Abstract
Objective
To assess at 24 months corrected age (CA) the neurological, respiratory, and general health status of children born prematurely from 27+0 to 33+6 weeks’ gestation who were treated in a first-in-human study with a new fully synthetic surfactant (CHF5633) enriched with SP-B and SP-C proteins.
Outcome measures
Children were assessed using Bayley Scales of Infant Development (BSID), with a score below normal defined as BSID-II Mental Development Index score <70, or BSID-III cognitive composite score <85. In addition, a health status questionnaire was used to check for functional disability including respiratory problems and related treatments, sensory and neurodevelopment assessments, communication skills as well as the number of hospitalizations.
Results
35 of 39 survivors had a neurodevelopmental assessment, 24 infants being evaluated by Bayley’s Scales and 11 by health status questionnaires only. 23 children had scores within normal limits and one had BSID-III <85. The remaining 11 were judged clinically to have normal development. Health status questionnaires detected only issues that would normally be expected in preterm-born children.
Conclusions
This assessment offers reassurance that treatment with CHF5633 surfactant was not associated with adverse neurodevelopmental, respiratory, or health outcomes by two years corrected age.
Introduction
CHF5633 is a new intratracheally administered, fully synthetic surfactant enriched with peptide analogs of human SP-B and SP-C proteins which was tested in a first-in-human study at two different doses (100 and 200 mg/kg) in two cohorts of 20 preterm neonates with respiratory distress syndrome (RDS). The main phase of the study from birth until hospital discharge demonstrated a good safety and tolerability profile of both endotracheal doses without detectable systemic absorption nor immunogenicity and with encouraging efficacy results [Citation1]. At 2 years of age, the neurological, respiratory, and general health status of study patients was evaluated in agreement with standard local practice at the participating centres.
Materials and methods
According to the initial study protocol and consent form, investigators invited families of preterm infants born between 27+0 and 33+6 weeks’ gestation with RDS who participated in the main initial phase of the study to attend a clinical visit at the recruiting site at 24 months (±3 months) corrected age (CA). The aim was where possible to perform a neurological clinical assessment based on the Bayley Scales of Infant Development (BSID) as well as a respiratory and general health status evaluation by questionnaire. Preterm infants who were included in the first-in-human study had to have clinical and radiological findings of mild to moderate RDS, needing a fraction of inspired oxygen concentration (FiO2) ≥0.35 on continuous positive airways pressure (CPAP) to maintain pre-ductal pulse oximeter oxygen saturation (SpO2) in the range 90–95%, and they were treated with endotracheal CHF5633 at a dose of either 100 or 200 mg/kg [Citation1]. BSID scores are a series of measurements to assess the motor, language, and cognitive development of infants and toddlers. According to the standard of practice and the Bayley scale version in use at each participating site, we used either the Mental Developmental Index (MDI) from Bayley Scale of Infant and Toddler Development - Second edition (BSID-II) or the Cognitive score from Bayley Scale of Infant and Toddler Development - Third edition (BSID-III).
In addition, a health status questionnaire was used to assess health and to check for functional disability, including diagnosis of cerebral palsy, need for ventricular shunting, presence of seizures or other neurological problems, visual, hearing, speech or language problems, need for home oxygen, pulmonary problems and related medications as well as re-hospitalizations during the first two years of life. When a formal neurodevelopmental assessment could not be conducted at the recruiting sites, the visit was replaced by a contact/phone call with the parents/carers or family pediatricians to collect as much information as possible based on the health status questionnaire.
Statistical analysis
This was a stand-alone assessment at 2 years of age of ex-premature infants who participated in a first-in-human trial mainly aimed at evaluating the safety of two CHF5633 surfactant doses. Because of the nature of Phase-I clinical trials, there were no controls. All clinical data were summarized by descriptive statistics by the dose administered to each treatment group. Continuous variables were summarized using the n, mean, standard deviation (SD), 95% confidence interval (CI), median, minimum, and maximum value. Categorical variables were summarized using frequency distributions. BSID scores below the normal range were defined as BSID-II MDI score <70 or BSID-III cognitive composite score <85 [Citation2].
Results
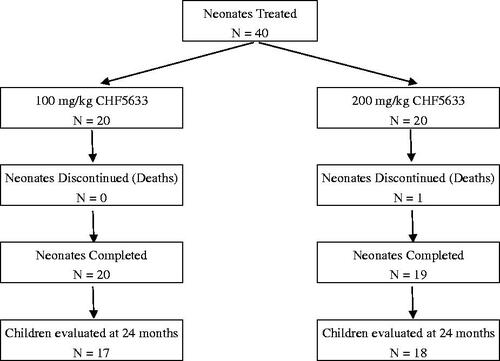
Overall, 35 of 39 surviving children were assessed (17/20 from the 100 mg/kg and 18/19 from the 200 mg/kg cohort) (). Most were Caucasian (94%) with a mean (SD) corrected age at the time of assessment of 25 (2.03) months. 51% were male. Four children from the original cohort of 39 survivors were not able to be assessed, for one child the parents refused consent and three other families had moved address and could not be contacted.
