Abstract
Objective
The primary objective of this study was to explore whether antisense oligonucleotides (ASOs) that reduce LncNR_040117 expression in patients with antiphospholipid antibody syndrome (APS)-induced recurrent pregnancy loss (RPL), and further decrease apoptosis and improve trophoblasts invasion through mitogen-activated protein kinase (MAPK) pathways. This paper aimed to provide a new strategy to treat APS-induced RPL.
Methods
In this study, we used quantitative reverse transcription-polymerase chain reaction (RT–qPCR) to analyze the expression level of LncNR 040117 in HTR-8/SVneo cells following transfection with ASOs. Then we utilized Western blotting to test the expression levels of interleukin-1β (IL-1β), intracellular cell adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1) and key molecules of MAPK pathways, including the extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinases (JNK) and p38. In addition, we examined the HTR-8/SVneo cells apoptosis by cell apoptosis assay, and migration and invasion by transwell antibody assay. Each experiment was repeated three times. The data are presented as the means ± SDs, and statistical comparisons were performed using Student’s t-test. p < 0.05 was considered significant.
Result
Transfected with ASOs, LncNR_040117 was downregulated in trophoblasts compared with APS-induced RPL patients. And LncNR_040117 low expression induced IL-1β and downstream adhesion molecules ICAM-1 and VCAM-1expression level decreased, as well as MAPK pathways downregulation, including the ERK pathway, JNK pathway and p38/MAPK pathway. Furthermore, all these changes resulted in decreased apoptosis and increased migration and invasion of trophoblasts.
Conclusion
This study indicated that ASOs that decrease LncNR_040117 expression can reduce apoptosis and enhance the invasion and migration of trophoblasts by regulating the MAPK pathway.
Introduction
Recurrent pregnancy loss (RPL) is defined as the spontaneous loss of two or more pregnancies in the USA [Citation1]. However, the causes of RPL are complex, and recent studies have found that more than half of all cases of RPL are due to immune diseases, such as antiphospholipid syndrome (APS), a noninflammatory autoimmune disease [Citation2]. According to many epidemiological studies [Citation3,Citation4], APS is the main risk factor for RPL (7–25%). Additionally, APS-induced RPL has a variety of clinical presentations in late pregnancy, such as complications including preeclampsia (PE) [Citation5] and fetal growth restriction (FGR) [Citation6]. Moreover, with the development of transcriptomic technology, research on long noncoding RNAs (lncRNAs), which are defined as RNA transcripts > 200 nucleotides that do not encode proteins [Citation7], is increasing. Studies have shown that upregulation or downregulation of some lncRNAs leads to miscarriage by regulating the physiological function of trophoblasts [Citation8] through increases or decreases in inflammatory pathways [Citation9,Citation10], such as the mitogen-activated protein kinase (MAPK) pathways [Citation10] and the nuclear factor-kappa B (NF-κB) pathways [Citation11]. Through the MAPK cascade signaling system, cells transduce extracellular stimuli into nuclear signals and control the expression of genes that are essential for cellular processes such as cell proliferation, differentiation, and stress responses [Citation12]. In addition, among all the signaling networks, the MAPK signal transmission pathway plays an important role in controlling various physiological processes in cells, such as cell growth, development, division and death. And extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinases (JNK) and p38 are members of the MAPK family. The ERK signaling pathway is involved in regulating cell growth, development and division [Citation13]; however, the JNK and p38/MAPK signaling pathways are mainly related to inflammation, stress and cell apoptosis [Citation14]. Moreover, MAPKs kinases that play an important role in stress and inflammation can be stimulated by interleukin-1β (IL-1β), a proinflammatory cytokine. And stimulation of the p38/MAPK pathway increases the expression of downstream adhesion molecules such as intracellular cell adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) [Citation12]. In addition, the mechanism of APS-induced RPL is placental dysfunction caused by reduced invasion of trophoblasts and impaired vascular recasts [Citation15], which are related to the clinical symptoms of APS-induced RPL, such as abortion, PE and FGR. Moreover, during the past 40 years, methods involving antisense oligonucleotides (ASOs), which are usually 12–30 nucleotides in length, can be chemically synthesized and can uniquely modulate RNA function by binding to only one target RNA [Citation16,Citation17] and have achieved steady progress. In our previous work, we isolated platelet-derived microparticles (PMPs) from healthy pregnancies and APS-induced RPL patients and confirmed that they could stimulate inflammatory reactions and damage placental function [Citation18]. We analyzed PMPs genes isolated from healthy pregnancies and APS-induced RPL patients by lncRNA chip analysis and found LncNR_040117 overexpression in APS-induced RPL patients. Therefore, the purpose of this study was to explore whether ASOs reduce LncNR_040117 expression in APS-induced RPL patients decrease angiogenesis and improve trophoblasts invasion through the MAPK pathways, aiming to provide a new strategy to treat APS-induced RPL.
