Abstract
Objective
The aim of this study was to determine if appropriately grown fetuses (those that are not small-for-gestational-age) with a raised umbilical artery pulsatility index (>95th centile) in the early third trimester are at increased risk of placental dysfunction and adverse outcome.
Methods
This is a 5-year retrospective cohort study using routinely collected data. Inclusion criteria were singleton, non-anomalous pregnancies having a growth scan with umbilical artery Doppler velocimetry between 28 + 0 and 33 + 6 weeks’ gestation. Small-for-gestational-age fetuses were excluded. Cases were classified as group 1 (those with an umbilical artery pulsatility index >95th centile at any scan during target window) or group 2 (those where the umbilical artery pulsatility index was ≤95th centile at all scans). p-Values and odds ratios were calculated. Logistic regression was used to compute odds ratios adjusted for baseline estimated weight z-score, gestational age at delivery, and labor induction.
Results
After exclusions, there were 202 pregnancies in group 1 and 7950 in group 2. Differences in baseline characteristics between the groups include age (median age was 30 for group 1 and 32 for group 2, p < .001), smoking (group 1 were more likely to smoke, p < .001) and labor induction (more common in group 1, p = .03). Among those delivering ≥34 + 0, group 1 were more likely to be small-for-gestational-age and have an abnormal cerebro-placental ratio at the final scan (OR 6.76, CI 4.23–10.80 and OR 5.07, CI 3.37–7.63 respectively), and to develop features of growth restriction (OR 9.85, CI 6.27–15.49). Group 1 were also more likely to deliver <37 + 0 weeks’ gestation (OR 1.71, CI 1.13–2.58) and to have birthweight <10th or <3rd centile (OR 5.26, CI 3.65–7.58 and OR 6.13, CI 3.00–12.54 respectively). These associations remained significant when adjusted for estimated weight at the initial scan.
Conclusions
These data suggest that raised umbilical artery pulsatility index in an appropriately grown fetus at 28 + 0 to 33 + 6 weeks’ gestation is associated with subsequent development of growth restriction markers and an increased risk of moderate and severe small-for-gestational-age at birth. This is independent of the estimated weight of these babies at the index scan.
Introduction
Stillbirth complicates 1 in 200 pregnancies in developed regions and 1 in 60 globally. Efforts to reduce stillbirth have produced modest results, with a particular focus on the identification of small-for-gestational-age (SGA) fetuses, a well-established risk factor [Citation1]. Umbilical artery (UA) Doppler velocimetry is then used to help determine which SGA fetuses are at most risk. In “high-risk” pregnancies, this reduces perinatal mortality [Citation2] and forms the basis of guidelines for the management of SGA [Citation3,Citation4]: those that are SGA with an abnormal UA pulsatility index (PI) are at sufficiently increased risk of adverse outcome that monitoring is intensive.
A common clinical problem, however, is where the fetus is not SGA, but the UA PI is nevertheless abnormal. It is generally believed that the degree of impedance to blood flow in the umbilical artery reflects the degree of placental dysfunction, and so it is biologically plausible to believe these fetuses may also be at increased risk of adverse outcomes. However, the management of such cases is unclear because the prognosis is largely unknown. More than 70% of babies with antepartum stillbirth are not SGA, particularly at term [Citation5]. Nevertheless, risk increases with decreasing estimated fetal weight (EFW) centile, and so is related to size [Citation6]. Growth velocity may be more important than actual size [Citation7]. Indeed, it has been suggested that 40–60% of stillbirths have fetal growth restriction (FGR) due to placental insufficiency [Citation8,Citation9].
We hypothesize that appropriate-for-gestational-age (AGA) babies with an incidental finding of raised UA PI are at increased risk of adverse outcomes compared with AGA babies where the UA PI is normal.
The aim of this study was to determine if appropriate-for-gestational-age (AGA) fetuses – those that are not SGA – with a raised (>95th centile) UA PI in the early third trimester are at increased risk of placental dysfunction and adverse outcome.
