Abstract
Objective
To investigate the relationship between general and central obesity in the first trimester of pregnancy and gestational diabetes and its predicted value.
Materials and methods
We recruited 813 women who registered at 6–12 weeks of gestation. Anthropometric measurements were done at the first antenatal visit. At 24–28 weeks of pregnancy, gestational diabetes was diagnosed using the 75 g oral glucose tolerance test. Binary logistic regression was used to determine odds ratios and 95% confidence intervals. The receiver-operating characteristic curve was used to evaluate the ability of obesity indices to predict the risk of gestational diabetes.
Results
Odds ratios (95% confidence intervals) of gestational diabetes across increasing quartiles of waist-to-hip ratio were 1.00, 1.54 (0.65–3.66), 2.63 (1.18–5.85), and 4.96 (2.27–10.85), respectively (p < .001), while those for waist-to-height ratio were 1.00, 1.21 (0.47–3.08), 2.99 (1.26–7.10), and 4.01 (1.57–10.19), respectively (p < .001). Areas under the curve for general and central obesity were similar. However, the area under the curve of body mass index combined with the waist-to-hip ratio was the biggest.
Conclusion
Higher waist-to-hip ratio and waist-to-height ratio in the first trimester of pregnancy are associated with increased risks of gestational diabetes in Chinese women. The combination of body mass index and waist-to-hip ratio in the first trimester of pregnancy is a good predictor for gestational diabetes.
Introduction
Gestational diabetes mellitus (GDM), a common complication of pregnancy, refers to “diabetes diagnosed in the second or third trimester of pregnancy that was not clearly overt diabetes prior to gestation” [Citation1]. GDM incidence is rising and poses serious risks to pregnant women and fetuses, including spontaneous abortion, preterm birth, gestational hypertension, fetal macrosomia, and hydramnios [Citation2]. Intrauterine hyperglycemia during pregnancy places the offspring at higher risk of long-term adverse outcomes like obesity and type 2 diabetes mellitus (T2DM) [Citation3]. The rate of T2DM development in women with GDM is 38% in the first postpartum year and 60% in the 16th postpartum year [Citation4]. Studies show that in China, about 18.9% of pregnant women are diagnosed with GDM [Citation5]. Obesity is a major risk factor for GDM [Citation6]. Body mass index (BMI) is used as a measurement of general obesity. However, BMI does not consider the regional distribution of fat in the body, i.e. subcutaneous versus visceral/central. Researches have implicated adipose tissue, especially intra-abdominal or visceral adipose tissue (VAT), relative to subcutaneous adipose tissue (SAT), in diabetes mellitus (DM) development [Citation7]. Chronic, low-grade inflammation has a causal role in the loss of insulin sensitivity in adipose tissue. Adipose tissue inflammation and dysfunction link obesity to DM pathogenesis [Citation8]. Thus, measures of central/abdominal obesity like waist circumference (WC), neck circumference, waist-to-hip ratio (WHR), and waist-to-height ratio (WHtR) have been proposed and extensively studied for their adverse cardiovascular and metabolic consequences [Citation9–10]. Excessive visceral and subcutaneous fat built up around the abdomen and stomach outperforms general obesity in predicting diabetes risk [Citation11]. However, whether the effects are generalizable to GDM remains uncertain. Here, we investigated if central obesity in the 1st trimester is associated with GDM.
Materials and methods
Study population
From January 2018 to July 2018, women in the 1st trimester of pregnancy (range: 6–12 weeks) attending their first antenatal visit at the International Peace Maternity and Child Health Hospital antenatal clinic were recruited into the study. Exclusion criteria were as follows: history of GDM and macrosomia in previous pregnancies, history of impaired glucose tolerance (IGT) and DM, multiple pregnancies, and polycystic ovary syndrome (PCOS).