Methods
Cell culture
HTR-8/SVneo cells were grown in Roswell Park Memorial Institute-1640 (RPMI-1640) with 10% fetal bovine serum (FBS). All cells were grown in 24-well plates at 5 × 104 cells per well in a humidified 5% CO2 incubator at 37 °C.
Transfection assay
ASO sequences are shown in Supplemental Table 1. HTR-8/SVneo cells were divided into two groups: the ASO sequences mixed (50 nM) transfection group (ASO three sequences mixed group) and the empty vector transfection group (NC group). When the cells reached 30% confluence, transfection was conducted with the ASOs by FECT™ CP Reagent. After 48 h, gene expression was analyzed by real-time fluorescent quantitative reverse transcription-polymerase chain reaction (RT–qPCR) to determine the transfection efficiency.
RT–qPCR
Total RNA was extracted from HTR-8/SVneo cells in a 12-well plate with RNAiso Plus. A NanoDrop One system was used to measure the RNA concentration. Reverse transcription of 1 μg of total RNA was performed with the Revert Aid First Strand cDNA Synthesis kit. A 2 μL volume of cDNA with 10 μL of SYBR Green was used in a final reaction volume of 20 μL. RT–qPCR was performed in triplicate by a LightCycler II 480 system. The data were analyzed using the ΔΔCt method. The primer sequences are shown in Supplemental Table 2.
Western blotting
Total proteins were extracted with cell lysis buffer. Equal amounts of proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) and then transferred onto polyvinylidene fluoride (PVDF) membranes. The membranes were incubated with primary antibodies against ERK, JNK, p38, IL-1β, ICAM-1, VCAM-1, tubulin or gapdh at 4 °C overnight, followed by incubation with peroxidase-linked secondary antibody for 1 h at room temperature. The membranes were processed using an enhanced chemiluminescence detection system. The experiments were repeated three times.
Cell apoptosis assay
Each group of cells was harvested by trypsinization and washed in phosphate buffered saline (PBS) and 1X binding buffer. The cells were then resuspended at 1–10 × 106 cells/mL with 1X staining buffer and stained with 5 mL of FITC-Annexin V/PI for 10–15 min at room temperature in the dark. Apoptotic cells were detected with flow cytometry following the manufacturer’s instructions.
Transwell invasion assay
Transwell chambers coated with 50 μL of Matrigel were used to calculate the invasion of HTR-8/SVneo cells. Cell suspensions of 100 μL containing 5 × 105 cells were seeded in the upper chamber (with no FBS), and the lower chamber was filled with 600 μL of culture medium containing 10% exosome-depleted FBS. After a 48 h incubation, cells adhered to the lower surface were fixed with 4% paraformaldehyde and dyed with crystal violet. Images were taken by a microscope (×20 objective), and five fields of each chamber were used to quantify the invasive cells [Citation29].
Statistical analysis
Each experiment was repeated three times. The data are presented as the means ± SDs, and statistical comparisons were performed using Student’s t test. p < 0.05 was considered significant.
