Abstract
Objective
To summarize currently available evidence on maternal, fetal, and neonatal outcomes of pregnant women infected with Coronavirus Disease 2019 (COVID-19).
Material and methods
PubMed, Google Scholar, CNKI, Wanfang Data, VIP, and CBMdisc were searched for studies reporting maternal, fetal, and neonatal outcomes of women infected with COVID-19 published from 1 January 2020 to 26 March 2020. The protocol was registered with the Open Science Framework (DOI: 10.17605/OSF.IO/34ZAV).
Results
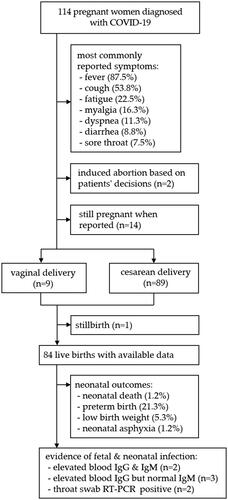
In total, 18 studies comprising 114 pregnant women were included in the review. Fever (87.5%) and cough (53.8%) were the most commonly reported symptoms, followed by fatigue (22.5%), diarrhea (8.8%), dyspnea (11.3%), sore throat (7.5%), and myalgia (16.3%). The majority of patients (91%) had cesarean delivery due to various indications. In terms of fetal and neonatal outcomes, stillbirth (1.2%), neonatal death (1.2%), preterm birth (21.3%), low birth weight (<2500 g, 5.3%), fetal distress (10.7%), and neonatal asphyxia (1.2%) were reported. There are reports of neonatal infection, but no direct evidence of intrauterine vertical transmission has been found.
Conclusions
The clinical characteristics of pregnant women with COVID-19 are similar to those of non-pregnant adults. Fetal and neonatal outcomes appear good in most cases, but available data only include pregnant women infected in their third trimesters. Further studies are needed to ascertain long-term outcomes and potential intrauterine vertical transmission.
Introduction
Coronavirus Disease 2019 (COVID-19) is an emerging, rapidly evolving situation. It is reported that pregnant women were also susceptible to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection [Citation1], which may increase the risk of adverse pregnancy outcomes. Currently, studies investigating the status of COVID-19 in obstetric population and its perinatal outcomes remain scarce. Moreover, the majority of published studies are case reports/series, which are written in Chinese, resulting in a gap of evidence. Therefore, we performed a systematic review to comprehensively summarize the perinatal outcomes in pregnant women with COVID-19.
Material and methods
We systematically reviewed available evidence in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines; the protocol was registered with the Open Science Framework (DOI: 10.17605/OSF.IO/34ZAV). We searched PubMed, Google Scholar, CNKI, Wanfang Data, VIP, and CBMdisc for potentially eligible studies published from 1 January 2020 to 26 March 2020, with no restriction on language. The following search (MeSH) terms were applied in each database accordingly: “pregnancy,” “infant, newborn,” “COVID-19,” “severe acute respiratory syndrome coronavirus 2,” and “coronavirus infections”; details can be found in the protocol. Moreover, we hand-searched (manual search) the reference lists of all identified studies and key journals in the related field. Observational studies, case reports/series, letters, and comments reporting maternal, fetal, and neonatal outcomes in pregnant women with COVID-19 were screened. The quality (risk of bias) of included studies was assessed using the Newcastle-Ottawa Scale and a modified tool for quality appraisal of case reports/series; details can be found in the protocol. All methodology procedures were conducted independently by two authors, and any discrepancies were discussed with an expert in the related field. The datasets of this study can be accessed in Mendeley Data (DOI: 10.17632/hvcjfsrjkb.2).
Results and discussion
The initial search identified 525 studies; three additional articles from manual search were identified. Finally, 18 studies (17 case reports/series and 1 case-control study) comprising 114 pregnant women were included. The flow diagram of the study selection process and the characteristics of included studies can be accessed in the datasets. All studies were rated as low-quality. Maternal, fetal, and neonatal outcomes are briefly summarized in .
