Abstract
Objective
Fetal growth restriction (FGR) is associated with perinatal adverse outcomes including intrauterine fetal death. Antenatally unidentified FGR has a higher risk of intrauterine fetal death than that identified antenatally. We, therefore, investigated the antenatal identification of FGR among intrauterine fetal deaths, and assessed the perinatal factors associated with the identification of FGR.
Methods
This retrospective and population-based study reviewed all stillbirths in Shiga Prefecture, Japan, from 2007 to 2016 with exclusion criteria of multiple births, births at unidentified gestational weeks or < 22 gestational weeks, and lethal disorders. We analyzed cases of FGR, using the Japanese clinical definition: Z-score of estimated fetal weight for gestational age <−1.5 standard deviations (SD).
Results
We identified 94 stillbirths with FGR among 429 stillbirths. Thirty-seven cases were antenatally identified during pregnancy management (39%). Dividing cases by a Z-score of −2.5 SD, 51 cases were classified as ≤−2.5 SD. Twenty-eight of the 51 cases (55%) with a Z-score <−2.5 SD were antenatally identified as having FGR, whereas 9 of the 43 cases (21%) with a Z-score ≥−2.5 SD were antenatally identified as having FGR (p = .002). Among cases with a Z-Score <−2.5 SD, 16 of 21 (76%) beyond 28 weeks’ gestation and 12 of 30 (40%) before 28weeks’ gestation were antenatally identified as having FGR (p = .023).
Conclusion
Fetal growth restriction leading to intrauterine fetal death in Japan was antenatally identified in less than half of cases. Antenatal identification of FGR was associated with the severity of growth restriction.
Introduction
Fetal growth restriction (FGR) is defined as failure to achieve a normal weight for gestational age in a fetus due to several factors [Citation1]. The etiology of FGR can be broadly categorized into maternal, fetal, placental and umbilical cord’s factors [Citation1]. Growth-restricted fetuses are more likely to develop adverse outcomes, such as severe fetal distress, cerebral damage, long-term neurological sequalae and intrauterine fetal death (IUFD), than those with a normal growth [Citation2]. The proportion of FGR in IUFD was reported to be 34–52% [Citation3–8], indicating that FGR is a major risk factor for IUFD.
Several population-based studies have shown that fetuses with FGR not detected antenatally have a higher risk of IUFD than those with FGR identified antenatally [Citation3–5,Citation9] Although many guidelines recommend screening for FGR during pregnancy, including ultrasound examinations [Citation9], an accurate antenatal diagnosis of FGR is very difficult due to the limited precision of fetal weight estimation using transabdominal ultrasonography [Citation10]. Studies have shown that the antenatal identification rate of FGR leading to IUFD was 12% [Citation5] in New Zealand, 18% [Citation3] in the UK, and 44% [Citation9] in France, rates that are considered insufficient. There are a limited number of population-based studies regarding antenatal identification of FGR leading to IUFD in developed countries. It would thus be useful to evaluate the rate of antenatal identification of FGR among IUFD in Japan, which has the lowest stillbirth rate in the world. To be specific, the stillbirth rate (vs. 1000 births) in 2019 was 1.5 in Japan, 2.2 in Australia, 2.7 in Germany, 3.0 in the UK, 2.8 in Canada, and 3.0 in the USA [Citation11].
Therefore, we investigated the rate of antenatal identification of FGR among IUFD in a regional population-based study in Japan. In addition, we also assessed perinatal factors associated with the antenatal identification of FGR.
Materials and methods
Data collection
This study was a population-based survey of stillbirth in Shiga Prefecture, Japan. There are approximately 13,000 births per year in Shiga. Two-thirds of them are delivered in 30 primary obstetric clinics, while the remaining cases are delivered at 11 general hospitals or 4 tertiary perinatal centers; all these cases were evaluated in this survey.
