Abstract
Objective
To compare Doppler blood flow velocity measures in the right and left proximal branch of the fetal pulmonary artery at 30 gestational weeks.
Methods
Doppler blood flow velocity waveforms were recorded in both fetal proximal pulmonary artery branches in 62 healthy fetuses at 30 gestational weeks. Pulsatility index, peak systolic velocity, time averaged maximum velocity, time velocity integral, fetal heart rate, acceleration- and ejection time with their ratio, time of one heart cycle and time velocity integral were recorded. Paired-samples t-test was used to compare measures from the right and left pulmonary branch.
Results
We observed significantly higher peak systolic velocity, time averaged maximum velocity, time velocity integral, acceleration time and acceleration time/ejection time ratio in the right compared to the left pulmonary artery (p < .001). Sampling angle, pulsatility index, fetal heart rate, ejection time and time of one heart cycle were similar in both pulmonary branches.
Conclusion
Our study conducted at 30 weeks gestational age found significantly different blood flow velocity waveform measures in the right and left pulmonary artery branches in contrast to previous reports of similar velocities.
Keywords:
Introduction
Doppler measurement of blood flow in fetal pulmonary arteries is a diagnostic tool used mainly to evaluate fetuses at risk of neonatal lung hypoplasia, particularly those with congenital diaphragmatic hernia [Citation1,Citation2]. Pulmonary artery Doppler measures have been found to be associated with respiratory outcome following preterm birth [Citation3], oligohydramnios [Citation4] and show potential in assessing lung maturity [Citation5]. Identification of fetuses with adverse respiratory outcome is important for parent counseling, optimizing postnatal care or, at some centers, for considering intrauterine interventions. Blood flow velocity waveforms in fetal right and left pulmonary arteries have previously been reported to have similar values [Citation6–9]. As part of the prospective population-based birth cohort study PreventADALL (Preventing Atopic Dermatitis and ALLergies in children) that included exploring associations between fetal lung growth and development, and postnatal lung function [Citation10], we conducted a cross-sectional sub-study of 449 pregnancies at 30 gestational weeks to analyze associations between blood flow in the pulmonary arteries of healthy fetuses and tidal flow volume at 3 months of age. We sought to perform the measurements in at least one of the pulmonary branches, but in 62 cases succeeded in measuring both. The aim of this article is to explore if Doppler blood flow velocity waveforms were similar in the right and left proximal pulmonary artery branches.
Methods
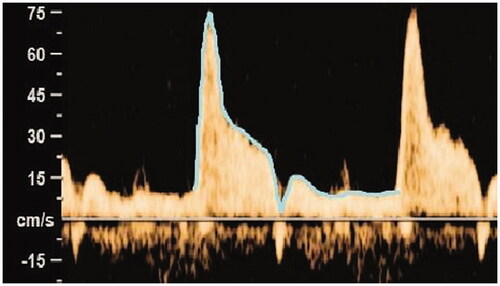
A single operator performed Doppler measurements by a GE Voluson E8 ultrasound system (GE Medical Systems, Zipf, Austria) using a 4–8 mHz transducer in 62 uncomplicated singleton pregnancies without fetal abnormality or growth disorder at gestational age 30.2 weeks (range 29.4–31.1 weeks). In the absence of fetal respiratory movements, Doppler blood flow velocity waveforms were obtained in the proximal parts of both pulmonary branches close to the pulmonary bifurcation. Sampling angle was as low as possible (always <25°) and sampling gate was 3 mm. The wallmotion filter was set to 160 Hz. Automatic tracing was used in 81% of the measurements, with manual tracing if the automatic was not possible. In 15 randomly selected women, the intraclass coefficient of 0.971–0.995 showed good agreement for pulsatility index, peak systolic velocity and time averaged maximum velocity values obtained by manual or automatic tracing. The dicrotic notch of reversed flow was not traced (). In the heart cycles used for the velocity measures, acceleration time (time from onset of the systole, to the systolic velocity peak), ejection time (duration of the systole), acceleration time/ejection time ratio and time of one heart cycle were measured. Time velocity integral was calculated as the product of time averaged maximum velocity and time of one heart cycle. For Doppler measurements we always used mean values from three consecutive heart cycles. Paired-samples t-test was used to compare Doppler blood flow velocity measures between the right and left pulmonary artery.
