Abstract
Background
Respiratory distress syndrome (RDS) is a common critical lung disease in newborn infants, especially those in premature infants with higher mortality rate. Early and correct diagnosis is the key to improve its prognosis. Previously, the diagnosis of RDS mainly relied on chest X-ray (CXR) findings, and it has been graded into four stages based on the progression and severity of CXR changes. This traditional diagnosing and grading method may lead to high misdiagnosis rate or delayed diagnosis. Recently, using ultrasound to diagnose neonatal lung diseases and RDS is becoming increasingly popular, and the technology is gaining higher sensitivity and higher specificity. The management of RDS under lung ultrasound (LUS) monitoring has achieved significant results, reducing the misdiagnosis rate of RDS, thereby reducing the probability of mechanical ventilation and the use of exogenous pulmonary surfactant, and making the success rate of treatment of RDS up to 100%.
Objective
The purpose of the article was to introduce the ultrasound grading methods and criteria of RDS, in order to promote the application of LUS in the diagnosis and treatment of RDS.
Methods
Literature (in English and Chinese) on the use of ultrasound in the diagnosis of neonatal RDS between 2008 and 2022 was selected for inclusion in this study.
Results
From the collected literature, the use of ultrasound in the diagnosis of RDS is increasing, and people's understanding of the ultrasound imaging findings of RDS is also changing. Among them, the research on ultrasound grading of RDS is the latest progress.
Conclusion
Ultrasound is accurate and reliable in the diagnosis and differential diagnosis of RDS. It is of great clinical value to master the ultrasound diagnosis and grading criteria of RDS.
1. Introduction
Neonatal respiratory distress syndrome (RDS) is one kind of severe pulmonary disease, caused by immature lung development and primary or secondary pulmonary surfactant (PS) deficiency for a variety of reasons, which result in the formation of an eosinophilic hyaline membrane and the atelectasis of the lungs from the alveolar wall to the terminal bronchiole wall; therefore, it is also called “hyaline membrane disease” (HMD) according to its pathological changes. As the result, progressive dyspnea, expiratory grunting, cyanosis and respiratory failure occur shortly after birth. The high incidence, rapid progression and high mortality of RDS not only cause serious harm to premature infants but also damage the health of full-term infants [Citation1–3]. According to literature, the incidence of RDS accounts for 1.72–8.2% of all live births in underdeveloped countries, including 23.8–37.3% of premature infants and 0.11% of full-term infants [Citation4–6]. The incidence of RDS was higher as gestational age decreased, occurring among 100% of infants at a gestational age of ≤26 weeks, 57.1% among those at ∼32 weeks and 3.7% among those at ∼36 weeks [Citation4]. In developed countries, such as the United States, it has been reported that the incidence of RDS among infants at gestational age of 34 weeks or less is 26% [Citation7], while, in the neonatal intensive care unit (NICU), full-term RDS accounted for 1.64–3.8% of the total hospitalized infants [Citation3,Citation6], and the mortality rate of full-term infants with RDS was 24 times higher than that of other hospitalized infants during the same period [Citation3]. RDS is also the common cause of neonatal pneumothorax, pulmonary hemorrhage, and persistent pulmonary hypertension in newborns (PPHN) [Citation8–10], all of which increase RDS mortality to 43.6%, while survivors also have an increased incidence of bronchopulmonary dysplasia (BPD) [Citation7,Citation8]. Therefore, early and accurate diagnosis and consequent adequate treatment are the keys to improving prognosis.
Recently, lung ultrasound (LUS) has been gradually used in the diagnosis of neonatal RDS, and it is even one of the earliest lung diseases diagnosed by ultrasound in the neonatal field. However, in the early stage, the study and understanding of LUS were not deep enough, and the understanding of ultrasound imaging characteristics of RDS may be wrong. Moreover, whether to perform ultrasound grading of RDS and how to do it have not been fully discussed. Our long-term research and clinical application experience in this field show that strengthening the correct understanding of the above problems will help to develop LUS better, and also help to guide the correct choice of clinical decision-making. The diagnosis and treatment of RDS under LUS monitoring have achieved remarkable results, significantly reducing the misdiagnosis rate of RDS, thereby reducing the probability of mechanical ventilation and the use of exogenous PS, and making the cure rate of RDS up to 100% [Citation11,Citation12]. To enable clinicians to better manage RDS with LUS, this article introduces the ultrasound grading criteria and methods of RDS in detail.