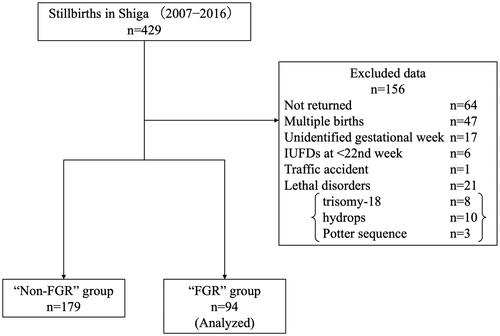
First, we directly investigated all stillbirth certificates with permission from the Japanese Ministry of Health, Labor and Welfare. Second, we prepared and sent a questionnaire to each facility that had submitted a stillbirth certificate. Basically, the obstetrician at each facility wrote and returned the answers to the questionnaire. A peer-review team involving experienced obstetricians and neonatologists then retrospectively reviewed the questionnaires returned from the facilities [Citation12]. There were 429 stillbirths after the 22nd gestational week in Shiga Prefecture from 2007 to 2016, and 85% of recipients (365/429) completed this survey. We first excluded the following 135 cases in this study (): cases with questionnaires not returned (n = 64); multiple births (n = 47); unknown gestational week of IUFD (n = 17); IUFD before the 22nd gestational week (22 + 0) (n = 6) and traffic accident (n = 1). We then divided these cases into two groups according to the criteria of FGR by birthweight of infants: the FGR and Non-FGR groups. We also excluded cases of lethal disorders (n = 13), including fetal hydrops (n = 8), trisomy-18 (n = 3) and Potter sequence (n = 2). We ultimately analyzed a total of 94 cases of stillbirths with FGR.
Definition of FGR
In the Japanese Obstetric Clinical Guideline, FGR is defined as an estimated fetal weight (EFW) with a Z-score of −1.5 standard deviations (SD) or an EFW below Z-score of −1.5 SD from the mean based on the fetal growth curve at a given gestational week. The precise date of IUFD could not be determined in the cases that were diagnosed with IUFD at the outpatient department. Therefore, there was some unknown period between the date of IUFD and delivery of a stillborn infant. In the current study, we used the birthweight of the infant at stillbirth and the gestational week at which IUFD was confirmed to determine FGR. We did not employ the Japanese neonatal anthropometric charts used for “small for gestational age (SGA),” which is defined in cases falling under the 10th percentile of the chart at a given gestational week. We considered it more appropriate to use a fetal growth curve to evaluate whether or not fetuses should have been diagnosed with FGR.
Calculation of Z-score
The Z-score was calculated using the mean and SD of the estimated fetal weight at each gestational week from the fetal growth curve defined by the Japanese Society of Ultrasound in Medicine [Citation13]. The mean and SD of the EFW for each gestational week and day were interpolated from the above values.
Antenatal identification of FGR
We classified cases that the health care provider described as FGR in the returned questionnaire as “antenatally identified FGR.” In this context, “antenatally identified FGR” refers to the cases in which the EFW measured by ultrasonography was compared with the fetal growth curve, and the healthcare provider made an overall judgment concerning the diagnosis of FGR. We divided subjects into two groups based on the antenatal identification of FGR prior to the diagnosis of IUFD: the identified group (group I) and the unidentified group (group U).
Association between identification of FGR and Z-score of infant weight
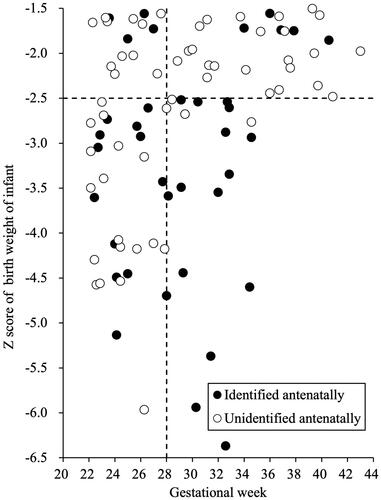
We created a scatter plot of stillbirth data using the gestational age as the horizontal axis and the Z-score of the birth weight of the stillborn baby as the vertical axis (). To evaluate the relationship between EFW and antenatal identification, we set the cutoff level for the Z-score at −2.5 SD, which is a critical value for the severity of FGR, and set the cutoff level for the gestational age as 28 weeks, which is super preterm.