All statistical analyses were conducted with the use of IBM© SPSS© statistics version 25.0 (SPSS Inc., Chicago, IL, U.S.A.) using p-values <.05 as statistically significant. Our study was approved by the Regional board for Medical and Health Research Ethics in South-Eastern Norway (2014/518) and Sweden (2014/2242-31/4), and registered at ClinicalTrials.gov NCT02449850.
Results
Baseline characteristics were as follows. Mean maternal age was 32.8 years (SD 3.9 years). Of the participants 39 (62.9%) were para 0. Seven women (11.3%) reported nicotine use during the pregnancy but none after the 6th gestational week. Mean gestational age at delivery was 40.5 weeks (SD 1.1 week). Mean birthweight was 3586 g (SD 489 g). Thirty three (53.2%) of the infants were boys. Mean 1 min Apgar score was 8.8 (SD 1.2) and mean 5 min Apgar score was 9.5 (SD 0.8). As reported in , we observed significantly higher peak systolic velocity, time averaged maximum velocity, time velocity integral, acceleration time and acceleration time/ejection time ratio in the right compared to the left pulmonary artery (p < .001). No significant differences were observed for sampling angle, pulsatility index, fetal heart rate, ejection time and time of one heart cycle.
Table 1. Results of Doppler recordings from both the right and left branch of the pulmonary artery in 62 fetuses at 30 gestational weeks, given as mean (95% confidence interval).
Discussion
Contrary to previous reports we found different Doppler blood flow velocity waveform measures in the right and left pulmonary artery branches. We are aware of only two studies that compared right and left pulmonary Doppler measures by paired statistical tests [Citation6,Citation7]. Another study [Citation11] indicated consistently higher acceleration time/ejection time ratio in the right pulmonary artery, though unsubstantiated by statistical calculations. Our findings are supported by the observations from healthy newborns showing higher peak systolic velocities in the right pulmonary artery, though the pulmonary circulation differs significantly in fetuses compared to neonates [Citation12]. Different blood velocities in the pulmonary branches could be explained by their different relation to ductus arteriosus and the main pulmonary trunk as a result of their embryological origin. The right pulmonary artery arises from the right sixth aortic arch. Left pulmonary artery and ductus arteriosus originate both from the left sixth aortic arch [Citation13] resulting in their closer proximity and more parallel direction. Right pulmonary artery arises with a more acute angle from the main trunk [Citation14]. Secondly, our study focused on the third trimester when pulmonary circulation is markedly different from the second trimester. Blood flow in the pulmonary artery is twice as high at 30 compared to 20 gestational weeks [Citation15]. With advancing gestational age the vasoreactivity of the pulmonary arteries and ductus arteriosus increases [Citation16,Citation17]. Consequently, the differences in blood perfusion of the pulmonary branches could become more prominent in the third compared to the second trimester of pregnancy. Another possible contribution to our observation is the narrow gestational age range, eliminating gestational age as a potential confounding factor. Our sampling gate was placed as close as possible to the pulmonary bifurcation. Rasanen et al. observed lower pulsatility index, lower peak systolic velocity and shorter acceleration time in the distal compared to the proximal branches of the pulmonary artery [Citation8]. We consider the risk of a systematic skew due to distal placement of the sampling gate on the left compared to the right side during the examination, as small. Moreover, our peak systolic velocity values correspond to those obtained by Rasanen et al. in the proximal pulmonary branches.
Our study is limited to assessing information on blood flow velocities in the pulmonary arteries at 30 gestational weeks only, and cannot be generalized to other periods of pregnancy. Nevertheless, given a sizeable study population and short gestational range, we are able to demonstrate variation in flow velocity waveforms between the right and left pulmonary branches, with implications for future research.
Previous reports of similar Doppler values in the right and left proximal pulmonary branches may have influenced later studies resulting in either sampling from the best accessible side [Citation18] or not specifying the sampling side when creating nomograms with reference values for different pregnancy weeks [Citation19].
Our observation of different Doppler blood flow velocity waveform measures in the right and left pulmonary artery in early third trimester should be considered in planning research studies for further insight into pathophysiologic mechanisms of cardio-pulmonary development.
Acknowledgments
We acknowledge the study participants as well as Håvard Ove Skjerven, Eva Maria Rehbinder, Christine Monceyron Jonassen and Björn Nordlund on behalf of the PreventADALL study team [Citation10].
Disclosure statement
The authors have no conflicts of interest to disclaim.