2. Current diagnosis methods of RDS
Traditionally, the diagnosis of RDS is based on typical history, clinical manifestations, arterial blood gas analysis and chest X-ray (CXR) findings [Citation1,Citation3,Citation6,Citation13]. CXR, in particular, is even considered the “Gold Standard” for the diagnosis of RDS. It has been graded to four different stages based on the progression and severity of CXR changes. Grade I: bilateral lung fields are generally opaque because of reduced aeration, with uniformly scattered fine granularity because of collapsed alveoli and reticular shadows caused by overaerated bronchioles. Grade II: in addition to the above findings, with more extensive air bronchograms extending into the middle-outer lung field. Grade III: lung field opacity increased significantly, the heart border and diaphragm edge are visible but blurred. Grade IV: the whole lung field diffuse opacification presents as “whiteout lung”, the heart border disappeared. However, since CXR is not specific for the diagnosis of different lung diseases, any lung disease can have the same or similar X-ray manifestations; not only RDS but also severe edema, pneumonia, pulmonary hemorrhage, and meconium aspiration syndrome (MAS) can lead to reticular shadows and “whiteout lung”. Therefore, CXR is not reliable in the diagnosis of RDS and even leads to a misdiagnosis rate as high as 62–77% for RDS [Citation14,Citation15]. In addition, X-ray exists inevitable radiation damage to the human body, including other infants and medical staff in the same ward, especially among developing premature infants and newborns, who are considerably more susceptible to ionizing radiation because of the rate at which their cells undergo mitosis is more rapid than that observed in adult populations [Citation16]. Increased radiosensitivity, greater mitotic activity, and a protracted period for consequences to manifest lead to a two- to -threefold higher risk of radiation-induced cancer per unit of dose among preterm infants than among the average population [Citation17]. A much recent long-term follow-up study showed that for every 10 mGy increase in the dose of radiation received in childhood, the risk of central nervous system tumors increased 1.05 times (95% CI: 1.01–1.09), and the risk of leukemia was increased by 1.17 times (95% CI: 1.09–1.26) [Citation18]. Therefore, it is imperative to find new methods that can replace X-ray to diagnose RDS.
Over the decade, using ultrasound to diagnose lung diseases has emerged and has been effectively applied in neonatal lung examination [Citation19–22], or even as the routine application or completely replacing the CXR in NICU [Citation23,Citation24]. A number of recently issued guidelines and consensuses can play an important role in promoting the application of LUS in the neonatal field [Citation25–28]. For neonatal RDS, it was also proven that using LUS to diagnose RDS is more accurate and reliable compared with CXR [Citation29–33].
3. The application of LUS in diagnosing RDS
3.1. Ultrasound has been successfully used in the diagnosis of RDS
From as early as 2008, Copetti et al. conducted a preliminary investigation on the using ultrasound to diagnose RDS and believes that ultrasound can be used for the diagnosis of RDS [Citation29], followed by Lovrenski [Citation30] and Liu et al. [Citation31], which further confirmed that diagnosing RDS by ultrasound has higher accuracy and specificity. Subsequently, the use of LUS in RDS diagnosis has become increasingly widespread [Citation32–41].
3.2. LUS diagnosing RDS with higher accuracy and reliability
Long-term clinical practice and increasing evidence have shown that the sensitivity and specificity of ultrasound in the diagnosis of RDS are significantly higher than those of CXR [Citation32,Citation33,Citation35,Citation40]. The evidence from systematic reviews and meta-analysis showed that the diagnostic sensitivity of LUS for RDS is 99–100%, and the specificity is 95–99% [Citation32,Citation33]. The radiation dosage, the body position and breathing movements of the patients have significant adverse effects on the results of CXR examinations leading to CXR is difficult to accurately reflect the lung lesions. Therefore, it is easy to lead to misdiagnosis due to different lung diseases that can have the same or similar X-ray findings.
4. The new terminologies for the diagnosis of RDS
The ultrasound terminologies related to RDS diagnosis and gradation, such as pleural line and lung sliding, A-line, lung point, double lung point (DLP), B-line, confluent B-line, compact B-lines, white lung as well as bamboo sign, etc., have been described in detail in the literature [Citation25,Citation26]; therefore, only two ultrasound terms need to be described more detailedly here, which are groundglass opacity signs (GOS) and snowflake signs (SFS).
4.1. Groundglass opacity signs
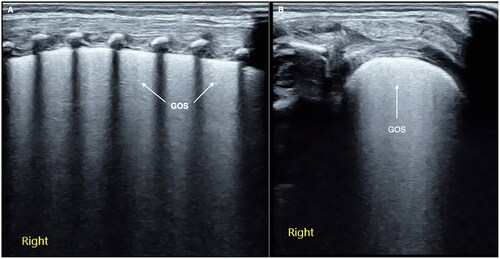
Groundglass opacity signs is one kind of mild lung consolidation with no obvious air-bronchograms, the echogenicity in the near-field is higher and then gradually decreases from shallow to deep field, which looks-like groundglass opacity was known as GOS [Citation42,Citation43] (). GOS is a characteristic ultrasound change in early stage (that is grade I) of RDS, which is easier to show when the probe is scanned in parallel along the intercostal space. For beginners or inexperienced doctors, GOS is easy to be mistaken for confluent B-lines or compact B-lines, which needs to be distinguished.