Statistical analyses
Continuous variables were shown as the mean (SD) or n (%) and assessed using the Mann-Whitney U test or Student’s t-test, depending on the results of the normality test. The frequency of other subjects and ratios are shown as n (%) and were assessed using a chi-squared test. The results of multivariable logistic regression analysis were presented as the adjusted odds ratio with 95% confidence interval (CI). p Values under .05 were considered to indicate statistical significance. All statistical analyses were performed using the R application software program (ver. 4.0.2) [Citation14].
Results
Background of stillbirths
As shown in , the mean maternal age was 30.3 years old, and 51 women (54%) were primipara among the stillbirths with FGR cases. Eighty-two cases (87%) of IUFD were identified at the outpatient department. The mean week of gestation for stillbirths with FGR cases was 29.3 weeks. The mean infant’s weight for stillbirths with FGR cases was 948 g. The mean Z-score for stillbirths with FGR cases was −2.84. Thirty-seven of 94 cases (39%) were antenatally identified as having FGR. In addition, stillbirths at tertiary centers accounted for 24% of these stillbirths with FGR.
Table 1. Background of stillbirth with FGR in this study.
Difference in background characteristics according to the antenatal identification of FGR
As shown in and described before, we divided subjects into two groups based on the antenatal identification of FGR prior to the diagnosis of IUFD: group I and group U. In groups I and U, the mean maternal age was 30.4 and 30.2 years old, the ratio of primiparity was 57% and 53%, the percentage of outpatients at the diagnosis of IUFD was 89% and 86%, the mean weight of stillborn babies was 859 and 1005 g, and the rate of managing pregnancies in tertiary centers was 32% and 19%, respectively. Furthermore, the mean gestational age was 29.3 weeks in each group. There was no significant difference in any of these parameters between the two groups. The mean Z-Score was −3.22 in group I and −2.60 in group U, showing a significantly smaller value in group I (p < 0.001).
Table 2. Difference of background according to antenatal identification of FGR.
Impact of the gestational age and Z-score on the antenatal identification of FGR
As shown in , which presents a scatter plot of stillbirth data, 51 cases were classified as being <−2.5 SD and 43 cases were classified as being ≥−2.5 SD. Twenty-eight of 51 cases (55%) with a Z-score < −2.5 SD were antenatally identified as having FGR, whereas 9 of 43 cases (21%) with a Z-score ≥ −2.5 SD were antenatally identified as having FGR (p = .002). Among cases with a Z-Score <−2.5 SD, 16 of 21 (76%) beyond 28 weeks’ gestation and 12 of 30 (40%) before 28 weeks’ gestation were antenatally identified as having FGR (p = .023).
Factors influencing on antenatal identification of FGR stillbirth cases
A multiple logistic regression model yielding adjusted odds ratio (aOR) and 95% confidence intervals (CIs) was used to identify factors associated with antenatal identification of FGR leading to IUFD. The model included almost all factors shown in : maternal age, parity, gestational age (<28 or ≥28 weeks), Z-score (<−2.5 or ≥−2.5), and institution where the pregnancy was managed before IUFD. As a result, only Z-score of infant birth weight was significantly associated with the antenatal identification of FGR. (aOR for Z-score <−2.5 SD vs. ≥−2.5 SD: 8.46; 95% CI 2.65–27.01, p < .001)
Discussion
FGR leading to IUFD in Japan was antenatally identified in less than half of cases during pregnancy management. Next, we also found that antenatal identification of FGR was associated with the severity of FGR.
We found that less than half (39%) of stillbirths with FGR were antenatally identified as having FGR during pregnancy management. The identification rate in the current study is higher than that in previous population-based reports (12% [Citation5] and 18% [Citation3]). In these studies, FGR was identified based on the EFW measured by ultrasonography in addition to Doppler velocimetry of the umbilical artery or an evaluation of the amniotic fluid. In Japan, however, FGR is typically identified based solely on the EFW measured during an antenatal pregnancy checkup. Possible reasons for the difference in the identification rate include the definition of FGR and the frequency of fetal weight estimation between Japan and other developed countries. Regarding the difference in the definition of FGR, the Japanese definition of FGR is ≤6.7% of the estimated fetal weight, which is nearly −1.5 SD of the Z-score, whereas this value is less than 10% in other developed countries. It might be easier to antenatally identify prominently smaller fetuses as having FGR with the Japanese definition (≤6.7%ile) than with other countries’ definitions (≤10%ile) when using ultrasound measurements of fetuses.