Details of clinical characteristics of pregnant women infected with COVID-19 and maternal outcomes can be found in Supplemental Table 1. Briefly, the most commonly reported symptoms were fever (87.5%) and cough (53.8%), less common symptoms included fatigue (22.5%), diarrhea (8.8%), dyspnea (11.3%), sore throat (7.5%), and myalgia (16.3%). Fever (70.2%) and cough (52.6%) were the most common onset symptoms. Two patients were asymptomatic at admission and developed typical symptoms few days later. Most patients (96.5%) had mild symptoms or were categorized into a mild or regular type of COVID-19 at admission, according to the Novel Coronavirus Pneumonia Diagnosis and Treatment Protocols published by the Chinese government. Six patients (5.3%) developed a severe or critical type, including one case of multiple organ failure and use of extracorporeal membrane oxygenation. The majority of patients (91%) had cesarean delivery, mostly due to preeclampsia, fetal distress, history of previous C-sections, and unknown risk of intrapartum mother-to-child transmission by vaginal delivery. Two cases of induced abortion, based on patients’ decisions, were reported. The only case-control study suggested no differences in preeclampsia, gestational diabetes mellitus, and premature rupture of membrane between COVID-19 and non-COVID-19 groups. In general, the clinical characteristics of pregnant women are similar to those of non-pregnant adults [Citation2], but more case-control studies are needed to ascertain the results.
Currently, there is no direct evidence suggesting that COVID-19 in pregnancy could lead to fetal infection via intrauterine vertical transmission. To date, no positive RT-PCR results were found in the amniotic fluid, placenta, or cord blood in the studied population, even in the only two cases published by Li et al. [Citation3] and Yu et al. [Citation4], in which COVID-19 was confirmed in two neonates at 36 h and three days of age (Supplemental Table 2). The lack of virologic evidence for vertical transmission raises the doubt whether these two neonates were infected after birth. Furthermore, Dong et al. [Citation5] and Zeng et al. [Citation6] detected virus-specific antibodies in neonatal blood samples collected after birth, but none of the infants had a positive RT-PCR result. As IgM does not transfer to the fetus via the placenta due to its larger macromolecular structure, we are concerned if the placentas in these cases were damaged. Alternatively, IgM could have been produced by the infant if the virus crossed the placenta. However, pathological analyses reported in the case series of Chen et al. [Citation7] suggested no morphological changes related to the infection in placentas, and a recent study [Citation8] suggested that there is a lack of susceptible cell subsets of SARS-CoV-2 at the maternal-fetal interface.
Seventeen studies comprising 84 live births reported fetal and neonatal outcomes (Supplemental Table 2). Adverse outcomes including stillbirth (1.2%), neonatal death (1.2%), preterm birth (21.3%), low birth weight (<2500 g, 5.3%), fetal distress (10.7%), and neonatal asphyxia (1.2%) were reported. The case-control study showed no significant differences in fetal and neonatal outcomes (fetal distress [p = .668], preterm birth [p = .686], and neonatal asphyxia [p = .441]) between groups. To date, available data on fetal and neonatal outcomes only include pregnant women infected in their third trimesters. It remains unknown whether infection in the first or second trimester would increase the risk of adverse fetal and neonatal outcomes. Therefore, future studies, focusing on the long-term outcomes in this population, are still warranted.
In this epidemic, it is essential to standardize screening, admission, and management of all suspected/confirmed pregnant women infected with COVID-19 and prepare maternity wards in the best possible way. Management should be performed in accordance with local, federal, and international guidelines, and strategies to quickly implement obstetric units have also been suggested [Citation9,Citation10]. Once a pregnant woman is suspected/confirmed COVID-19 infection, maternal care and childbirth would become complicated and challenging. Studies have presented with preventive healthcare patterns and safe delivery strategies to handle pregnant women with COVID-19 [Citation11,Citation12]. An expert consensus for managing pregnant women and neonates born to mothers with suspected/confirmed COVID-19 infection has also been published to guide clinical practice, which demonstrated the route of delivery and delivery timing should be individualized according to obstetrical indications and maternal-fetal status [Citation10]. Neonates are suggested to be isolated for at least 14 days, and direct breastfeeding is not recommended until the recovery of confirmed mothers or rejection of probable infection [Citation13].
This review is limited by the low quality and retrospective nature of included studies. There is inconsistence of the number of each item being calculated. Moreover, some patients were still pregnant and hospitalized when these case reports were finalized.