Figure 1. GOS. Perpendicular scanning shows that the pleural line is thickened and blurred, the echogenicity in the near-field is enhanced, and the echogenicity in the far-field is significantly weakened, looking like groundglass opacity (A). Parallel scanning shows that the ground glass-like performance is more obvious in the near field (B). GOS is a characteristic ultrasound finding of grade I RDS.
4.2. Snowflake signs
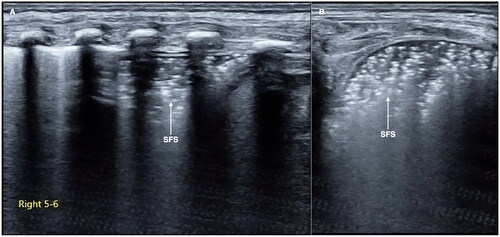
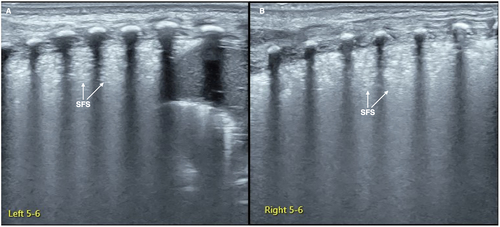
Snowflake signs is one kind of lung consolidation with no significant hepatoid change but with obvious air-bronchograms. SFS is characterized by some obvious spot, patches, or thin-line air-bronchograms in the consolidated areas, forming a snowflake-like manifestation under the ultrasound, so it is known as SFS [Citation42,Citation43] (). SFS is a characteristic ultrasound finding of progressive stage (grade II or above) RDS.
Figure 2. SFS. On the B-mode ultrasound, we can see a kind of specific lung consolidation, which is characterized by an obvious spot and patchy or thin line air-bronchograms in the lesion area, forming a snowflake-like manifestation, known as SFS. SFS is a characteristic ultrasound finding of grade II and above RDS.
5. Ultrasound characteristics and diagnostic criteria of RDS
The related issues, including the instrument and probe selection, parameter adjustment, scanning method, etc., should follow the published guidelines and specifications [Citation25–28]. In general, as with other lung diseases, the diagnosis of RDS by ultrasound requires the use of a high-end ultrasound equipment with a high-frequency (>10.0 MHz) linear transducer, otherwise, it may be misdiagnosed due to poor resolution [Citation28]. The transducer should perpendicular or parallel to the ribs when scanning [Citation25,Citation28]. In general, scanning with the probe perpendicular to the ribs can make a definite diagnosis in most cases, but scanning with the probe parallel to the intercostal space is more helpful to detect GOS in mild RDS.
5.1. Normal lung ultrasound imaging of newborn infants
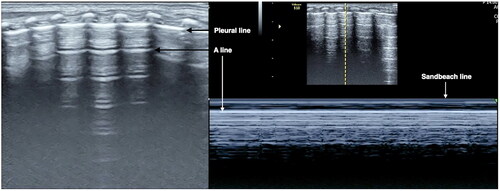
Normal newborn lungs show bamboo signs in two-dimensional gray scale mode (B-mode), sandy beach signs on M-mode ultrasound and lung sliding under real-time ultrasound. Newborns within seven days of birth can see a little B lines, seven days later can still has B lines is belong to abnormal [Citation25,Citation26] ().
5.2. Ultrasound diagnosis criteria for RDS
As mentioned above, LUS has higher accuracy, reliability, and specificity in the diagnosis of RDS compared with CXR. The diagnosis of RDS by LUS is mainly based on the following characteristics and criteria [Citation25,Citation32,Citation42–44].
Lung consolidation accompanied by air-bronchograms: Lung consolidation is the most important and necessary sign in the diagnosis of RDS by ultrasound. The degree of consolidation is related to the disease severity, which is characterized by the following features: (1) early-stage or mild RDS can be characterized by the GOS. It is easier to find this kind of minor lesion when parallel-scanning during the examination (). (2) Progress-stage or severe RDS is characterized by SFS [Citation42–44] (). SFS-like lung consolidation is the most characteristics of RDS and the ultrasound imaging performance. (3) Lung consolidation in infants with mild RDS is limited to the pleural, involving within a small range and being localized. Conversely, consolidation areas may extend to deeper and larger parts of the lung fields in more severe RDS and even lead to large area of atelectasis. (4) Usually, the consolidation areas are bilaterally visible in different lung fields. Nevertheless, they may be limited to certain intercostal spaces on one side of the lungs.
The pleural line is abnormal and the A-lines disappear. The pleural line abnormality can manifest as the thickened pleural line, being interrupted or disappeared. Abnormal pleural line and A-line disappearance were found in almost all RDS patients.