Additional information
Funding
References
- Cruz-Martinez R, Moreno-Alvarez O, Hernandez-Andrade E, et al. Contribution of intrapulmonary artery Doppler to improve prediction of survival in fetuses with congenital diaphragmatic hernia treated with fetal endoscopic tracheal occlusion. Ultrasound Obstet Gynecol. 2010;35(5):572–577.
- Fuke S, Kanzaki T, Mu J, et al. Antenatal prediction of pulmonary hypoplasia by acceleration time/ejection time ratio of fetal pulmonary arteries by Doppler blood flow velocimetry. Am J Obstet Gynecol. 2003;188(1):228–233.
- Kim SM, Park JS, Norwitz ER, et al. Acceleration time-to-ejection time ratio in fetal pulmonary artery predicts the development of neonatal respiratory distress syndrome: a prospective cohort study. Am J Perinatol. 2013;30(10):805–812.
- Laudy JA, Tibboel D, Robben SG, et al. Prenatal prediction of pulmonary hypoplasia: clinical, biometric, and Doppler velocity correlates. Pediatrics. 2002;109(2):250–258.
- Azpurua H, Norwitz ER, Campbell KH, et al. Acceleration/ejection time ratio in the fetal pulmonary artery predicts fetal lung maturity. Am J Obstet Gynecol. 2010;203(1):40.e1-8–40.e8.
- Chaoui R, Taddei F, Rizzo G, et al. Doppler echocardiography of the main stems of the pulmonary arteries in the normal human fetus. Ultrasound Obstet Gynecol. 1998;11(3):173–179.
- Moreno-Alvarez O, Hernandez-Andrade E, Oros D, et al. Association between intrapulmonary arterial Doppler parameters and degree of lung growth as measured by lung-to-head ratio in fetuses with congenital diaphragmatic hernia. Ultrasound Obstet Gynecol. 2008;31(2):164–170.
- Rasanen J, Huhta JC, Weiner S, et al. Fetal branch pulmonary arterial vascular impedance during the second half of pregnancy. Am J Obstet Gynecol. 1996;174(5):1441–1449.
- Laudy JAM, De Ridder MAJ, Wladimiroff JW. Doppler Velocimetry in Branch Pulmonary Arteries of Normal Human Fetuses during the Second Half of Gestation. Pediatr Res. 1997;41(6):897–901.
- Lodrup Carlsen KC, Rehbinder EM, Skjerven HO, et al. Preventing Atopic Dermatitis and ALLergies in Children-the PreventADALL study. Allergy. 2018;73(10):2063–2070.
- Yamamoto Y, Hirose A, Howley L, et al. Parameters of fetal pulmonary vascular health: baseline trends and response to maternal hyperoxia in the second and third trimesters. Ultrasound Obstet Gynecol. 2017;50(5):618–623. Nov
- Du ZD, Roguin N, Barak M, et al. Doppler echocardiographic study of the pulmonary artery and its branches in 114 normal neonates. Pediatr Cardiol. 1997;18(1):38–42.
- Sadler TW. Cardiovascular system. In: Sadler TW, Langman J, editors. Langman's medical embryology. 12th ed. London: Lippincott Williams & Wilkins; 2012. p. 162–200.
- Gardin JM, Sung HW, Yoganathan AP, et al. Doppler flow velocity mapping in an in vitro model of the normal pulmonary artery. J Am Coll Cardiol. 1988;12(5):1366–1376.
- Rasanen J, Wood DC, Weiner S, et al. Role of the pulmonary circulation in the distribution of human fetal cardiac output during the second half of pregnancy. Circulation. 1996;94(5):1068–1073.
- Rasanen J, Wood DC, Debbs RH, et al. Reactivity of the human fetal pulmonary circulation to maternal hyperoxygenation increases during the second half of pregnancy: a randomized study. Circulation. 1998;97(3):257–262. 27
- Moise KJ. Jr. Effect of advancing gestational age on the frequency of fetal ductal constriction in association with maternal indomethacin use. Am J Obstet Gynecol. 1993;168(5):1350–1353.
- Herren H, Araujo Junior E, Martins WP, et al. Reference ranges of Doppler parameters of foetal pulmonary artery segments between 19 and 39 weeks of gestation. J Matern Fetal Neonatal Med. 2016;29(1):85–90.
- Fittschen M, Reinhard I, Wellek S, et al. Advanced dynamic Doppler flow of the pulmonary artery in a normal population: reference values from 18 to 41 weeks of gestation calculated by automatic Doppler waveform analysis. Arch Gynecol Obstet. 2014;289(5):973–980.