Regarding the difference in the frequency of fetal weight estimation, the frequency is greater in Japan than in other developed countries. Fetal weight estimation is typically performed every 2 weeks after 24 gestational weeks and every week after 36 gestational weeks in Japan [Citation15], whereas it is performed once every 3–4 weeks at most throughout pregnancy in other developed countries [Citation16]. Frequent fetal weight estimation may increase the reliability of estimation by taking into account the fetal growth rate [Citation17,Citation18], although there are negative opinions [Citation19,Citation20] concerning the appropriateness of frequent estimation, which might result in confusion due to variations in the measurement technique itself [Citation16]. In addition, Ego et al. reported a relatively high identification rate of 44% [Citation9], which is largely due to the definition of FGR identification. That study classified the cases with slowed growth or referrals to specialists as antenatally identified cases of FGR, regardless of the estimated fetal weight, whereas FGR was identified mainly by the estimated fetal weight in other developed countries, including Japan. After reassessments using only the estimated fetal weight, the identification rate was corrected to 25% in this report [Citation9], which seems to be comparable to that in other reports (12% [Citation5] and 18% [Citation3]).
We also found that the antenatal identification of FGR was associated with the severity of FGR, namely with the Z-score of fetal weight at each gestational week. The identification rate of FGR was significantly higher when the Z-score was smaller, which is consistent with previous reports [Citation9,Citation19,Citation21,Citation22]. This result is considered reasonable, as it means that the greater the deviation from the normal, the more easily the condition is identified. In addition, among severe cases with a Z-score of <−2.5 SD, the identification rate was significantly higher beyond 28 weeks’ gestation than before 28 weeks’ gestation (). This may be because the measurements were performed more frequently as gestational age increased, thus increasing the reliability of fetal measurements for the reasons mentioned above.
Several limitations associated with the present study warrant mention. First, this study was limited by the inability to determine the accurate date of IUFD. It is impossible to determine when the IUFD occurred after the last confirmation of fetal viability at the outpatient department or inpatient ward. Due to the difference between the date of birth and that of fetal death, we could overestimate the numbers of FGR. Although the maximum interval during the above checkups may have been two weeks, the interval of most cases was restricted to within a few days, as most IUFDs were confirmed after the appearance of indicative symptoms, such as decreased fetal movement. Our overestimation might not have been very significant. Second, all data were limited to cases of stillbirth, and we were unable to study the antenatal identification rate of FGR leading to livebirths. Since the identification rate of FGR leading to livebirths is unknown, whether or not the identification rate of FGR leading to stillbirth is indeed relatively low is unclear. If the identification rate of FGR leading to livebirths could be studied under similar conditions, then its impact on IUFD would be expected to be clearer. Third, there may have been some examiner bias, as various skilled healthcare providers identified FGR. However, despite these limitations, we were able to demonstrate the current situation in Japan, where pregnancies are managed at facilities of various levels, including primary obstetric clinics, general hospitals, and tertiary perinatal centers. Finally, some proportion of our data, including unclaimed cases, multiple pregnancies etc., were omitted from the population. Furthermore, data on the maternal educational level and body mass index, which might have affected the risk of stillbirth or measurement of the EFW, were not available.
Conclusion
The antenatal identification rate of FGR leading to IUFD in Japan is considered insufficient. Stillbirths might be prevented if antenatal identification of FGR is improved. Further research concerning the antenatal identification of FGR to reduce IUFD is needed.
Acknowledgement
We would like to express our appreciation to the peer-review team members.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics and the Society for Maternal-Fetal Medicin. ACOG Practice Bulletin No. 204: fetal growth restriction. Obstet Gynecol. 2019;133:e97–e109.
- Wennergren M, Wennergren G, Vilbergsson G. Obstetric characteristics and neonatal performance in a four-year small for gestational age population. Obstet Gynecol. 1988;72:615–620.
