy outcome was a normal livebirth, similar to what we found in our study. The second most likely possibility is a miscarriage/fetal loss, but this is strongly dependent on the specific RAT, gestational age at the time of testing and referral indication. A cfDNA result indicating trisomy 16, 15 or 22 appears to be strongly associated with fetal loss as most are meiotic in origin or associated with a low PAPPA [Citation32].
Unlike other studies, the strength of ours is that it was conducted on a non-biased, low-risk population, as a first-line screening test. Among the 24 cases with RATs, PIs and RAMs, only 3 PIs were confirmed during invasive procedures or postnatally and none had an adverse clinical outcome. This is in line with the recently published study by Grati et al. related to the association between confined placental mosaicism (CPM) involving RATs and adverse pregnancy outcome [Citation33]. The authors concluded that, excluding T16, the incidence of adverse outcome for pregnancies carrying a CPM involving RATs is very low. The main advantage claimed for detecting RATs through cfDNA is their association with pregnancy complications. Since RATs identified through genome-wide cfDNA screening are mostly attributable to cpm, the conclusion was that such screening is of minimal benefit.
Implications for clinical practice
By using a HB-cfDNA test, failure rate after two attempts was 4.3%. These women are usually advised to undergo an invasive procedure. Adding the 0.8% of patients with incidental findings, more than 5% of patients are now advised to undergo an invasive procedure with this approach, which bring us to an FPR comparable to a first- trimester combined test screening, while the FPR due to NIPT has been repeatedly reported to be around 0.1% [Citation1].
Further, the TAT was quite long since about half of the women did not obtain their results within 1 week. Due to the high redraw rate, about 10% of patients had a TAT of 1 month or more. Such a TAT is much longer than the 96 hours recommended by the Belgium Ministry of Public Health.
Of the 25 nonconfirmed findings in fetal tissues, 10 (40%) were unexpected findings related or possibly related to maternal conditions (3 SCAs, 1 MAT and 6 partial (micro)deletion/duplication) confirming the complex nature of positive results and counseling and management difficulties [Citation31–33].
Home-brew laboratories are those operating at academic genetic centers in Belgium and it therefore seems advisable to audit their results and reevaluate their approach of offering NIPT, since the purpose of NIPT is mainly to decrease the FPR and maternal anxiety, and this goal is unlikely to be met with the current setting.
Further, it is clear that there are observations in favor and against the GW approach to cfDNA testing. However, the utility of discovering incidental findings during NIPT is questionable: they are rare, may lead to unnecessary anxiety in the parents, unnecessary diagnostic procedures and pregnancy interruptions, and genetic counseling could be very challenging also in absence of professional society endorsed steps for optimal patient management [Citation32]. GW assessment through cfDNA is not endorsed by any professional societies and the question of whether the benefits of providing GW screening exceed the harms therefore remains unanswered. Moreover, healthcare systems using cfDNA testing should invest in patient counseling and informed consent because pregnant women do not always understand the importance and implications of prenatal screening. In a recent survey, about half of the women undergoing NIPT did not understand that the test was meant to screen for trisomy 21 [Citation34].
Recently, Di Renzo et al. acknowledged that GW cfDNA testing may help elucidate the background of certain pregnancy complications [Citation35,Citation36]. The authors added that clinicians must weigh the potential benefits of such a screening against potential harms to patients prior to implementation in clinical practice. That is the reason why much more research is needed prior to its generalized use in the clinical care of pregnant women.
Limitations
The main limitation of our study is that the between-laboratory comparisons of cfDNA tests using the two different methodologies were not performed on the same women. Ideally, the best approach would have been to have a blood sample from the same women analyzed by the two laboratories, blinded to each other’s results. Such a study is unlikely to be performed because of logistical and/or financial reasons. One could argue that the second best approach would have been to conduct a study with two populations with the same characteristics. However, the probability of having statistically matched population cohorts for all confounder variables is very unlikely. Although the two populations that we studied were relatively similar, there were still some differences. To overcome these limitations, we opted to use a propensity score analysis to allow matching of both populations for the various factors known to influence failure rate.