Measurements and data collection
Body height and weight were measured using standard instruments with the participant standing without shoes, with both feet touching, wearing light clothing, and arms hanging freely. WC and hip circumference (HC) were measured by uniformly-trained study personnel using an inelastic tape placed at the level of the umbilicus for WC and at the site of maximum extension of the buttocks for HC at the end of normal respiration with the participants standing erect on a horizontal plane [Citation12]. BMI was calculated using the formula: weight (kg) divided by height (meters) squared (kg/m2). WHR was calculated as WC (meters) divided by HC (meters). WHtR was calculated as WC (meters) divided by height (meters). Based on guidelines by the “working group on obesity in China” [Citation13], BMI = <18.5 kg/m2 indicates underweight, BMI = 18.5–23.9 kg/m2 indicates normal weight, BMI = 24.0–27.9 kg/m2 indicates overweight, and BMI = ≥28 kg/m2 indicates obesity. Maternal age was categorized into 3 groups: <35, 35–39, and ≥40 years. Blood pressure was measured on the right arm using a calibrated mercury sphygmomanometer after at least 5–10 min of rest. We used maternal BMI, WC, and HC measured at the first antenatal visit instead of pre-pregnancy data. Mean maternal body composition values are reported to remain largely unchanged in the 1st trimester of pregnancy [Citation14]. Other clinical data like maternal age, history of pregnancy and childbirth, gestational age, mode of conception, education level, smoking status, and health history were collected by the medical worker at the first antenatal visit.
Diagnostic criteria for GDM
According to IADPSG criteria [Citation15], GDM can be diagnosed using the following criteria when using the 75 g oral glucose tolerance test (OGTT) at 24–28 weeks: fasting plasma glucose ≥5.1 mmol/L, 1 h plasma glucose ≥10.0 mmol/L or 2 h plasma glucose ≥8.5 mmol/L.
Statistical analyses
Statistical analyses were performed on SPSS version 25 (IBM, Armonk, NY, USA) Continuous variables were expressed as mean ± standard deviation (x¯ ± s) or median with interquartile range (IQR). T-test (for normal distributions) and Mann–Whitney rank-sum test (non-normal distributions) were used for between-group comparisons. Categorical variables were expressed as rates, percentage, or constituent ratio, and statistical significance assessed using the Chi-square test. p < .05 (two-tailed) indicated statistically significant differences. Binary logistic regression was conducted to obtain odds ratios (OR) and their 95% confidence intervals (CI) for GDM-associated factors. WC, WHR, and WHtR values were divided into quartiles and used for linear trend tests. Receiver operating characteristics curve (ROC) analysis was used to evaluate the diagnostic value of adiposity measures in pregnant women with GDM.
Results
Characteristics of the study population
Of the 859 pregnant women who met the eligibility criteria, 813 were included in the final analysis after excluding pregnant women with early spontaneous abortion (n = 9) and unfinished OGTT at 24–28 weeks (n = 37) (). Of these, 108 (13.3%) were diagnosed with GDM. The baseline demographic characteristics of the GDM group and non-GDM group are summarized in . The 2 groups did not differ significantly with regards to education level, smoking habit, and gestational week (all p > .05). Compared to those without GDM, women with GDM were older and had higher systolic/diastolic blood pressure, BMI, WC, WHR, and WHtR at the first antenatal visit (all p < .001). Relative to the non-GDM group, the proportions of those at a maternal age >35 years, those with previous births, those who underwent assisted reproductive technique (ART), and those with a BMI >24.0 kg/m2 were higher in the GDM group (p < .001, p = .003, p = .002, and p = .001, respectively).
Figure 1. Flow diagram of study participants. GDM: gestational diabetes mellitus; IGT: impaired glucose tolerance; DM: diabetes mellitus; PCOS: polycystic ovary syndrome; OGTT: oral glucose tolerance test.
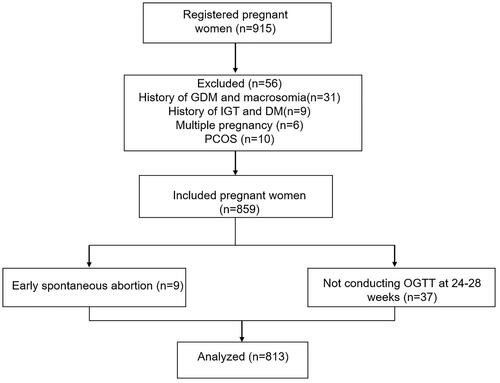
Table 1. Demographic and clinical data of pregnant women.