Results
ASOs decrease LncNR_040117 expression in trophoblasts
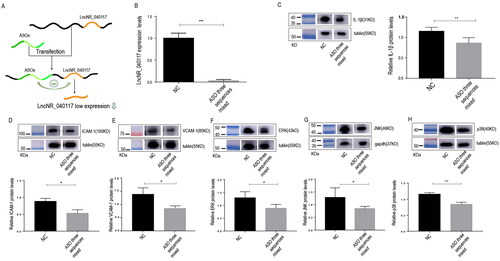
The transfection efficiency was examined by RT–qPCR 48 h after transfection. LncNR_040117 mRNA expression was significantly decreased in the ASO three sequences mixed group compared with the NC group (p < 0.001) (). We examined the transfection efficiency by detecting their expression levels. In addition, to further verify that ASOs can effectively reduce LncNR_040117 expression, we also detected the protein expression level, which is regulated by LncNR_040117. Based on our previous results and other references, we found that LncRNAs may regulate MAPK pathways; therefore, we detected the expression level of an inflammatory factor (IL-1β), which is the key molecule that stimulates MAPK pathways. The results showed that compared with the NC group, the IL-1β expression level was significantly decreased in the ASO three sequences mixed group (p < .01) (). All the results indicated that ASOs decrease LncNR_040117 expression in trophoblasts.
Figure 1. ASOs decrease LncNR_040117 expression and reduce the expression of downstream adhesion molecules and key molecules of the MAPK pathways in trophoblasts. Based on our previous work, LncNR_040117 is overexpressed in a pregnant woman with APS-induced RPL, and we tried to decrease LncNR_040117 expression by ASO transfection (A). HTR-8/SVneo cells were transfected with ASOs by FECT™ CP Reagent. After 48 h, the LncNR_040117 expression level was measured by qRT–PCR (B), and inflammatory factors were detected by western blotting (C). Western blotting was used to detect the expression levels of downstream adhesion molecules, such as ICAM-1 and VCAM-1 (D, E), and key molecules of the MAPK pathways, such as ERK, JNK and p38 (F, G and H), after ASO transfection for 48 h. The data are presented as the mean ± SD of three independent experiments. *p < 0.05, **p < 0.01, ***P < 0.001.
Low LncNR_040117 expression reduces inflammatory factor expression and downregulates the MAPK pathways
To further study the mechanism by which inflammatory factor expression is downregulated, we detected the downstream adhesion molecules and key molecules of the MAPK pathways by western blotting. The results showed that the expression of downstream adhesion molecules, such as ICAM-1 (p < 0.05) () and VCAM-1 (p < 0.05) (), and key molecules of the MAPK pathways, such as ERK (p < 0.05) (), JNK (p < 0.05) (), and p38 (p < 0.01) (), was decreased in the ASO three sequences mixed group compared with the NC group. These results showed that low LncNR_040117 expression could both reduce inflammatory factors expression and downregulate the MAPK pathways.
Low LncNR_040117 expression decreases trophoblasts apoptosis
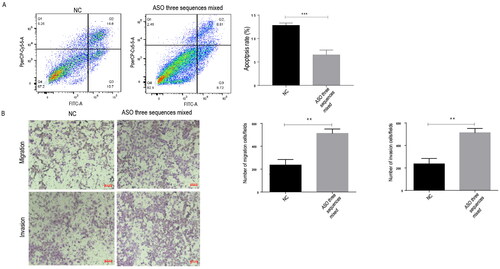
Furthermore, we investigated the effects of ASOs on cell apoptosis by FITC-Annexin V/PI staining and flow cytometry. The results showed that the percentage of apoptotic cells in the LncNR_040117 low expression group (ASO three sequences mixed group) was 6.81%, which was much lower than that in the NC group, 16.8% (p < 0.001) (). These results indicated that low LncNR_040117 expression decreased trophoblast cell apoptosis.
Figure 2. Trophoblasts cells apoptosis decreased and invasion and migration increased through LncNR_040117 downregulation by ASOs treatment. After transfection with ASOs for 48 h, apoptosis in HTR-8/SVneo cells with low LncNR_040117 expression and normal HTR-8/SVneo cells was detected by flow cytometry (A). Then, transwell assays were applied to measure the migration and invasion capacity of LncNR_040117 low-expression HTR-8/SVneo cells treated with ASOs and normal HTR-8/SVneo cells (B). The scale bar is 100 μm. The data are presented as the mean ± SDs of three independent experiments. **p < 0.01, ***p < 0.001.
Low LncNR_040117 expression enhances trophoblasts invasion and migration
The invasion and migration of trophoblasts with low LncNR_040117 expression were assessed by a transwell assay. The invasion and migration () of the LncNR_040117 low expression group (ASO three sequences mixed group) were much higher than those of the NC group (p < 0.01). These results indicated that low LncNR_040117 expression enhances trophoblast cell invasion and migration.