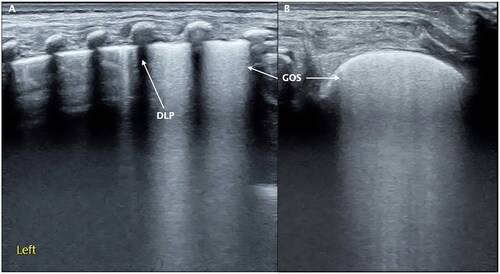
Double lung point: There can be a DLP during acute-stage of mild RDS or the recovery stage of severe RDS [Citation25,Citation42]. The DLP has been believed to reveal specificity and sensitivity ultrasound signs of transient tachypnea of the neonate (TTN) [Citation45]. This sign was later found to appear in only about 50% of TTN patients and was also present in other lung diseases, such as RDS and other lung diseases [Citation42,Citation46,Citation47].
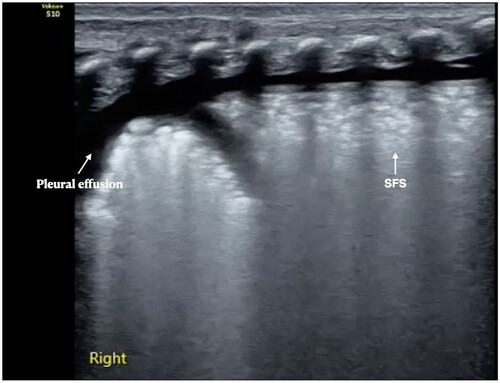
Pleural effusion: About 20% of patients may have different degrees of unilateral or bilateral pleural effusion [Citation31]. Pleural effusion is a new recognition of neonatal RDS after the development of LUS.
The inconsistencies in the lesion degree and pathological properties of different parts of the lung: LUS studies have proved that the lesion degree and pathological properties may be different in different fields of the bilateral lungs or even on the same side of the lung (e.g. one side of the lung has consolidations, while the other side does not have this pathological lesion, or one lung field of the same side of the lung shows consolidation, while the other lung fields display edema or pleural effusion).
In the past era, which relied on X-ray for diagnosis of lung diseases, it was never documented that pleural effusion could be present in neonates with RDS, and it was considered that the degree and characterization of bilateral lung lesions in RDS were homogenous. However, the development of LUS has changed our traditional concept of lung diseases, like RDS.
6. Ultrasound grading criteria and methods for RDS
There is no difficulty in diagnosing typical RDS using LUS, but it is still easy to misdiagnose early-stage or mild RDS, that is, to mistake mild or early-stage RDS for TTN, especially for inexperienced doctors. As the latter is a benign, self-limited condition and, generally, no special treatment is required, this misdiagnosis will inevitably have adverse consequences for the infant patients. To facilitate the management of RDS, according to the results of our multicenter prospective study [Citation42], it is necessary to classify RDS according to the ultrasound findings and the presence of severe complications. The diagnosis and management of RDS under ultrasound monitoring has achieved remarkable results [Citation11,Citation12]: (1) the misdiagnosis of RDS was avoided. (2) The dose of exogenous PS was reduced. (3) It can reduce the probability of ventilator use and shorten the duration of mechanical ventilation. (4) The occurrence of BPD was avoided. (5) It can shorten the hospitalization time of infants and save hospitalization expenses.
6.1. Grade I RDS
Grade I RDS is the mild RDS or the early stage of RDS, in which the lung consolidation presents as GOS-like consolidation on ultrasound [Citation42,Citation43] ( and ).
6.2. Grade II RDS
Grade II RDS is moderate RDS in which the lung consolidation presents as SFS-like consolidation on ultrasound but does not yet involve all of the lung fields [Citation42,Citation43]. It means that SFS can involve one to two intercostal spaces and can also involve 11 lung regions, but it has not yet involved 12 lung regions ( and ).
6.3. Grade III RDS
Grade III RDS is the severe RDS. Those with any of the following criteria belong to this category [Citation42,Citation43]: (1) the SFS-like lung consolidation has already involved all of the lung fields, that is 12 regions of the lung on ultrasound (). (2) The SFS-like lung consolidation does not involve all of the lung fields but has already led to serious complications, such as pulmonary hemorrhage and/or pneumothorax and/or PPHN and/or a large area of atelectasis, etc. ( and ).
Figure 6. Grade III RDS-SFS-like consolidation invoked all of the lung fields. An infant with a gestational age of 29 weeks and birth weight of 1210 g experienced serious respiratory distress shortly after birth. LUS showed that the SFS-like lung consolidation involved all of the lung fields of the bilateral lungs, caused by grade III (i.e. severe) RDS.