Central obesity indicators for the risk of GDM
Next, we assessed the association between central obesity levels and GDM risk using binary logistic regression (). The multivariale model 1 (after adjustment for maternal age, number of deliveries, and mode of conception), revealed that GDM increased with increasing quartile levels of WC, WHR, and WHtR (all p for trend <.001). The multivariale model 2 (adjusted also for BMI), revealed that GDM risk increased with increasing quartile levels of WHR and WHtR (WHR [OR (95% CI)]: 1.00, 1.54 (0.65–3.66), 2.63 (1.18–5.85), 4.96 (2.27–10.85); WHtR [OR (95% CI)]: 1.00, 1.21 (0.47–3.08), 2.99 (1.26–7.10), 4.01 (1.57–10.19), respectively, all p for trend <.001). However, the trend was not statistically significant in the WC cohort (p for trend = .56).
Table 2. Association between central obesity measures and gestational diabetes.
Prediction of obesity indicators for GDM
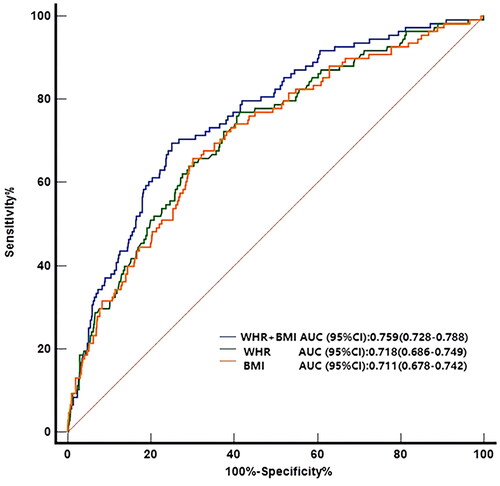
ROC curves analysis revealed that general obesity (BMI) and central obesity (WC, WHR, and WHtR) did not differ significantly in the prediction of GDM (BMI [AUC (95% CI)]: 0.711 (0.678–0.742); WC [AUC (95% CI)]: 0.707 (0.674–0.738); WHR [AUC (95% CI)]: 0.718 (0.686–0.749); WHtR [AUC (95% CI)]: 0.722 (0.690–0.753), respectively, p > .05). However, BMI in combination with WHR was a better predictor of GDM relative to either alone, or other indicator combinations (AUC = 0.759, 95%CI 0.728–0.788, p < .05, ).
Discussion
Past studies have shown the importance of central obesity in determining GDM risk [Citation16–17]. Here, we assessed the importance of central obesity in future GDM during the 1st trimester. WC, WHR, and WHtR are the primary parameters for determining central obesity. Han Qian et al. suggested that the risk of GDM increased slightly with increasing WC up to 76 cm and then linearly rose steeply from 78.5 cm [Citation18]. Different anatomical landmarks used to determine the exact location for measuring WC have been utilized in clinical studies. However, it is known from the literatures that no one of these measures was consistently better than the others [Citation19]. In our study, the tape was placed at the level of the umbilicus to obtain WC. There have been some studies that show WC is a risk factor for developing GDM, and the combination of WC and other variables can be used to predict the disease in the early stages [Citation20–21]. With regards to WC, we found that women with GDM had higher WC than those without GDM.
Snijder MB et al. found independent and opposite associations between HC and diabetes and dyslipidemia [Citation22]. This could suggest that a larger HC could protect against metabolic syndrome. Previous studies have shown that higher WHR and WHtR increases the risk of GDM [Citation12,Citation23]. As a result, we used WHR and WHtR to evaluate abdominal obesity in pregnant women during the 1st trimester. To exclude potential GDM confounders like the history of IGT, DM, GDM and macrosomia, multiple pregnancy, and PCOS, we used a prospective approach. Our findings indicate that the odds of GDM increased with one quartile increment in WHR and WHtR. The relative odds of GDM onset attenuated and the trend of the odds remained statistically significant after adjusting for confounders. However, the trend was not statistically significant in the WC cohort after the confounder of BMI was added. Vazquez et al. [Citation24] found that the correlation between BMI, WC, and WHR was 0.88 (BMI/WC), 0.34 (BMI/WHR), and 0.44 (WC/WHR), respectively. Possibly, a collinear relationship reduces the accuracy of the model.