Material and methods
This is a retrospective cohort study at a single tertiary center at the John Radcliffe Hospital, Oxford, UK, over a 5-year period between January 2014 to September 2019. Routinely collected data were used. Inclusion criteria were singleton pregnancies dated by crown rump length, who gave birth at the unit and had a non-anomalous fetus that had undergone a complete growth scan, with UA PI measurement, between 28 + 0 and 33 + 6 weeks’ gestation. This gestation window was chosen because it is at this time that the umbilical artery is most useful in SGA babies: later, a large number of at-risk pregnancies have a normal umbilical artery Doppler [Citation10] and the cerebroplacental ratio (CPR) is more useful [Citation11,Citation12]. Ultrasound at this gestation is clinically indicated, so performed only in pregnancies considered “high risk” according to local protocols, and this includes both routine and non-routine scans. Routine scans were arranged for those with accepted risk factors for FGR following local protocols based on current recommendations from Saving Babies’ Lives Version 2 [Citation13]. Routine scans were also arranged for those with preexisting hypertensive disease requiring treatment, previous pregnancy loss after 16 weeks’ gestation, gestational diabetes mellitus, preexisting diabetes mellitus, and preexisting medical conditions such as antiphospholipid syndrome. Non-routine scans were undertaken on an ad hoc basis for suspected or evolving pregnancy complications: local protocols dictate that non-routine can be arranged in cases of new hypertension arising in pregnancy, vaginal bleeding, symphysio-fundal height ≥3 cm less than the gestational age in weeks, persistent reduction in fetal movements, and any concern about fetal wellbeing subject to agreement by a senior clinician. All growth scans performed beyond 23 + 6 weeks routinely included assessment of the UA PI. From October 2016, an additional routine growth scan between 35 + 0 and 36 + 6 weeks’ gestation was offered in all cases, which included an assessment of the middle cerebral artery (MCA) and cerebro-placental ratio (CPR). All scan findings were available to clinicians involved in care provision.
Management of scan findings prior to 37 + 0 weeks was according to RCOG Guidelines [Citation4]. In situations without an established protocol (including AGA with raised UA PI) management decisions were guided by senior clinicians. After 37 + 0 weeks, all SGA babies and those with abnormal Doppler indices were risk assessed and managed according to a published algorithm [Citation14].
Pregnancies were dated using Crown Rump length before 14 weeks (except in cases of in vitro fertilization where the date of embryo transfer was available). Ultrasound examinations were conducted by accredited sonographers or clinical fellows, using Voluson E6 and E8 ultrasound machines (GE Healthcare) with a 2–8 Hz convex probe. Measurements were recorded prospectively using commercially available archiving software (Viewpoint, GE Healthcare) and transferred using DICOM. Doppler measurements were obtained during a period of no fetal movement, in the absence of fetal tachycardia and maintaining a low angle of insonation in a free loop of cord. The lowest PI of three satisfactory measurements was used. EFW was calculated from head circumference, abdominal circumference and femur length measurements using Hadlock’s 1985 equation [Citation15]. The gestation specific z-score for EFW was calculated according to the method described by Hadlock, and AGA was defined as EFW ≥10th centile [Citation16]. Scan reports presented the UA PI centile according to Acharya to clinicians [Citation17]. However, for the purposes of analysis, the gestation specific z-score for UA PI was calculated according to the method described by Ciobanu, and abnormal UA PI was defined as >95th centile [Citation18]. For outcomes, birthweight was defined using UK 90 standards [Citation19]; CPR <5th centile was defined using equations from Ciobanu et al. [Citation18], and fetal growth restriction (FGR) according to ISUOG Consensus Criteria [Citation20]. Data were collected prospectively and merged according to a unique identifier from neonatal (Badgernet), maternity (Cerner) and ultrasound (Viewpoint, GE Healthcare) records.
Two groups of pregnancies were compared (Appendix A). AGA fetuses with an UA PI >95th centile at any scan during the target gestation window were allocated to group 1. The first scan with such findings was assessed. Pregnancies where any previous scans showed the fetus to be SGA were excluded, but those where any subsequent scan showed SGA were not. Group 2 comprised pregnancies scanned in the same gestation window where the fetus was AGA but with an UA PI ≤ 95th centile at all scans performed during the window.
STROBE guidelines were followed.
A summary of the statistical analysis protocol is provided in Appendix B. Ethical approval was granted on 27/07/2017: (IRAS project ID 222260; REC reference: 17/SC/0374).
Results
Of 9112 eligible pregnancies, 202 (2.2%) met criteria for Group 1 and 7950 (87.3%) for Group 2 (the reference group) (Appendix C). The remaining 960 (10.5%) pregnancies were SGA and were excluded. No babies in Group 1 had absent/reversed end diastolic flow in the umbilical artery at the index scan.