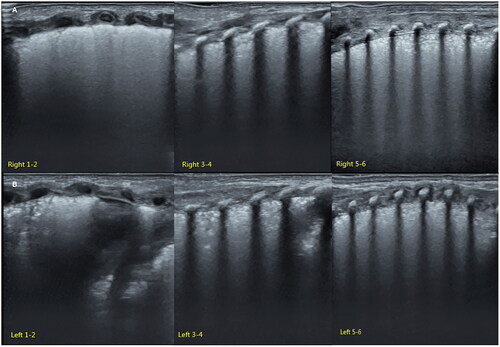
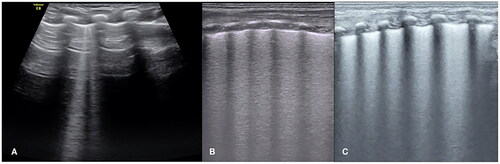
Figure 7. Grade III RDS: RDS result in pneumothorax. An infant with a gestational age of 28 weeks and birth weight of 1020 g experienced serious respiratory distress shortly after birth. LUS showed an SFS-like lung consolidation in his left lung (A). Bamboo signs on B-mode ultrasound (B) and stratosphere signs on M-mode ultrasound (C) in his right lung. Lung sliding disappeared in real-time ultrasound.
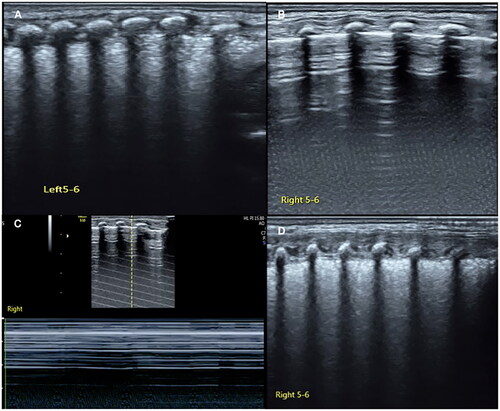
Figure 8. RDS complicated with pulmonary hemorrhage. A term RDS patient, bloody fluid emerging from the endotracheal tube during ventilator treatment. LUS examination revealed SFS-like lung consolidation with significant pleural effusion. The bloody fluid was withdrawn by thoracentesis. It was confirmed pulmonary hemorrhage due to RDS.
7. The distinguish of RDS from TTN and other lung diseases
7.1. The identification of RDS and TTN
Both RDS and TTN are common causes of neonatal dyspnea. They may have similar clinical manifestations, blood gas changes and CXR findings, which make it difficult to distinguish them from each other [Citation48]. This leads to a misdiagnosis rate as high as 62–77% [Citation14,Citation15]. LUS, however, can distinguish them very easily and clearly. According to our long-term clinical practice and the literature, TTN mainly manifests as various degrees of lung edema, while RDS mainly manifests as various degrees of lung consolidations [Citation33,Citation46–52], which can avoid misdiagnosis and mistreatment. Therefore, the identification of RDS and TTN is truly the identification of GOS and confluent B-lines.
For beginners and less experienced doctors, it is easy to mistake GOS for B-lines, especially the confluent B-lines. The following attributes help to distinguish them from each other: (1) echogenicity fading: there is a significant echogenicity fading in the lung fields from shallow field to deep field of GOS on ultrasound, while the echogenicity of B-lines or confluent B-lines shows no fading. (2) Pathologically: B-lines and confluent B-lines represent lung edema, while GOS is a kind of mild lung consolidation. (3) Parallel scanning is more helpful to distinguish these two signs ().
7.2. The identification of RDS and other lung diseases with lung consolidation
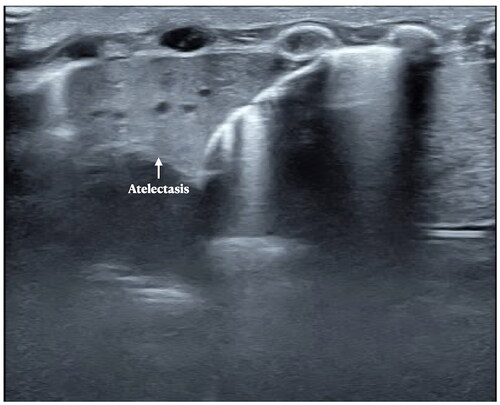
Several kinds of lung diseases, such as pneumonia, MAS, pulmonary hemorrhage, atelectasis, etc., can also have significant respiratory difficulty, they are easy to be misdiagnosed in clinics and X-rays; however, LUS can distinguish them easily. Although lung consolidations are also the main LUS findings of other kinds of lung diseases such as pneumonia, MAS, and pulmonary hemorrhage [Citation53–57], etc., as noted above, the DOS- and SFS-like consolidation are the specific ultrasound signs of RDS that are not present in other diseases [Citation42,Citation43,Citation53–57]. In pneumonia, MAS or pulmonary hemorrhage, lung consolidation usually manifests as a typical hepatoid change, with irregular and serrated boundaries, which is easy to identify under ultrasound (). In atelectasis, however, the boundaries of consolidation are more regular (), while in severe atelectasis, lung pulsed can be detected by real-time ultrasound.