With regards to Bayley assessments, a total of 18/35 children were assessed with BSID-III and 6/35 with version II. Bayley’s scores were not available for 11/35 children. For three of them, Bayley’s assessment was not available at their site at all, and for the remaining 8, it was not their site’s standard of care if the infant had an apparently normal development. In these cases, parent interviews and clinical assessment were used for a global qualitative assessment of the child’s development. Overall the mean (SD) BSID-II MDI score was 105 (16) and the BSID-III cognitive composite score was 95 (12). Only one child was below the normal range in the 100 mg/kg group with a BSID-III cognitive composite score of 70. Another child (200 mg/kg group) was diagnosed with spastic bilateral cerebral palsy caused by cystic periventricular leukomalacia, one child (100 mg/kg group) had permanent hearing impairment, well managed using hearing aids. A total of 3/17 children (18%) in the 100 mg/kg group and 4/18 children (22%) children in the 200 mg/kg group had some difficulties with speech or language development ().
Table 1. Outcomes of Cognitive Development Assessment and Health Status Questionnaire.
None of the children in either dosing cohort ever needed home oxygen. A total of 6/17 children (35%) in the 100 mg/kg group and 9/18 children (50%) in the 200 mg/kg group required at least one re-hospitalization after discharge for a mean (SD) of 5.3 (4.4) nights and 6.9 (7.3) nights respectively. The mean (SD) number of hospitalizations per patient was similar between the treatment groups: 1.5 (0.55) for the 100 mg/kg and 1.6 (0.88) for the 200 mg/kg cohort. 1/17 children (5.9%) in the 100 mg/kg cohort required mechanical ventilation and 3/18 children (16.7%) in the 200 mg/kg cohort received intensive care during re-hospitalization. One child in the 100 mg/kg cohort and two in the 200 mg/kg cohort were on regular medications for wheezing. No other abnormalities sometimes found in premature-born children were detected (ventricular shunt, motor impairment, nystagmus, strabismus) at the 24-month follow up.
Discussion
This is a descriptive analysis of the results of a stand-alone clinical assessment at approximately 24 months CA on the developmental and the general health status of children who had participated as preterm neonates with RDS in the first-in-human, two dosing cohort (100 and 200 mg/kg) clinical trial of a new synthetic surfactant CHF5633 administered within 48 h of life [Citation1]. Follow-up studies conducted in similar late preterm neonates treated with commercially available surfactants have not to date identified any increases in major neurodevelopmental or pulmonary sequelae in surviving children [Citation3, Citation4]. However considering that this was a first-in-human study, we deemed it appropriate to conduct a 24-month stand-alone clinical assessment in accordance with the standard practice at each participating site to evaluate the long-term outcomes in this mild to moderate RDS population to confirm no evidence of harm.
As this was a first-in-human trial, there was no control arm with whom this cohort could be compared. It must be borne in mind that the original study group was preterm infants born between 27+0 and 33+6 weeks’ gestation who had been treated for RDS. The risk of cerebral palsy increases with decreasing gestation, the prevalence being about 6% in babies born between 28 and 31 weeks’ gestation, therefore it is not surprising that one of the CHF5633 dosing cohorts, went on to develop spastic diplegia, and unlikely that this was causally related to the drug [Citation5]. Likewise, there is also a well-known association between prematurity and expressive and receptive language delay [Citation6].
Overall, one child from the 100 mg/kg group, who had a good short-term response to CHF5633, was below the normal range on the BSID-III assessment, however, without any other major abnormalities nor re-hospitalizations in the first 24 months of life.
The average MDI (BSID-II) score was 105 and the average Cognitive composite score (Bayley-III) was 95 in this population. Bayley-III scores usually up to 7 points higher than MDI scores [Citation7]. There were apparently no big differences in clinical characteristics and disease severity between the two groups at birth. Considering the low number of children evaluated with the BSID-II compared to BSID-III (6 vs. 18, respectively) in different sites, the explanation of this difference is not clear and is likely to have occurred by chance. Re-hospitalizations during this period were also checked. No relevant associated neurological or developmental issues were observed in the two treatment groups.