Demographic and index scan details are presented in Appendix D. The proportion of smokers was higher in Group 1 (p < .001), the median maternal age was younger (p < .001), but there were no other significant demographic differences. The index scans were performed at a similar gestation in both groups (30 weeks’ gestation). EFW z-score was significantly lower in group 1 (p < .001), and growth velocity (change in z-score since anomaly scan/days since anomaly scan) was also significantly lower (p < .001); showing that Group 1, although still AGA, were smaller and had slower apparent growth since the anomaly scan. Induction of labor was more common in group 1 (p .03) and the median gestational age at birth for group 1 was two days earlier than group 2 (p .004). Although statistically significant, the observed difference in gestational age at birth is unlikely to be of clinical significance.
Group 1 had a significantly increased risk of being born SGA (OR 3.94, CI 2.80–5.53), including severe SGA (OR 4.91, CI 2.65–9.08), and being born preterm (OR 1.71, CI 1.13–2.58). The risk of SGA remained after adjustment for the EFW z score at the index scan (OR 2.43, CI 1.64–3.59), suggesting that it was not simply because these babies were smaller to start with. There was no difference in adverse outcomes, including after adjustment for intervention ().
Table 1. Perinatal outcomes.
Of the 8152 pregnancies, 4550 (55.8%) continued beyond 34 + 0 weeks and had at least one further complete growth scan (). Group 1 pregnancies were not more likely to undergo a further scan, but had significantly higher rates of SGA (OR 6.76, CI 4.23–10.80), severe SGA (OR 13.32, CI 6.59–26.91), and FGR (OR 9.85, CI 6.27–15.49) according to the ISUOG Delphi consensus definition [Citation20]. In some cases, Doppler velocimetry was repeated without fetal biometry: of the 4606 (56.5%) cases that continued beyond 34 + 0 and had both UA and MCA Doppler measurements repeated, UA PI was significantly more likely to be >95th centile (OR 18.79, CI 11.51–30.66), and the CPR was more likely to be <5th centile (OR 5.07, CI 3.37–7.63). This effect was little altered by adjustment for EFW at the index scan. MCA PI was also more likely to be <5th centile, but this effect was not statistically significant.
Table 2. Findings of final ultrasound scans ≥34 weeks.
Discussion
These findings suggests that a raised UA PI in an early third trimester AGA fetus is associated with subsequent development of FGR markers and increased risk of severe birthweight SGA. This is independent of the lower mean EFW of these babies: these fetuses are not merely smaller but are risk of deterioration in growth and placental function. Indeed, this slowed growth has already started at the time of the index scan.
It was not our remit to determine whether and to what extent umbilical artery doppler can be used to screen for SGA or adverse outcomes. We wished to inform practice when faced with the relatively common conundrum of Group 1. The lack of association with adverse outcomes may be because these outcomes are relatively rare or could be due to intervention; and this is reflected in the higher rates of preterm birth, labor induction, and cesarean section. These fetuses do not appear to be at immediate risk and may not require monitoring at intervals appropriate for an SGA baby with an abnormal UA PI. Yet we conclude that such a finding necessitates further assessment for FGR as it is associated with an increased risk of markers of long term adverse neonatal outcome.
This finding aligns with the relatively sparse literature. Goffinet et al. examined 192 AGA fetuses with an UA resistance index >90th centile of the study population, which comprised 2016 low-risk pregnancies scanned at 28 weeks between 1988 and 1990 [Citation21]. Raised UA resistance index was associated with a 2 and 3-fold increase in birthweight below the 10th and 3rd centiles respectively. These 30-year-old data are consistent with our findings. Key differences are the low-risk population, the likely poorer accuracy of ultrasound because of subsequent improvements in technology, and the different reference ranges. Al Hamayel et al., in a study of fetuses with an EFW >10th centile, compared 98 women who had a raised UA PI to 2646 who did not [Citation22]. They found a 2-fold increase in the risk of SGA at birth, although the gestation at assessment was unclear. Valino et at (2016), in a screening study of 8268 pregnancies, show that abnormal UA PI at 30–34 weeks was a risk factor for subsequent low birthweight that was independent of the EFW [Citation23]. In a retrospective study of 2485 pregnancies, Khalil et al. demonstrated that among term births with Doppler assessment at 34 + 0 to 35 + 6 (later than in our study), UA PI was higher among babies requiring neonatal unit admission, despite no difference in EFW percentile [Citation24]. Mone et al. further showed that an abnormal UA in AGA fetuses at 28 weeks, although not at 32 and 34 weeks, was associated with impaired cognitive assessments of information processing and memory [Citation25].