Conclusions
Our study shows that laboratories performing cfDNA tests using the HB-cfDNA approach seem to have quite high failure rates, even after redraw, as compared to targeted technology transfer laboratories, and quite long TAT. The argument of choosing a WG cfDNA approach as a first-line screening to diagnose extra anomalies and improve clinical care seems to be invalid. Clinicians are required to look critically at different providers and make informed choices about testing to ensure quality patient care.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Gil MM, Accurti V, Santacruz B, et al. Analysis of cell-free DNA in maternal blood in screening for aneuploidies: updated meta-analysis. Ultrasound Obstet Gynecol. 2017;50(3):302–314.
- Gil MM, Galeva S, Jani J, et al. Screening for trisomies by cfDNA testing of maternal blood in twin pregnancy: update of The Fetal Medicine Foundation results and meta-analysis. Ultrasound Obstet Gynecol. 2019;53(6):734–742.
- Grossman TB, Bodenlos KL, Chasen ST. Abnormal nuchal translucency: residual risk with normal cell-free DNA screening. J Matern Fetal Neonatal Med. 2019;1–6. DOI:https://doi.org/10.1080/14767058.2019.1568405.
- White K, Wang Y, Kunz LH, et al. Factors associated with obtaining results on repeat cell-free DNA testing in samples redrawn due to insufficient fetal fraction. J Matern Fetal Neonatal Med. 2019;1–6. DOI:https://doi.org/10.1080/14767058.2019.1594190.
- Kostenko E, Chantraine F, Vandeweyer K, et al. Clinical and economic impact of adopting noninvasive prenatal testing as a primary screening method for fetal aneuploidies in the general pregnancy population. Fetal Diagn Ther. 2019;45(6):413–423.
- Gammon BL, Jaramillo C, Riggan KA, et al. Decisional regret in women receiving high risk or inconclusive prenatal cell-free DNA screening results. J Matern Fetal Neonatal Med. 2018;1–7. DOI:https://doi.org/10.1080/14767058.2018.1519541.
- Rego de Sousa MJ, Albuquerque M, Ribeiro R, et al. Evaluation of Noninvasive prenatal Testing (NIPT) guidelines using the AGREE II instrument. J Matern Fetal Neonatal Med. 2018;1–9. DOI:https://doi.org/10.1080/14767058.2018.1494716.
- Chen KM, White K, Shabbeer J, et al. Maternal age trends support uptake of non-invasive prenatal testing (NIPT) in the low-risk population. J Matern Fetal Neonatal Med. 2019;32(23):4039–4042.
- Gil MM, Brik M, Casanova C, et al. Screening for trisomies 21 and 18 in a Spanish public hospital: from the combined test to the cell-free DNA test. J Matern Fetal Neonatal Med. 2017;30(20):2476–2482.
- Norwitz ER, Levy B. Noninvasive prenatal testing: the future is now. Rev Obstet Gynecol. 2013;6(2):48–62.
- Hartwig TS, Ambye L, Sørensen S, et al. Discordant non-invasive prenatal testing (NIPT) – a systematic review. Prenat Diagn. 2017;37(6):527–539.
- Curnow KJ, Wilkins-Haug L, Ryan A, et al. Detection of triploid, molar, and vanishing twin pregnancies by a single-nucleotide polymorphism-based noninvasive prenatal test. Am J Obstet Gynecol. 2015;212(1):79.e1–79.e9.
- Bianchi DW. Should we “open the kimono” to release the results of rare autosomal aneuploidies following noninvasive prenatal whole genome sequencing? Prenat Diagn. 2017;37(2):123–125.
- Pertile MD, Halks-Miller M, Flowers N, et al. Rare autosomal trisomies, revealed by maternal plasma DNA sequencing, suggest increased risk of feto-placental disease. Sci Transl Med. 2017;9(405). DOI:https://doi.org/10.1126/scitranslmed.aan1240.
- Poon LL, Leung TN, Lau TK, et al. Differential DNA methylation between fetus and mother as a strategy for detecting fetal DNA in maternal plasma. Clin Chem. 2002;48(1):35–41.
- Defrag SR. (DEtection of fetal FRaction and Gender) [WWW document]. [cited 30 Sept 2016]. Available from: https://github.com/rstraver/wisecondor/blob/master/defrag.py
- Kim SK, Hannum G, Geis J, et al. Determination of fetal DNA fraction from the plasma of pregnant women using sequence read counts. Prenat Diagn. 2015;35(8):810–815.