The number and type of health problems reported by parents were the sorts of issues that would normally be expected in the enrolled study population of preterm born children. Re-hospitalisation within the early years of life is a well-recognized complication of prematurity. In a cohort of survivors born at less than 32 weeks in Australia, around two-thirds required readmission, mostly for respiratory morbidity [Citation8]. The CHF5633 cohort’s readmission rates were less than this, presumably on the basis that only relatively well preterm neonates were selected for inclusion in the initial first-in-human trial phase, however, it is reassuring that the children were no more likely to need hospital care over the first 2 years than expected. The absence of an excess of children with wheeze requiring treatment was also reassuring.
In conclusion, clinical outcomes observed at two years corrected age indicate that the administration of CHF5633 at doses of 100 and 200 mg/kg of birth weight was not associated with impaired neurodevelopmental, respiratory, or health consequences, taking into account the level of prematurity of the babies recruited in the initial phase of this first-in-human study. These data will hopefully be confirmed in the on-going 24-month assessment of the children evaluated in the phase II trial comparing the effectiveness and safety of CHF5633 with a natural surfactant in an even more preterm population of neonates with higher RDS severity.
Authors’ contributions
Dr. Sweet recruited patients, helped with data analysis, drafted the initial manuscript, and approved the final manuscript as submitted. Profs. Speer, Stenson, Clarke, Goelz, Singer Turner, Straňák, Plavka recruited patients and approved the final manuscript. Drs. Fabbri, Varoli, Piccinno, and Del Buono assisted with 24-month data collection and analysis and approved the final manuscript. Dr. Santoro supported data management and statistical analysis and approved the final manuscript.
Acknowledgments
The authors would like to thank the following Investigators for their help in collecting 24-month data or previously recruited study patients: Samir Gupta (University of Durham & North Tees University Hospital, Stockton-on-Tees, UK), Suzanne Schmidtke (Asklepios Klinik Barmbek, Abteilung Neonatologie, Hamburg, Germany), Monika Wolf (Sektion Neonatologie und Pädiatrische Intensivmedizin, Universitätsklinikum Eppendorf, Hamburg), Alison Walker (Neonatal Unit, Royal Maternity Hospital, Belfast). The authors would also like to thank the patients and their families for their participation in the study as well as Chiesi Farmaceutici S.p.A. (Parma, Italy) for the support in conducting this study, and Pharm-Olam International (The Brackens, Ascot, UK) for the monitoring activities, data collection and management, and statistical analysis.
Disclosure statement
Laura Fabbri, Debora Santoro, Annalisa Piccinno, Dorothea Del Buono, and Guido Varoli are full employees of Chiesi Farmaceutici S.p.A., sponsor of the study. D. Sweet has previously acted in an advisory capacity for Chiesi Pharmaceuticals UK. C. P. Speer is a consultant for Chiesi Farmaceutici S.p.A. (Italy). M. Turner serves as a consultant to Chiesi Farmaceutici S.p.A. (Italy) with respect to the development of CHF5633 on behalf of the University of Liverpool without deriving any personal benefit from this consultancy. The remaining authors have no conflict of interest to declare.
Additional information
Funding
References
- Sweet DG, Turner M, Straňák Z, et al. A first-in-human clinical study of a new SP-B and SP-C enriched synthetic surfactant (CHF5633) in preterm babies with respiratory distress syndrome. Arch Dis Child Fetal Neonatal Ed. 2017;102(6):F497–F503.
- Johnson S, Moore T, Marlow N. Using the Bayley-III to assess neurodevelopmental delay: which cut-off should be used? Paediatric Research. 2014;75:671–674.
- Halliday HL. Surfactants: past, present and future. J Perinatol. 2008;28(1):S47–S56.
- Sinn JK, Ward MC, Henderson-Smart DJ. Developmental outcome of preterm infants after surfactant therapy: systematic review of randomized controlled trials. J Paediatr Child Health. 2002;38(6):597–600.
- Himpens E, Van den Broeck C, Oostra A, et al. Prevalence, type, distribution, and severity of cerebral palsy in relation to gestational age: a meta-analytic review. Dev Med Child Neurol. 2008;50(5):334–340.
- Ribeiro LA, Zachrisson HD, Schjolberg S, et al. Attention problems and language development in preterm low-birth-weight children: cross-lagged relations from 18 to 36 months. BMC Pediatr. 2011;11:59.
- Bayley N. Bayley scales of infant and toddler development. 3rd ed. San Antonio (TX): Pearson; 2006.
- Hong T, Bolisetty S, Bajuk B, et al. A population study of respiratory rehospitalisation in very preterm infants in the first 3 years of life. J Paediatr Child Health. 2016;52(7):715–721.