More recently, systematic review and meta-analysis has assessed fetal umbilical artery Doppler velocimetry as a tool for universal screening in the third trimester and the authors conclude that UA Doppler has moderate predictive accuracy for birthweight SGA, but not for indicators of neonatal morbidity [Citation26]. However, outside of the context of universal screening, this does not address the significance of abnormal UA PI with AGA in a clinically indicated third trimester scan.
Our findings add weight to the increasing emphasis on FGR rather than on cutoffs of absolute EFW. Reliance on SGA alone in the early third trimester risks missing a small cohort of babies who later develop established risk factors for serious adverse outcomes. While our evidence is not sufficient to recommend universal screening in an unselected population, it suggests that UA velocimetry does have utility whenever ultrasound assessment of fetal growth is indicated, including for babies that are not SGA.
This study is strengthened by its relatively large sample, prospective data collection and use of DICOM to prevent transcription errors. Our comparison groups were carefully specified, with index scans at similar gestations and with a similar frequency of subsequent scans. We nevertheless acknowledge potential limitations. We used cutoffs of umbilical artery Doppler rather than a continuous variable: this was to directly address the question posed. Our numbers were insufficient to examine serious adverse events of antepartum origin; this further prevented us from analyzing whether Group 1 had different outcomes from Group 2 according to whether they had had a further scan. The length of the study (>5 years) means that local practice changed during the study timeframe. Specifically, a routine growth scan between 35 + 0 and 36 + 6 weeks’ gestation was introduced, although, since allocation to Group 1 and 2 is independent of this factor, this should not be a source of bias. The study population was not unselected, in that the index scans were clinically indicated, and findings should not necessarily be applied to situations where universal screening of low-risk women at this gestation is undertaken. Equally, our findings are likely therefore more translatable to a general obstetric population without universal ultrasound in the early third trimester, and our rate of ultrasound (23.2%) was not dissimilar to the proportion of clinically indicated scans in a recent UK study [Citation27]. Finally, not all pregnancies with a raised UA had a repeat assessment, likely because the reference chart used for analysis [Citation18] was more up-to date than that used for clinical decision making [Citation17]. This meant that the UA PI centiles presented to clinicians at the time were slightly different to those presented in this study, but this also has the advantage of helping to reduce the effects of intervention paradox since the PI value representing the 95th centile is lower for the new charts.
We conclude that raised UA PI in AGA fetuses in the early third trimester is associated with increased risk of both birthweight SGA and other late pregnancy markers of abnormal placental function.
Acknowledgements
The authors are grateful to the women whose data has made this work possible, and to Matias Costa Viera for contributing methodological suggestions.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- Lawn JE, Blencowe H, Waiswa P, et al. Stillbirths: rates, risk factors, and acceleration towards 2030. Lancet. 2016;387(10018):587–603.
- Alfirevic Z, Stampalija T, Dowswell T. Fetal and umbilical doppler ultrasound in high-risk pregnancies. Cochrane Database Syst Rev. 2017;6(6):CD007529.
- Lees CC, Stampalija T, Baschat A, et al. ISUOG practice guidelines: diagnosis and management of small-for-gestational-age fetus and fetal growth restriction. Ultrasound Obstet Gynecol. 2020;56(2):298–312.
- Green-Top Guideline No. 31. The investigation and management of the small-for-gestational-age fetus. London: Royal College of Obstetricians & Gynaecologists; 2013.
- Nohuz E, Riviere O, Coste K, et al. Prenatal identification of small-for-gestational age and risk of neonatal morbidity and stillbirth. Ultrasound Obstet Gynecol. 2020;55(5):621–628.
- Moraitis AA, Wood AM, Fleming M, et al. Birth weight percentile and the risk of term perinatal death. Obstet Gynecol. 2014;124(2 Pt 1):274–283.
- Sovio U, White IR, Dacey A, et al. Screening for fetal growth restriction with universal third trimester ultrasonography in nulliparous women in the pregnancy outcome prediction (POP) study: a prospective cohort study. Lancet. 2015;386(10008):2089–2097.
- Froen JF, Gardosi JO, Thurmann A, et al. Restricted fetal growth in sudden intrauterine unexplained death. Acta Obstet Gynecol Scand. 2004;83(9):801–807.
- Draper EG, Smith LK, Kurinczuk JJ, et al. MBRRACE-UK perinatal mortality surveillance report, UK perinatal deaths for births from January to December 2017. Leicester: the Infant Mortality and Morbidity Studies, Department of Health Sciences, University of Leicester; 2019.