Conclusions
Based on currently available data, the clinical characteristics of pregnant women with COVID-19 are similar to those of non-pregnant adults. There is no evidence that COVID-19 infected pregnant women are more likely to develop severe pneumonia or death. The maternal outcomes observed in late pregnancy and the fetal and neonatal outcomes appear good in most cases. In the absence of more robust data, active and intensive management might be best practice. Long-term outcomes and potential intrauterine vertical transmission need further analysis.
Supplemental_Table_2.docx
Download MS Word (17.4 KB)Supplemental_Table_1.docx
Download MS Word (19.7 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Liu Y, Chen H, Tang K, et al. Clinical manifestations and outcome of SARS-CoV-2 infection during pregnancy. J Infection. Forthcoming. [cited 2020 Apr 2]:[10 p.]. Author's manuscript available at DOI:https://doi.org/10.1016/j.jinf.2020.02.028
- Fang Z, Yi F, Wu K, et al. Clinical Characteristics of Coronavirus Pneumonia 2019 (COVID-19). An Updated Systematic Review. [cited 2020 Apr 2]. Available from: https://www.medrxiv.org/content/https://doi.org/10.1101/2020.03.07.20032573v2
- Li M, Xu M, Zhan W, et al. [Report of the first cases of mother and infant infections with 2019 novel coronavirus in Xinyang City Henan Province]. Chin J Infect Dis. 2020. [cited 2020 Apr 2]; [8 p.]. DOI:https://doi.org/10.3760/cma.j.issn.1000-6680.2020.02.000
- Yu N, Fang Z, Wu J, et al. [Novel Coronavirus pnuemonia in pregnancy: perinatal outcomes]. Progress Obstet Gynecol. 2020. [cited 2020 Apr 2]; [6 p.]. DOI:https://doi.org/10.13283/j.cnki.xdfckjz.2020.03.004
- Dong L, Tian J, He S, et al. Possible Vertical Transmission of SARS-CoV-2 From an Infected Mother to Her Newborn. JAMA. 2020[cited 2020 Apr 2]; [3 p.]. DOI:https://doi.org/10.1001/jama.2020.4621
- Zeng H, Xu C, Fan J, et al. Antibodies in Infants Born to Mothers With COVID-19 Pneumonia. JAMA. 2020 [cited Apr 2]; [2 p.]. DOI:https://doi.org/10.1001/jama.2020.4861
- Chen S, Huang B, Luo DJ, et al. Pregnant women with new coronavirus infection: a clinical characteristics and placental pathological analysis of three cases]. Chin J Patho. 2020;49(0):E005.
- Zheng Q, Duan T, Jin L. Single-cell RNA expression profiling of ACE2 and AXL in the human maternal–Fetal interface. Reprod Dev Med. 2020;4(1):7.
- Capanna F, Haydar A, McCarey C, et al. Preparing an obstetric unit in the heart of the epidemic strike of COVID-19: quick reorganization tips. J Matern-Fetal Neo M. 2020 [cited 2020 Apr 2]; [8 p.]. DOI:https://doi.org/10.1080/14767058.2020.1749258
- Chen D, Yang H, Cao Y, et al. Expert consensus for managing pregnant women and neonates born to mothers with suspected or confirmed novel coronavirus (COVID-19) infection. Int J Gynecol Obstet. 2020;149(2):130–136.
- Qi H, Luo X, Zheng Y, et al. Safe Delivery for COVID-19 Infected Pregnancies. BJOG-Int J Obstet Gy. 2020 [cited 2020 Apr 2]; [8 p.]. DOI:https://doi.org/10.1111/1471-0528.16231
- Mirzadeh M, Khedmat L. Pregnant women in the exposure to COVID-19 infection outbreak: the unseen risk factors and preventive healthcare patterns. J Matern-Fetal Neo M. 2020 [cited 2020 Apr 2]; [3 p.]. DOI:https://doi.org/10.1080/14767058.2020.1749257
- Wang J, Qi H, Bao L, et al. A contingency plan for the management of the 2019 novel coronavirus outbreak in neonatal intensive care units. Lancet Child Adolesc Health. 2020;4(4):258–259.