- Straver R, Oudejans CB, Sistermans EA, et al. Calculating the fetal fraction for noninvasive prenatal testing based on genome-wide nucleosome profiles. Prenat Diagn. 2016;36(7):614–621.
- Dahl F, Ericsson O, Karlberg O, et al. Imaging single DNA molecules for high precision NIPT. Sci Rep. 2018;8(1):4549.
- Bevilacqua E, Jani JC, Letourneau A, et al. Cell-free DNA analysis in maternal blood: differences in estimates between Laboratories with Different Methodologies Using a propensity score approach. Fetal Diagn Ther. 2019;45(5):302–311.
- Sparks AB, Wang ET, Struble CA, et al. Selective analysis of cell-free DNA in maternal blood for evaluation of fetal trisomy. Prenat Diagn. 2012;32(1):3–9.
- Sparks AB, Struble CA, Wang ET, et al. Noninvasive prenatal detection and selective analysis of cell-free DNA obtained from maternal blood: evaluation for trisomy 21 and trisomy 18. Am J Obstet Gynecol. 2012;206(4):319.e1–319.e9.
- Brison N, Van Den Bogaert K, Dehaspe L, et al. Accuracy and clinical value of maternal incidental findings during noninvasive prenatal testing for fetal aneuploidies. Genet Med. 2017;19(3):306–313.
- Bayindir B, Dehaspe L, Brison N, et al. Noninvasive prenatal testing using a novel analysis pipeline to screen for all autosomal fetal aneuploidies improves pregnancy management. Eur J Hum Genet. 2015;23(10):1286–1293.
- Imai K, Ratkovic M. Robust estimation of inverse probability weights for marginal structural models. J Am Stat Assoc. 2015;110(511):1013–1023.
- Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424.
- Funk MJ, Westreich D, Wiesen C, et al. Doubly robust estimation of causal effects. Am J Epidemiol. 2011;173(7):761–767.
- Galeva S, Gil MM, Konstantinidou L, et al. First-trimester screening for trisomies by cfDNA testing of maternal blood in singleton and twin pregnancies: factors affecting test failure. Ultrasound Obstet Gynecol. 2019;53(6):804–809.
- Malan V, Bussières L, Winer N, et al. Effect of cell-free DNA screening vs direct invasive diagnosis on miscarriage rates in women with pregnancies at high risk of trisomy 21: a randomized clinical trial. JAMA. 2018;320(6):557–565.
- Revello R, Sarno L, Ispas A, et al. Screening for trisomies by cell-free DNA testing of maternal blood: consequences of a failed result. Ultrasound Obstet Gynecol. 2016;47(6):698–704.
- Benn P, Grati FR. Genome-wide non-invasive prenatal screening for all cytogenetically visible imbalances. Ultrasound Obstet Gynecol. 2018;51(4):429–433.
- Benn P, Malvestiti F, Grimi B, et al. Rare autosomal trisomies: comparison of detection through cell-free DNA analysis and direct chromosome preparation of chorionic villus samples. Ultrasound Obstet Gynecol. 2019;54(4):458–467.
- Grati FR, Ferreira J, Benn P, et al. Outcomes in pregnancies with a confined placental mosaicism and implications for prenatal screening using cell-free DNA. Genet Med. 2019. DOI:https://doi.org/10.1038/s41436-019-0630-y.
- Abousleiman C, Lismonde A, Jani JC. Concerns following rapid implementation of first-line screening for aneuploidy by cell-free DNA analysis in the Belgian healthcare system. Ultrasound Obstet Gynecol. 2019;53(6):847–848.
- Di Renzo GC, Luis Bartha J, Bilardo CM. More research is needed prior to the implementation of genome-wide cell-free DNA testing in specific populations (Response to letter L19-020A: confined placental trisomy detection through cell-free DNA in the maternal circulation: benefit for pregnancy management). Am J Obstet Gynecol. 2019;221(3):287.
- Di Renzo GC, Bartha JL, Bilardo CM. Expanding the indications for cell-free DNA in the maternal circulation: clinical considerations and implications. Am J Obstet Gynecol. 2019;220(6):537–542.