- Figueras F, Eixarch E, Gratacos E, et al. Predictiveness of antenatal umbilical artery Doppler for adverse pregnancy outcome in small-for-gestational-age babies according to customised birthweight centiles: population-based study. BJOG. 2008;115(5):590–594.
- Kalafat E, Khalil A. Clinical significance of cerebroplacental ratio. Curr Opin Obstet Gynecol. 2018;30(6):344–354.
- Vollgraff Heidweiller-Schreurs CA, De Boer MA, Heymans MW, et al. Prognostic accuracy of cerebroplacental ratio and middle cerebral artery Doppler for adverse perinatal outcome: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2018;51(3):313–322.
- Saving babies’ lives version two. A care bundle for reducing perinatal mortality: NHS England. 2019. Available from: https://www.england.nhs.uk/publication/saving-babies-lives-version-two-a-care-bundle-for-reducing-perinatal-mortality/
- Veglia M, Cavallaro A, Papageorghiou A, et al. Small-for-gestational-age babies after 37 weeks: impact study of risk-stratification protocol. Ultrasound Obstet Gynecol. 2018;52(1):66–71.
- Hadlock FP, Harrist RB, Sharman RS, et al. Estimation of fetal weight with the use of head, body, and femur measurements–a prospective study. Am J Obstet Gynecol. 1985;151(3):333–337.
- Hadlock FP, Harrist RB, Martinez-Poyer J. In utero analysis of fetal growth: a sonographic weight standard. Radiology. 1991; Oct181(1):129–133.
- Acharya G, Wilsgaard T, Berntsen GK, et al. Reference ranges for serial measurements of umbilical artery Doppler indices in the second half of pregnancy. Am J Obstet Gynecol. 2005;192(3):937–944.
- Ciobanu A, Wright A, Syngelaki A, et al. Fetal medicine foundation reference ranges for umbilical artery and middle cerebral artery pulsatility index and cerebroplacental ratio. Ultrasound Obstet Gynecol. 2019;53(4):465–472.
- Freeman JV, Cole TJ, Chinn S, et al. Cross sectional stature and weight reference curves for the UK, 1990. Arch Dis Child. 1995;73(1):17–24.
- Gordijn SJ, Beune IM, Thilaganathan B, et al. Consensus definition of fetal growth restriction: a Delphi procedure. Ultrasound Obstet Gynecol. 2016;48(3):333–339.
- Goffinet F, Paris J, Heim N, et al. Predictive value of Doppler umbilical artery velocimetry in a low risk population with normal fetal biometry. A prospective study of 2016 women. Eur J Obstet Gynecol Reprod Biol. 1997;71(1):11–19.
- Al Hamayel NA, Baghlaf H, Blakemore K, et al. Significance of abnormal umbilical artery Doppler studies in normally grown fetuses. Matern Health Neonatol Perinatol. 2020;6:1.
- Valino N, Giunta G, Gallo DM, et al. Biophysical and biochemical markers at 30-34 weeks’ gestation in the prediction of adverse perinatal outcome. Ultrasound Obstet Gynecol. 2016;47(2):194–202.
- Khalil AA, Morales-Rosello J, Elsaddig M, et al. The association between fetal Doppler and admission to neonatal unit at term. Am J Obstet Gynecol. 2015;213(1):57 e1–57 e7.
- Mone F, McConnell B, Thompson A, et al. Fetal umbilical artery Doppler pulsatility index and childhood neurocognitive outcome at 12 years. BMJ Open. 2016;6(6):e008916.
- Moraitis AA, Bainton T, Sovio U, et al. Fetal umbilical artery doppler as a tool for universal third trimester screening: a systematic review and meta-analysis of diagnostic test accuracy. Placenta. 2021;108:47–54.
- Gaccioli F, Lager S, Sovio U, et al. The pregnancy outcome prediction study (POP): investigating the relationship between serial prenatal ultrasonography, biomarkers, placental phenotype and adverse pregnancy outcomes. Placenta. 2017;59:S17–S25.
Appendix A. Terms and definitions.
Appendix B.
Statistical analyses.