- Gardosi J, Madurasinghe V, Williams M, et al. Maternal and fetal risk factors for stillbirth: population based study. BMJ. 2013;346:f108.
- Lindqvist PG, Molin J. Does antenatal identification of small-for-gestational age fetuses significantly improve their outcome? Ultrasound Obstet Gynecol. 2005;25(3):258–264.
- Stacey T, Thompson JMD, Mitchell EA, et al. Antenatal care, identification of suboptimal fetal growth and risk of late stillbirth: findings from the Auckland stillbirth study. Aust N Z J Obstet Gynaecol. 2012;52(3):242–247.
- Bukowski R, Hansen NI, Willinger M, et al. Fetal growth and risk of stillbirth: a population-based case-control study. PLoS Med. 2014;11(4):e1001633.
- Poon LCY, Volpe N, Muto B, et al. Birthweight with gestation and maternal characteristics in live births and stillbirths. Fetal Diagn Ther. 2012;32:156–165.
- Frøen JF, Gardosi JO, Thurmann A, et al. Restricted fetal growth in sudden intrauterine unexplained death. Acta Obstet Gynecol Scand. 2004;83(9):801–807.
- Ego A, Monier I, Skaare K, et al. Antenatal detection of fetal growth restriction and risk of stillbirth: population-based case–control study. Ultrasound Obstet Gynecol. 2020;55(5):613–620.
- Dudley NJ. A systematic review of the ultrasound estimation of fetal weight. Ultrasound Obstet Gynecol. 2005;25(1):80–89.
- GHO | By category | Stillbirth rate - Data by country [Internet]. [cited 2022 Dec 15]. Available from: https://apps.who.int/gho/data/view.main.STILLBIRTHv?lang=en.
- Koshida S, Ono T, Tsuji S, et al. Recommendations for preventing stillbirth: a regional population-based study in Japan during 2007–2011. Tohoku J Exp Med. 2015;235(2):145–149.
- Shinozuka N. Fetal biometry and fetal weight estimation: JSUM standardization. Ultrasound Rev Obstet Gynecol. 2002;2(3):156–161.
- R Development Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2020.
- Minakami H, Maeda T, Fujii T, et al. Guidelines for obstetrical practice in Japan: Japan Society of Obstetrics and Gynecology (JSOG) and Japan Association of Obstetricians and Gynecologists (JAOG) 2014 edition. J Obstet Gynaecol Res. 2014;40(6):1469–1499.
- Committee on Practice Bulletins—Obstetrics and the American Institute of Ultrasound in Medicine. Practice bulletin no. 175: ultrasound in pregnancy. Obstet Gynecol. 2016;128:e241–e256.
- Hiersch L, Melamed N. Fetal growth velocity and body proportion in the assessment of growth. Am J Obstet Gynecol. 2018;218(2S):S700–S711.e1.
- Reboul Q, Delabaere A, Luo ZC, et al. Prediction of small-for-gestational-age neonate by third-trimester fetal biometry and impact of ultrasound–delivery interval. Ultrasound Obstet Gynecol. 2017;49(3):372–378.
- Ciobanu A, Formuso C, Syngelaki A, et al. Prediction of small-for-gestational-age neonates at 35–37 weeks’ gestation: contribution of maternal factors and growth velocity between 20 and 36 weeks. Ultrasound Obstet Gynecol. 2019;53(4):488–495.
- Tarca AL, Hernandez-Andrade E, Ahn H, et al. Single and serial fetal biometry to detect preterm and term small- and large-for-gestational-age neonates: a longitudinal cohort study. PLoS One. 2016;11(11):e0164161.
- Ciobanu A, Khan N, Syngelaki A, et al. Routine ultrasound at 32 vs 36 weeks’ gestation: prediction of small-for-gestational-age neonates. Ultrasound Obstet Gynecol. 2019;53(6):761–768.
- Roma E, Arnau A, Berdala R, et al. Ultrasound screening for fetal growth restriction at 36 vs 32 weeks’ gestation: a randomized trial (ROUTE). Ultrasound Obstet Gynecol. 2015;46(4):391–397.