Our analysis of the ability of obesity indicators to predict GDM during the 1st trimester revealed that contrary to expectation, general obesity (BMI) and central obesity (WC, WHR, and WHtR) performed similarly. One unanticipated result was that combining BMI with WHR was a better predictor of GDM relative to either indicator alone or other indicator combinations. Comparison of these obesity indices with each other for the predictive value of GDM revealed that the AUC of WHtR was numerically higher than that of other indices but the difference was statistically insignificant. This outcome differs from that of Sina et al. who found that WHtR was a better predictor of GDM relative to other anthropometric indices in Australian Aboriginal women [Citation25], which is consistent with similar diabetes studies in other populations [Citation26–27]. This difference may be due to different regions, study population, and anthropometric measurements methods. It should be noted that the ability of various obesity indicators to predict diabetes varies with ethnicity, likely due to differences in the percentage of total body fat and body fat distribution [Citation12,Citation28–30]. Asians have significantly more abdominal fat accumulation than Europeans, especially visceral adipose tissue [Citation31]. Given that high BMI played an irreplaceable role in future GDM, we hypothesized that combining BMI and other obesity indices as a collective risk assessment tool may improve their ability to predict GDM. Intriguingly, combining BMI and WHR was a better predictor of GDM compared to either indicator alone or other indicator combinations. This finding may guide the design of interventions that reduce BMI and WHR in women of child-bearing age to prevent or reduce the risk of developing GDM.
This study is limited by its small sample size and measurement bias while conducting anthropometric assessments despite measurements being done by trained researchers. Additionally, because the study was done in Shanghai, one of the most developed cities in China, the generalizability of our findings to other communities needs validation.
In conclusion, high WHR and WHtR levels in the 1st trimester are associated with an increased risk of GDM in Chinese women and the combination of BMI and WHR in the 1st trimester is a good GDM predictor. The finding has important clinical and public health implications and warrants follow-up investigation. Early GDM detection is likely to improve short-term pregnancy outcomes. Pre-pregnancy interventions that reduce these anthropometric measures may reduce GDM and associated adverse outcomes. Larger studies are needed to construct better GDM prediction models with better sensitivity and specificity.
Author contributions
Qunying Cai: design, planning, data analysis, and manuscript writing; Shu Shi: design, planning, and manuscript writing; Weiwei Cheng: design; Hong Shen: design and data analysis; Baoying Ye: data collection.
Ethical approval
The study was approved by the institutional Medical Ethics Committee of the International Peace Maternity and Child Health Hospital (approved 8/June/2017, (GKLW)2016-39).
Consent form
All human participants gave written informed consent before the study began.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- American Diabetes Association. 2 Classification and diagnosis of diabetes: standards of medical care in diabetes-2021. Diabetes Care. 2021;44(Suppl 1):S15–S33.
- Chiefari E, Arcidiacono B, Foti D, et al. Gestational diabetes mellitus: an updated overview. J Endocrinol Invest. 2017;40(9):899–909.
- El Hajj N, Schneider E, Lehnen H, et al. Epigenetics and life-long consequences of an adverse nutritional and diabetic intrauterine environment. Reproduction. 2014;148(6):R111–R120.
- O'Dea A, Tierney M, McGuire BE, et al. Can the onset of type 2 diabetes be delayed by a Group-Based lifestyle intervention in women with prediabetes following gestational diabetes mellitus (GDM)? findings from a randomized control mixed methods trial. J Diabetes Res. 2015;2015:798460.
- Wei Y, Yang H, Zhu W, et al. International association of diabetes and pregnancy study group criteria is suitable for gestational diabetes mellitus diagnosis: further evidence from China. Chin Med J. 2014;127(20):3553–3556.
- Chu SY, Callaghan WM, Kim SY, et al. Maternal obesity and risk of gestational diabetes mellitus. Diabetes Care. 2007;30(8):2070–2076.
- Wenderott JK, Flesher CG, Baker NA, et al. Elucidating nanoscale mechanical properties of diabetic human adipose tissue using atomic force microscopy. Sci Rep. 2020;10(1):20423.
- Dahik VD, Frisdal E, Goff L. W. Rewiring of lipid metabolism in adipose tissue macrophages in obesity: impact on insulin resistance and type 2 diabetes. IJMS. 2020;21(15):5505.
- Borel AL, Coumes S, Reche F, et al. Waist, neck circumferences, waist-to-hip ratio: which is the best cardiometabolic risk marker in women with severe obesity? The SOON cohort. PLOS One. 2018;13(11):e0206617.
- Lam BC, Koh GC, Chen C, et al. Comparison of body mass index (BMI), body adiposity index (BAI), waist circumference (WC), waist-to-hip ratio (WHR) and waist-to-height ratio (WHtR) as predictors of cardiovascular disease risk factors in an adult population in Singapore. PLOS One. 2015;10(4):e0122985.