Analysis was performed using SPSS (version 26). Demographic characteristics, ultrasound findings and pregnancy, birth and neonatal outcomes were summarized in the two groups with median and interquartile range (IQR) for continuous variables and count and proportion for categorical variables, and compared by means of Mann-Whitney U test or chi-square test as appropriate. Where missing values occurred, calculations were performed using only pregnancies with data as the denominator. Differences between the two groups were compared using odds ratios (OR), with 95% confidence intervals. Logistic regression was used to adjust for covariates and adjusted odds ratios were calculated. Two regressions were performed: the first using EFW z-score at the time of the index scan as a covariate, and the second using labor induction and gestational age at delivery. This was performed to investigate the effect of EFW z-score at the time of the index scan, as well as timing and mode of birth, on the outcomes of interest.
Appendix C.
Summary of exclusions and group allocation.
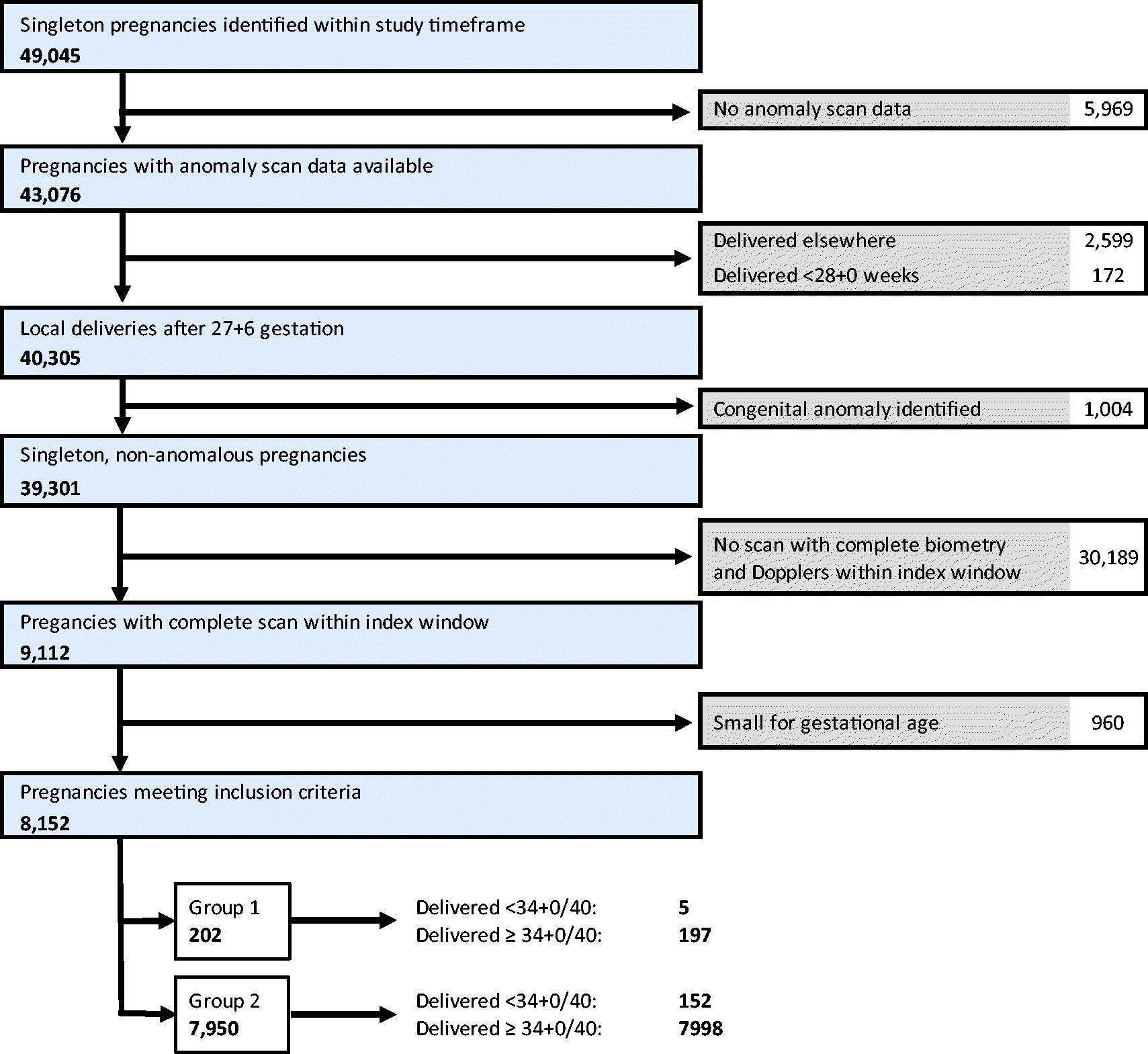
Appendix D. Cohort characteristics.