Figure 10. LUS manifestations in pneumonia. Patient with pneumonia. LUS showed large area lung consolidation with air – air-bronchograms, as well as irregular and serrated boundaries. We can see the consolidation is much different from that in RDS patients. The ultrasound findings of MAS were similar to those of pneumonia. Their identification needs to be combined with medical history, such as the time of onset, the presence of meconium contamination of amniotic fluid, etc.
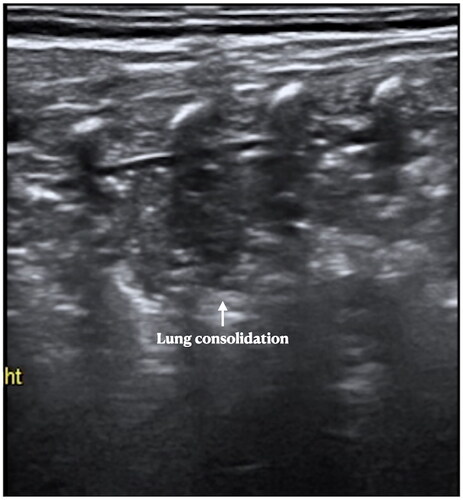
Figure 11. LUS manifestations in pneumonia. Patient with atelectasis. LUS showed significant lung consolidation with regular boundaries. We can see also the consolidation is much different from that in RDS patients and pneumonia. Atelectasis is not an independent disease, but a complication of other lung diseases such as pneumonia, MAS, RDS, etc.
8. Conclusions
In conclusion, LUS is of great value not only in the diagnosis and differential diagnosis of RDS but also in the evaluation and observation of dynamic changes of RDS due to its convenient operation at bedside, which is more conducive to the correct and timely management of infants with RDS. We also introduce the grading criteria and methods of RDS by ultrasound for the first time in English papers, it is hoped that this method can be applied in clinical practice, and its rationality and scientificity can be further evaluated through more high-quality research.
It is important to emphasize that during scanning practice, it is not appropriate to establish a subjective relationship between LUS and X-ray gradation because they are different evaluation systems and have no corresponding relationship between them.
Author contributions
Dr. JL contributed to the study conception, ultrasound examination, and literature collection and analysis, and wrote and approved the manuscript.
Ethical approval
Not applicable.
Consent form
Not applicable.
Disclosure statement
No potential conflict of interest was reported by the author.
Data availability statement
The data set used and analyzed are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Rubarth LB, Quinn J. Respiratory development and respiratory distress syndrome. Neonatal Netw. 2015;34(4):231–238.
- Chowdhury N, Louise Giles B, Dell SD. Full-term neonatal respiratory distress and chronic lung disease. Pediatr Ann. 2019;48(4):e175–e181.
- Liu J, Shi Y, Dong JY, et al. Clinical characteristics, diagnosis and management of respiratory distress syndrome in full-term neonates. Chin Med J. 2010;123(19):2640–2644.
- Ghafoor T, Mahmud S, Ali S, et al. Incidence of respiratory distress syndrome. J Coll Physicians Surg Pak. 2003;13(5):271–273.
- Jaberi E, Roksana M. A study on preterm births during 2013–2015, Shiraz, Iran. J Obstet Gynaecol. 2018;38(1):22–26.
- Alfarwati TW, Alamri AA, Alshahrani MA, et al. Incidence, risk factors and outcome of respiratory distress syndrome in term infants at academic centre, Jeddah, Saudi Arabia. Med Arch. 2019;73(3):183–186.
- Donda K, Vijayakanthi N, Dapaah-Siakwan F, et al. Trends in epidemiology and outcomes of respiratory distress syndrome in the United States. Pediatr Pulmonol. 2019;54(4):405–414.
- Lee M, Wu K, Yu A, et al. Pulmonary hemorrhage in neonatal respiratory distress syndrome: radiographic evolution, course, complications and long-term clinical outcomes. J Neonatal Perinatal Med. 2019;12(2):161–171.
- Vibede L, Vibede E, Bendtsen M, et al. Neonatal pneumothorax: a descriptive regional Danish study. Neonatology. 2017;111(4):303–308.
- Apiliogullari B, Sunam GS, Ceran S, et al. Evaluation of neonatal pneumothorax. J Int Med Res. 2011;39(6):2436–2440.
- Liu J, Zhang X, Wang Y, et al. The outcome- or cost-effectiveness analysis of LUS-based care or CXR-based care of neonatal lung diseases: the clinical practice evidence from a level III NICU in China. Diagnostics. 2022;12(11):2790.