Discussion
APS is a systemic autoimmune disease characterized by persistent antiphospholipid antibodies (aPLs), thrombosis and pathological pregnancy, such as RPL. However, the exact pathogenic mechanism of APS is not completely elucidated, and one of the causes is that aPLs induce thrombosis by activating endothelial cells and platelets and impede placentation by directly damaging trophoblasts [Citation19]. Therefore, one of the key pathogenic mechanisms of APS-induced RPL is a decrease in trophoblasts abilities. This is the first study to explore whether ASOs decrease lncRNA expression to improve trophoblast cell abilities.
Microparticles (MPs) play an important role in autoimmune diseases, and PMPs are a kind of MPs derived from platelets. In our previous work, we found that compared to healthy gestational week-matched women at gestational weeks 6–10, PMPs isolated from patients with APS-induced RPL augmented the expression of inflammatory cytokines (tumor necrosis factor-α, TNF-α)/adhesion molecules (ICAM-1 and VCAM-1) [Citation20] and resulted in increased trophoblast apoptosis and decreased invasion and angiogenesis [Citation18], and all of these factors led to impaired trophoblast function, which is characteristic of this type of pathological pregnancy [Citation3,Citation17,Citation21]. In addition, lncRNAs, which are usually located in the cytoplasm, nucleus or nucleoli, do not encode proteins. LncRNAs not only regulate RNA or protein transcription, post transcription, and degradation but also participate in various physiological and pathological processes as scaffolds and play key roles in the field of reproduction [Citation22]. Wang et al. study showed that upregulation or downregulation of some lncRNAs leads to miscarriage by regulating the physiological function of trophoblasts [Citation8]. Therefore, to study the ASO-mediated regulation of the change in the expression level of lncRNAs and the regulation of the physiological function of trophoblast cells, we performed further experiments. First, in our previous work, we further investigated LncNR_040117 in PMPs is overexpressed in the APS-induced RPL group compared with the normal group by lncRNA chip analysis. Based on these results, we selected the normal group, in which the expression level of LncNR_040117 is lower than that in APS-induced RPL patients, as research subjects rather than directly researching the APS-induced RPL group. Therefore, we transfected normal trophoblasts with ASOs to obtain the results of the present study. In addition, in the present study, to study placental trophoblasts biological behavior, we employed the HTR-8/SVneo cell line, which was derived by transfecting cells that grew out of chorionic villi explants of the human first-trimester placenta, as a cell model for our study.
As our RT–qPCR results showed, ASOs efficiently reduced LncNR_040117 expression at the nucleic acid level compared to that in the normal group, which was lower than that in the APS-induced RPL group. Furthermore, to verify that ASOs can effectively reduce LncNR_040117 expression at the protein level, we further detected the protein expression level, which is regulated by lncNR_040117. Zhao et al. study showed that lncRNAs could regulate MAPK pathways to cause the dysfunction of trophoblasts and the occurrence of RPL [Citation23]. In addition, our previous results study showed that PMPs of APS-induced RPL patients could activate MAPK pathways [Citation18]. Additionally, environmental stresses, such as osmotic shock, ultraviolet irradiation, lipopolysaccharide (LPS), cytotoxic chemicals, and proinflammatory cytokines, such as tumor necrosis factor (TNF) and interleukin 1 (IL-1), can stimulate ERK, JNK and p38, which are kinases of the MAPK pathways [Citation12]. Therefore, we detected the expression level of an inflammatory factor (IL-1β), which is the key molecule that stimulates the MAPK pathways. Our results showed that IL-1β expression was lower after ASO treatment than in the normal group. Moreover, p38/MAPK pathway stimulation increases the expression of downstream adhesion molecules, which are classified into two major families: selectins and the Ig superfamily, such as ICAM-1 and VCAM-1 [Citation14]. In summary, IL-1β could stimulate the MAPK pathways and further regulate the expression of adhesion molecules, such as ICAM-1 and VCAM-1. Therefore, we detected the expression levels of downstream adhesion molecules and key molecules of the MAPK pathways. Our results also indicated that the expression levels of ICAM-1, VCAM-1 and key molecules of the MAPK pathways, such as ERK, JNK and p38, were both decreased after treatment with ASOs. In other words, all our results showed that after ASOs transfection, low LncNR_040117 expression reduced IL-1β, ICAM-1 and VCAM-1 expression and downregulated the ERK, JNK and p38/MAPK pathways. Moreover, we deduced that MAPK pathways downregulation may be induced by low IL-1β expression and that decreased ICAM-1 and VCAM-1 expression levels may be induced by MAPK pathways downregulation.
