Abstract
Objective
This study’s aim was to determine the prevalence of chromosomal anomalies in fetuses with isolated and non-isolated aberrant right subclavian artery (ARSA) and to evaluate its association with other congenital anomalies.
Methods
From September 2018 to October 2021, 668 ARSA cases were diagnosed by prenatal ultrasound in our hospital; cases with missed visits and a lack of chromosomal findings were excluded and 363 cases were eligible for enrollment. General information, ultrasound presentation, chromosomal findings and pregnancy outcomes were retrospectively analyzed.
Results
Among the 363 cases, 296 were isolated, and 67 were associated with structural abnormalities or soft marker abnormalities. The proportion of fetuses with chromosomal abnormalities in the isolated ARSA group was significantly lower than that in the non-isolated ARSA group (p < .001). In the non-isolated ARSA group, 22 cases were combined with other soft marker abnormalities and 45 cases were combined with structural abnormalities. The most frequent structural abnormality coexisting with ARSA was cardiac malformations (38.81%).
Conclusion
The most common combined malformation in ARSA is intracardiac malformation. Isolated ARSA has a low risk of chromosomal abnormalities, so invasive chromosomal testing is not recommended. Non-isolated ARSA has a high incidence of chromosomal abnormalities, so early karyotyping should be recommended.
Introduction
An aberrant right subclavian artery (ARSA) is the most common congenital aortic arch anomaly. Usually, it does not cause any clinical symptoms, but in rare cases, it can cause dysphagia or chronic cough upon pressing the esophagus or trachea. It has been reported that ARSA is associated with chromosomal abnormalities such as trisomy 21, 22q11.2 microdeletion syndrome, and Turner syndrome [Citation1–4]. Even in chromosomally normal fetuses, the possibility of other associated fetal structural abnormalities in fetuses associated with ARSA remains, and cardiac anomalies are the most frequently reported structural abnormalities coexisting with ARSA. We retrospectively analyzed the clinical data, prenatal ultrasound characteristics, genetic results, and clinical outcomes of these 363 fetuses to assess the association between fetal chromosomal abnormalities and structural abnormalities with ARSA.
Methods
We conducted a retrospective study on ARSA cases diagnosed by prenatal ultrasound in Chongqing Health Center for Women and Children from September 2018 to October 2021. Examinations were performed using a Voluson E6, E8, E10 ultrasound system (RAD 4-8-D probe, GE Medical Systems, Milwaukee, WI, USA). All ARSA fetuses were diagnosed by at least two physicians (with more than 5 years of experience in prenatal ultrasound diagnosis) and confirmed by at least one follow-up examination. Each pregnant woman signed an informed consent form. Hospital ethical approval was obtained for the study design. In all cases, screening for chromosomal defects had been undertaken by a combination of maternal age, fetal nuchal translucency (NT) thickness, maternal serum-free beta-human chorionic gonadotropin (β-hCG), and pregnancy-associated plasma protein-A (PAPP-A) at 11 to 13 + 6 weeks.
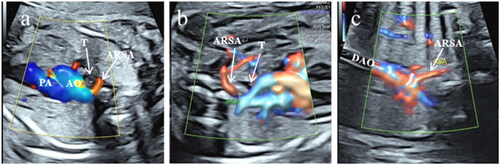
Each pregnant woman was scanned in the supine or lateral position. The ultrasonic diagnosis of ARSA was as follows. (1) In the three-vessel and tracheal view, ARSA was detected as an additional vessel arising from the junction of the aortic arch and ductus arteriosus, and color Doppler showed a "C"-shaped vascular ring (). (2) After the three-vessel and tracheal view was visualized, the probe was swept cephalically to display the bilateral subclavian artery sections. The color Doppler velocity was adjusted downward to properly reveal the subclavian arteries. The course of the normal left and right subclavian arteries was curved and symmetrical, and the right subclavian artery normally crossed anterior to the trachea and immediately adjacent to the right innominate vein. The gap between the right subclavian artery and the right innominate vein is relatively widened during ARSA, and the right subclavian artery travels straight and lies posterior to the trachea traveling to the right shoulder (). (3) Coronal view of the aortic arch shows the right subclavian artery originating from the beginning of the descending aorta and proceeding toward the right shoulder ().
Figure 1. Color Doppler ultrasound images of fetal aberrant right subclavian artery (ARSA). (a) In the three-vessel and tracheal view, ARSA was detected as an additional vessel arising from the junction of the aortic arch and ductus arteriosus, and color Doppler showed a "C-shaped” vascular ring. (b) In the bilateral subclavian artery sections, the right subclavian artery travels straight and lies posterior to the trachea during ARSA. (c) Coronal view of the aortic arch showing the right subclavian artery originating from the beginning of the descending aorta and proceeding toward the right shoulder. PA: pulmonary artery, AO: aorta, T: trachea, DAO: descending aorta.