- Hartz AJ, Rupley DC Jr, Kalkhoff RD, et al. Relationship of obesity to diabetes: influence of obesity level and body fat distribution. Prev Med. 1983;12(2):351–357.
- Basraon SK, Mele L, Myatt L, et al. Relationship of early pregnancy waist-to-hip ratio versus body mass index with gestational diabetes mellitus and insulin resistance. Am J Perinatol. 2016;33(1):114–121.
- Zhou BF. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults–study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci. 2002;15(1):83–96.
- Fattah C, Barry S, O'connor N, et al. Maternal leptin and body composition in the first trimester of pregnancy. Gynecol Endocrinol. 2011;27(4):263–266.
- Weinert LS. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy: comment to the international association of diabetes and pregnancy study groups consensus panel. Diabetes Care. 2010;33(7):e97–e98.
- Balani J, Hyer SL, Shehata H, et al. Visceral fat mass as a novel risk factor for predicting gestational diabetes in obese pregnant women. Obstet Med. 2018;11(3):121–125.
- Yao D, Chang Q, Wu QJ, et al. Relationship between maternal central obesity and the risk of gestational diabetes mellitus: a systematic review and meta-analysis of cohort studies. J Diabetes Res. 2020;2020:6303820.
- Han Q, Shao P, Leng J, et al. Interactions between general and central obesity in predicting gestational diabetes mellitus in Chinese pregnant women: a prospective population-based study in Tianjin, China. J Diabetes. 2018;10(1):59–67.
- Klein S, Allison DB, Heymsfield SB, et al. Waist circumference and cardiometabolic risk: a consensus statement from shaping America’s Health: Association for Weight Management and Obesity Prevention; NAASO, the Obesity Society; the American Society for Nutrition; and the American Diabetes Association. Am J Clin Nutr. 2007;85(5):1197–1202.
- Wang J, Lv B, Chen X, et al. An early model to predict the risk of gestational diabetes mellitus in the absence of blood examination indexes: application in primary health care centres. BMC Pregnancy Childbirth. 2021;21(1):814.
- Popova PV, Grineva EN, Gerasimov AS, et al. The new combination of risk factors determining a high risk of gestational diabetes mellitus. Minerva Endocrinol. 2015;40(4):239–247.
- Snijder MB, Zimmet PZ, Visser M, et al. Independent and opposite associations of waist and hip circumferences with diabetes, hypertension and dyslipidemia: the AusDiab study. Int J Obes. 2004;28(3):402–409.
- Nevill AM, Stewart AD, Olds T, et al. A new waist-to-height ratio predicts abdominal adiposity in adults. Res Sports Med. 2020;28(1):15–26.
- Vazquez G, Duval S, Jacobs DR, et al. Comparison of body mass index, waist circumference, and waist/hip ratio in predicting incident diabetes: a meta-analysis. Epidemiol Rev. 2007;29(1):115–128.
- Sina M, Hoy WE, Callaway L, et al. The associations of anthropometric measurements with subsequent gestational diabetes in aboriginal women. Obes Res Clin Pract. 2015;9(5):499–506.
- Jayawardana R, Ranasinghe P, Sheriff MH, et al. Waist to height ratio: a better anthropometric marker of diabetes and cardio-metabolic risks in South Asian adults. Diabetes Res Clin Pract. 2013;99(3):292–299.
- Zhang F, Wan Q, Cao H, et al. Identical anthropometric characteristics of impaired fasting glucose combined with impaired glucose tolerance and newly diagnosed type 2 diabetes: anthropometric indicators to predict hyperglycaemia in a community-based prospective cohort study in southwest China. BMJ Open. 2018;8(5):e019735.
- Resnick HE, Valsania P, Halter JB, et al. Differential effects of BMI on diabetes risk among black and white Americans. Diabetes Care. 1998;21(11):1828–1835.
- Abate N, Chandalia M. The impact of ethnicity on type 2 diabetes. J Diabetes Complications. 2003;17(1):39–58.
- Nakagami T, Qiao Q, Carstensen B, et al. Age, body mass index and type 2 diabetes-associations modified by ethnicity. Diabetologia. 2003;46(8):1063–1070.
- Razak F, Anand S, Vuksan V, et al. Ethnic differences in the relationships between obesity and glucose-metabolic abnormalities: a cross-sectional population-based study. Int J Obes. 2005;29(6):656–667.