In addition, among the numerous intracellular signaling pathways, the MAPK pathways transduce extracellular stimuli into nuclear signals and control the expression of genes to play a more important role in cell proliferation, differentiation, apoptosis, angiogenesis and tumor metastasis than other pathways [Citation12]. Above all, ERK plays a role in signaling cascades and transmits extracellular signals to intracellular targets. Then, through sequential activation of three to five layers of protein kinases, such as MAPK kinase kinase kinase (MAP4K), MAPK kinase (MAP3K), MAPK kinase (MAPKK), MAPK and MAPK-activated protein kinases (MAPKAPK), the ERK pathway is involved in regulating cellular biological functions, such as cell proliferation, cell differentiation, cell cycle regulation, cell apoptosis and tissue formation [Citation12]. In addition, the JNK pathway plays a complex role in multiple forms of cell death, including necrosis and autophagy [Citation24]. Moreover, the p38 family has four kinds of kinases, p38α (MAPK14), p38β (MAPK11), p38γ (stress-activated protein kinase (SAPK) 3, ERK 6 or MAPK12) and p38δ (SAPK4 or MAPK13). The four kinds of kinases are encoded by different genes and have different tissue expression patterns, with p38α being ubiquitously expressed at significant levels in most cell types, whereas the others seem to be expressed in a more tissue-specific manner. Additionally, the p38/MAPK pathway participates in numerous biological processes, including responses to stress and inflammation, as well as the regulation of proliferation, differentiation and survival in particular cell types. ICAM-1 mediates tight adhesion of neutrophils and is involved in fibrinogen-induced vascular smooth muscle cell migration. In addition, a study by Donghyuk Kim et al. showed that the p38/MAPK-dependent pathway plays a critical role in neutrophil chemotaxis through the regulation of the cell surface [Citation25]. Similarly, in the placenta, these changes altered placental function, and many studies have shown that aPLs can induce RPL by stimulating MAPK pathways. Several studies have also confirmed this view. For example, Lu et al. showed that aPLs induced reactive oxygen species (ROS) production and neutrophil extracellular traps (NETs) formation by activating the ERK and p38/MAPK pathways to cause RPL [Citation26]. Likewise, in our previous work, we also found that MAPK pathways played a significant role in APS-induced RPL. We found that APS patient PMPs induce RPL by activating the p38/MAPK pathway [Citation18].
Moreover, trophoblast dysfunction is one of the pathological bases of APS-induced RPL. Studies have confirmed that increased levels of proinflammatory/prothrombotic cytokines and adhesion molecules lead to inhibited placental angiogenesis and decreased trophoblast invasion, which results in trophoblast dysfunction.
Bettina Engel et al. confirmed this theory and found that the secretory ICAM-1 (sVCAM-1) plasma level was correlated with the frequency of abortions, and sVCAM-1 can be considered a positive predictive parameter for abortions in patients with APS [Citation27]. In summary, LncNR_040117 regulates MAPK pathways and downstream inflammatory factors and changes trophoblasts biological behavior and vascular functions. Moreover, placental function mainly depends on trophoblasts, which are important components of villi. Favorable placental function plays a vital role in a successful pregnancy. In addition, biological behavior [Citation17], such as the proliferation and differentiation of trophoblasts, is an essential element in the development of the placenta [Citation5], and invasion of trophoblasts is a crucial factor in remodeling of the placental vasculature [Citation15,Citation21]. Similarly, our results indicated that low LncNR_040117 expression reduced trophoblasts apoptosis and enhanced trophoblasts invasion and migration by regulating downstream inflammatory factor expression levels, which may be caused by downregulating MAPK pathways.