- Liu J, Fu W, Qin SJ. Lung ultrasound to guide the administration of exogenous pulmonary surfactant in respiratory distress syndrome of newborn infants: a retrospective investigation study. Front Pediatr. 2022;10:952315.
- Liszewski MC, Stanescu AL, Phillips GS, et al. Respiratory distress in neonates: underlying causes and current imaging assessment. Radiol Clin North Am. 2017;55(4):629–644.
- Brice JE, Walker CH. Changing pattern of respiratory distress in newborn. Lancet. 1977;2(8041):752–754.
- Rocha G, Rodrigues M, Guimarães H. Respiratory distress syndrome of the preterm neonate—placenta and necropsy as witnesses. J Matern Fetal Neonat Med. 2011;24(1):148–151.
- Bahreyni Toossi MT, Malekzadeh M. Radiation dose to newborns in neonatal intensive care units. Iran J Radiol. 2012;9(3):145–149.
- Linet MS, Kim KP, Rajaraman P. Children’s exposure to diagnostic medical radiation and cancer risk: epidemiologic and dosimetric considerations. Pediatr Radiol. 2009;39(S1):4–26.
- Foucault A, Ancelet S, Dreuil S, et al. Childhood cancer risks estimates following CT scans: an update of the French CT cohort study. Eur Radiol. 2022;32(8):5491–5498.
- Liu J. Lung ultrasonography for the diagnosis of neonatal lung disease. J Matern Fetal Neonat Med. 2014;27(8):856–861.
- Sharma D, Farahbakhsh N. Role of chest ultrasound in neonatal lung disease: a review of current evidences. J Matern Fetal Neonat Med. 2019;32(2):310–316.
- Miller LE, Stoller JZ, Fraga MV. Point-of-care ultrasound in the neonatal ICU. Curr Opin Pediatr. 2020;32(2):216–227.
- De Luca D. Respiratory distress syndrome in preterm neonates in the era of precision medicine: a modern critical care-based approach. Pediatr Neonatol. 2021;62:S3–S9.
- Chen SW, Fu W, Liu J, et al. Routine application of lung ultrasonography in the neonatal intensive care unit. Medicine. 2017;96(2):e5826.
- Gao YQ, Qiu RX, Liu J, et al. Lung ultrasound completely replaced chest X-ray for diagnosing neonatal lung diseases: a 3-year clinical practice report from a neonatal intensive care unit in China. J Matern Fetal Neonat Med. 2022;35(18):3565–3572.
- Liu J, Copetti R, Sorantin E, et al. Protocol and guidelines for point-of-care lung ultrasound in diagnosing neonatal pulmonary diseases based on international expert consensus. J Vis Exp. 2019;145:e58990.
- Liu J, Kurepa D, Feletti F, et al. International expert consensus and recommendations for neonatal pneumothorax ultrasound diagnosis and ultrasound-guided thoracentesis procedure. J Vis Exp. 2020;157(157):e60836.
- Singh Y, Tissot C, Fraga MV, et al. International evidence-based guidelines on point of care ultrasound (POCUS) for critically ill neonates and children issued by the POCUS Working Group of the European Society of Paediatric and Neonatal Intensive Care (ESPNIC). Crit Care. 2020;24(1):65.
- Liu J, Guo G, Kurepa D, et al. Specification and guideline for technical aspects and scanning parameter settings of neonatal lung ultrasound examination. J Matern Fetal Neonat Med. 2022;35(5):1003–1016.
- Copetti R, Cattarossi L, Macagno F, et al. Lung ultrasound in respiratory distress syndrome: a useful tool for early diagnosis. Neonatology. 2008;94(1):52–59.
- Lovrenski J. Lung ultrasonography of pulmonary complications in preterm infants with respiratory distress syndrome. Ups J Med Sci. 2012;117(1):10–17.
- Liu J, Cao HY, Wang HW, et al. The role of lung ultrasound in diagnosis of respiratory distress syndrome in newborn infants. Iran J Pediatr. 2015;25(1):e323.
- Ma HR, Liu J, Yan WK. Diagnostic value of lung ultrasound for neonatal respiratory distress syndrome: a meta-analysis and systematic review. Med Ultrason. 2020;22(3):325–333.
- Wu J, Wang Y, Zhao A, et al. Lung ultrasound for the diagnosis of neonatal respiratory distress syndrome: a meta-analysis. Ultrasound Q. 2020;36(2):102–110.
- Elsayed YN. Lung ultrasound as a new technique for diagnosis of neonatal respiratory diseases. Neonatal Netw. 2018;37(4):224–232.
- Liu J, Cao HY, Wang XL, et al. The significance and the necessity of routinely performing lung ultrasound in the neonatal intensive care units. J Matern Fetal Neonat Med. 2016;29(24):4025–4030.