Although JNK and p38/MAPK pathways have important roles in the signaling mechanisms that orchestrate cellular responses to many types of stresses, they also control the proliferation, differentiation, survival and migration of specific cell types. JNK can be activated by the upstream mitogen-activated protein kinase kinase (MKK) 4 and MKK7, and MKK3 and MKK6 can highly specifically phosphorylate p38; moreover, p38α can also be phosphorylated by MKK4, an activator of the JNK [Citation28]. Therefore, it has been reported that JNK and p38/MAPK pathways can exert antagonistic effects on cell proliferation and survival, but they depend not only on different cell type specificities but also on the signal intensity and duration and the crosstalk between other signaling pathways [Citation13]. However, a study by Zhao et al. found that the JNK and p38/MAPK signaling pathways can both be activated and ultimately cause the dysfunction of trophoblast cells and the occurrence of recurrent pregnancy loss [Citation23]. Our study showed that both the JNK and p38/MAPK pathways are stimulated and mainly explored p38/MAPK pathway downstream adhesion molecule expression levels. We did not compare the expression levels of the two pathways. Therefore, we do not know whether signal integration by the JNK and p38/MAPK pathways also occurs in trophoblasts. Moreover, RNA molecules are very unstable in blood circulation, and these molecules are easily degraded by nucleases, cleared by the kidneys and show a nonspecific distribution. Consequently, this factor limited ASO efficacy in vivo. Further studies are necessary to identify a delivery system that will carry ASOs to trophoblasts in vivo, as proven by animal experiments. Overall, our results showed that ASOs could reduce apoptosis and enhance the invasion and migration of trophoblasts by decreasing lncRNA expression.
Conclusion
In conclusion, we demonstrated that ASOs efficiently reduced LncNR_040117 expression compared to that in the normal group. Furthermore, low LncNR_040117 expression reduced IL-1β and downstream ICAM-1 and VCAM-1 expression levels and downregulated the ERK, JNK and p38/MAPK pathways. Consequently, after treatment with ASOs, cell apoptosis was lower than that of the normal group, which was far lower than that of the APS-induced RPL group, and invasion and migration were enhanced compared with those of the normal group, which were superior to those of the APS-induced RPL group. Our findings indicate that ASOs might be a potential treatment for APS-induced RPL by decreasing LncNR_040117 in trophoblasts.
Authors’ contribution
X.T.W. and Q. Z. led the conception and design of the study. Q. Z. and D. W. performed the experiments. D. W. and Q. Z. was responsible for the analysis of the data. D. W. and Q. Z. wrote the paper. All authors have reviewed and approved the manuscript.
Supplemental Material
Download MS Word (16.9 KB)Acknowledgements
We thank Dr. Q Zhou for analysing platelet-derived microparticles (PMPs) in the peripheral blood of patients with APS-induced RPL by a lncRNA chip.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Amer Soc Reprod, M. Definitions of infertility and recurrent pregnancy loss: a committee opinion. Fertility and Sterility. 2020;113(3):533–535.
- Carp H, Dardik R, Lubetsky A, et al. Prevalence of circulating procoagulant microparticles in women with recurrent miscarriage: a case-controlled study. Hum Reprod. 2004;19(1):191–195.
- Kutteh WH. Antiphospholipid antibody syndrome and reproduction. Curr Opin Obstet Gynecol. 2014;26(4):260–265.
- Santos TdS, Ieque AL, de Carvalho HC, et al. Antiphospholipid syndrome and recurrent miscarriage: a systematic review and meta-analysis. J Reprod Immunol. 2017;123:78–87.
- Nishizawa H, Hasegawa K, Suzuki M, et al. The etiological role of allogeneic fetal rejection in pre-eclampsia. Am J Reprod Immunol. 2007;58(1):11–20.
- Heazell AEP, Sharp AN, Baker PN, et al. Intra-uterine growth restriction is associated with increased apoptosis and altered expression of proteins in the p53 pathway in villous trophoblast. Apoptosis. 2011;16(2):135–144.
- Bridges MC, Daulagala AC, Kourtidis A. LNCcation: lncRNA localization and function. J Cell Biol. 2021;220(2):e202009045.
- Wang Y, Liu H-Z, Liu Y, et al. Downregulated MALAT1 relates to recurrent pregnancy loss via sponging miRNAs. Kaohsiung J Med Sci. 2018;34(9):503–510.