- Corsini I, Parri N, Ficial B, et al. Lung ultrasound in the neonatal intensive care unit: review of the literature and future perspectives. Pediatr Pulmonol. 2020;55(7):1550–1562.
- Migliaro F, Salomè S, Corsini I, et al. Neonatal lung ultrasound: from paradox to diagnosis and beyond. Early Hum Dev. 2020;150:105184.
- Mazmanyan P, Kerobyan V, Shankar-Aguilera S, et al. Introduction of point-of-care neonatal lung ultrasound in a developing country. Eur J Pediatr. 2020;179(7):1131–1137.
- Gregorio-Hernández R, Arriaga-Redondo M, Pérez-Pérez A, et al. Lung ultrasound in preterm infants with respiratory distress: experience in a neonatal intensive care unit. Eur J Pediatr. 2020;179(1):81–89.
- Oktem A, Yigit S, Oğuz B, et al. Accuracy of lung ultrasonography in the diagnosis of respiratory distress syndrome in newborns. J Matern Fetal Neonat Med. 2021;34(2):281–286.
- Louis D, Belen K, Farooqui M, et al. Prone versus supine position for lung ultrasound in neonates with respiratory distress. Am J Perinatol. 2021;38(2):176–181.
- Liu J, Li J, Shan RY, et al. Ultrasound diagnosis and grading of neonatal respiratory distress syndrome: a multicenter prospective study. Chin Pediatr Emerg Med. 2020;27(11):801–807.
- The Society of Pediatrics, Asia-Pacific Health Association; The Division of Critical Ultrasound, Society of Pediatrics, Asia-Pacific Health Association; The World Interactive Network Focused On Critical Ultrasound China Branch; The Critical Ultrasound Group of Neonatal Specialty Committee; the Cross¯Straits Medicine Exchange Association; The Ultrasound Diagnostic Group of Neonatal Specialty Committee; Chinese Maternal and Child Health Association; Editorial Board of Chinese Pediatric Emergency Medicine. Expert consensus on ultrasound diagnosis and gradation of neonatal respiratory distress syndrome. Chin Pediatr Emerg Med. 2021;28(7):545–551.
- Liu J, Qiu RX, Ren XL. The ultrasonic imaging characteristics of neonatal respiratory distress syndrome (RDS): the new concept of lung ultrasound to diagnose RDS. Chest. 2020;157(6):A318.
- Copetti R, Cattarossi L. The ‘double lung point’: an ultrasound sign diagnostic of transient tachypnea of the newborn. Neonatology. 2007;91(3):203–209.
- Liu J, Chen XX, Li XW, et al. Lung ultrasonography to diagnose transient tachypnea of the newborn. Chest. 2016;149(5):1269–1275.
- Raimondi F, Yousef N, Rodriguez-Fanjul J, et al. A multicenter lung ultrasound study on transient tachypnea of the neonate. Neonatology. 2019;115(3):263–268.
- Alhassen Z, Vali P, Guglani L, et al. Recent advances in pathophysiology and management of transient tachypnea of newborn. J Perinatol. 2021;41(1):6–16.
- Liu J, Wang Y, Fu W, et al. Diagnosis of neonatal transient tachypnea and its differentiation from respiratory distress syndrome using lung ultrasound. Medicine. 2014;93(27):e197.
- Ibrahim M, Omran A, AbdAllah NB, et al. Lung ultrasound in early diagnosis of neonatal transient tachypnea and its differentiation from other causes of neonatal respiratory distress. J Neonatal Perinatal Med. 2018;11(3):281–287.
- Liu J, Qiu RX, Ren XL, et al. The differentiation diagnosis of respiratory distress syndrome and transient tachypnea of the newborn by lung ultrasound. Chest. 2019;155(4):232A.
- Ma HR, Liu J, Yan WK. Accuracy and reliability of lung ultrasound to diagnose transient tachypnoea of the newborn: evidence from a meta-analysis and systematic review. Am J Perinatol. 2022;39(9):973–979.
- Liu J, Ma HR, Fu W. Lung ultrasound to diagnose pneumonia in neonates with fungal infection. Diagnostics. 2022;12(8):1776.
- Ma HR, Deng BY, Liu J, et al. Lung ultrasound to diagnose infectious pneumonia of newborns: a prospective multicenter study. Pediatr Pulmonol. 2023;58(1):122–129.
- Liu J, Cao HY, Fu W. Lung ultrasonography to diagnose meconium aspiration syndrome of the newborn. J Int Med Res. 2016;44(6):1534–1542.
- Ren XL, Fu W, Liu J, et al. Lung ultrasonography to diagnose pulmonary hemorrhage of the newborn. J Matern Fetal Neonat Med. 2017;30(21):2601–2606.
- Liu J, Chen SW, Liu F, et al. The diagnosis of neonatal pulmonary atelectasis using lung ultrasonography. Chest. 2015;147(4):1013–1019.