- Wang L, Tang H, Xiong Y, et al. Differential expression profile of long noncoding RNAs in human chorionic villi of early recurrent miscarriage. Clin Chim Acta. 2017;464:17–23.
- Wang H, Cao Q, Ge J, et al. LncRNA-regulated infection and inflammation pathways associated with pregnancy loss: genome wide differential expression of IncRNAs in early spontaneous abortion. Am J Reprod Immunol. 2014;72(4):359–375.
- Huang Z, Du G, Huang X, et al. The enhancer RNA lnc-SLC4A1-1 epigenetically regulates unexplained recurrent pregnancy loss (URPL) by activating CXCL8 and NF-kB pathway. Ebiomedicine. 2018;38:162–170.
- Guo Y-J, Pan W-W, Liu S-B, et al. ERK/MAPK signalling pathway and tumorigenesis. Exp Ther Med. 2020;19(3):1997–2007.
- Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer. 2009;9(8):537–549.
- Lin C-C, Lee I-T, Hsu C-H, et al. Sphingosine-1-phosphate mediates ICAM-1-dependent monocyte adhesion through p38 MAPK and p42/p44 MAPK-dependent akt activation. PLoS ONE. 2015;10(3):e0118473.
- Guo W, et al. Decreased human leukocyte antigen-G expression by miR-133a contributes to impairment of proinvasion and proangiogenesis functions of decidual NK cells. Front Immunol. 2017;8:741.
- Bennett CF. Therapeutic antisense oligonucleotides are coming of age. Annu Rev Med. 2019;70:307–321.
- Mayhew TM. Turnover of human villous trophoblast in normal pregnancy: what do we know and what do we need to know? Placenta. 2014;35(4):229–240.
- Zhou Q, Lian Y, Zhang Y, et al. Platelet-derived microparticles from recurrent miscarriage associated with antiphospholipid antibody syndrome influence behaviours of trophoblast and endothelial cells. Mol Hum Reprod. 2019;25(8):483–494.
- Rodrigues VO, Soligo A, Pannain GD. Antiphospholipid antibody syndrome and infertility. Rev Bras Ginecol Obstet. 2019;41(10):621–627.
- Nizamutdinova IT, Oh HM, Min YN, et al. Paeonol suppresses intercellular adhesion molecule-1 expression in tumor necrosis factor-alpha-stimulated human umbilical vein endothelial cells by blocking p38, ERK and nuclear factor-kappaB signaling pathways. Int Immunopharmacol. 2007;7(3):343–350.
- Maltepe E, Fisher SJ. Placenta: the forgotten organ. Annu Rev Cell Dev Biol. 2015;31:523–552.
- Fatica A, Bozzoni I. Long non-coding RNAs: new players in cell differentiation and development. Nat Rev Genet. 2014;15(1):7–21.
- Zhao H, et al. LncRNA LINC01088 inhibits the function of trophoblast cells, activates the MAPK-signaling pathway and associates with recurrent pregnancy loss. Mol Hum Reprod. 2021;27(8):gaab047.
- Weston CR, Davis RJ. The JNK signal transduction pathway. Curr Opin Cell Biol. 2007;19(2):142–149.
- Kim D, Haynes CL. The role of p38 MAPK in neutrophil functions: single cell chemotaxis and surface marker expression. Analyst. 2013;138(22):6826–6833.
- Lu Y, Dong Y, Zhang Y, et al. Antiphospholipid antibody-activated NETs exacerbate trophoblast and endothelial cell injury in obstetric antiphospholipid syndrome. J Cell Mol Med. 2020;24(12):6690–6703.
- Engel B, Müller G, Roch B, et al. Serum of patients with antiphospholipid syndrome induces adhesion molecules in endothelial cells. Atheroscler Suppl. 2017;30:141–148.
- Cuadrado A, Nebreda AR. Mechanisms and functions of p38 MAPK signalling. Biochem J. 2010;429(3):403–417.
- Zhao H-J, Klausen C, Li Y, et al. Bone morphogenetic protein 2 promotes human trophoblast cell invasion by upregulating N-cadherin via non-canonical SMAD2/3 signaling. Cell Death Dis. 2018;9(2):174.