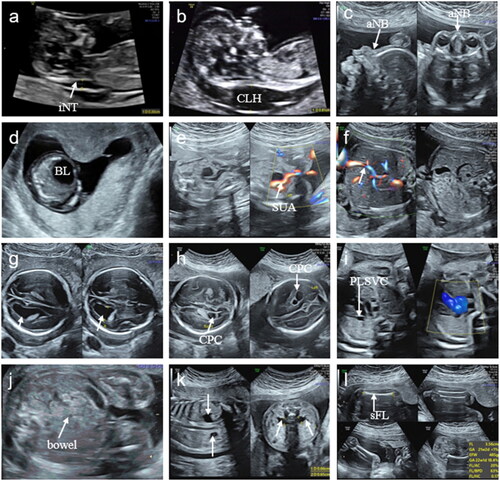
The fetuses were categorized as having an isolated aberrant right subclavian artery (iARSA) and a non-isolated aberrant right subclavian artery (niARSA). iARSA was defined as ARSA in a fetus with no other structural abnormalities or soft marker abnormalities, except for ARSA during pregnancy and postnatal by ultrasound and MRI. niARSA was defined based on prenatal and postnatal ultrasound or MRI findings, postnatal physical examination, and autopsy findings. niARSA was subdivided into ARSA combined with other soft marker abnormalities, ARSA combined with a single malformation, and ARSA combined with multiple malformations. Other soft markers combined in our study included NT/nuchal fold (NF)thickening, absent or hypoplastic nasal bone, cervical lymphatic hygroma, megalocystis, mild ventriculomegaly, single umbilical artery, persistent left superior vena cava, persistent right umbilical vein, choroid plexus cyst, echogenic bowel, short femur and humerus, and mild pyelectasis ().
Figure 2. Soft markers combined in this study (a) NT thickening. (b) cervical lymphatic hygroma. (c) absent nasal bone. (d) megalocystis. (e) single umbilical artery. (f) persistent right umbilical vein(arrow).(g) mild ventriculomegaly(arrow). (h) choroid plexus cyst. (i) persistent left superior vena cava. (j) echogenic bowel. (k) mild pyelectasis (arrow). (l) short femur or humerus. iNT: increased NT, CLH: cervical lymphatic hygroma, aNB: absent nasal bone, BL: bladder, SUA: single umbilical artery, CPC: choroid plexus cyst, PLSVC: persistent left superior vena cava, sFL: short femur length.
All pregnant women with ARSA were advised to attend the prenatal genetic counseling clinic in our hospital or other prenatal diagnostic centers. The clinicians provided specific recommendations on the chromosomal testing modalities for the pregnant women based on the prenatal test results, maternal age, previous adverse maternal history, and their own wishes. In this study, there were 246 cases of noninvasive prenatal testing, 116 cases of amniocentesis and 1 case of fetal genetic testing after induction of labor.
The clinical data, ultrasound reports, prenatal diagnosis, and outcomes of each case were collected. If the patient did not attend follow-up visits, the follow-up data were obtained by a telephone interview. Statistical analyses were performed using SPSS 25.0, and the χ2 test was applied, with a p value < .05 considered significant.
Results
Over the study period of three years, a total of 668 fetuses with ARSA were diagnosed. Cases with missing chromosomal results or that were lost to follow-up were excluded, and 363 cases were included in this study. The mean maternal age was 29.7 ± 4.5 (range 20 to 46) years, and the mean gestational age at ultrasound assessment was 24 ± 2 (range 13 to 33+2) weeks.
There were 296 pregnant women in the iARSA group and 67 in the niARSA group. As shown in , the general information of the two groups was compared, and the difference was not statistically significant (all p > .05).
Table 1. Characteristics of cases with ARSA and niARSA.
The niARSA group was divided into 3 groups: 22 combined with other soft index abnormalities, 29 combined with a single structural abnormality, and 16 combined with multiple structural abnormalities. The total number of structural abnormalities was 67 sites, and the most common abnormality was in the cardiovascular system ().
Table 2. Structural anomalies in the niARSA.
Among the ARSA cases, the incidence of chromosomal abnormalities was 6.1% (22/363). Among them, there were 7 cases of numerical chromosomal aberrations (including 4 cases of trisomy 21 syndrome and 3 cases of 47, XNN karyotype) and 13 cases of structural chromosomal aberrations (including 5 cases of microduplication, 4 cases of microdeletion, 1 case of chimerism, 1 case of chromosomal heteromorphism, 1 case of heterozygous mutation, and 1 case of loss of heterozygosity). The remaining 2 patients were followed up by telephone, and the specific type of chromosomal abnormality was unknown. The distribution of chromosomal abnormalities in each specific group is shown in .
Table 3. Clinical characteristics, chromosomal findings and pregnancy outcomes of fetuses in the iARSA group with chromosomal abnormalities.
Table 4. Clinical characteristics, ultrasound findings, chromosomal findings and pregnancy outcomes of fetuses in the niARSA group with chromosomal abnormalities.
There were 10 cases (3.4%) of chromosomal abnormalities in the iARSA group and 12 cases (17.9%) in the niARSA group. The difference between the two groups was statistically significant (x2 = 13.69, p < .001). In the comparative analysis of the iARSA group and the subgroups of niARSA (), the incidence of chromosomal abnormalities increased with increasing group size, and a two-by-two comparison was performed to determine whether there was a significant difference between the niARSA combination plus multiple malformations and the iARSA group. The differences between the remaining groups were not statistically significant.
Table 5. Number of chromosomal abnormalities in each ARSA group.
Among all the ARSA fetuses, 334 were born, 28 were induced abortions (3 in the iARSA group and 25 in the niARSA group), and 1 was a 24-week refractory abortion. All live births survived with a good prognosis. There were 137 males and 217 females among all ARSA fetuses.
Discussion
The normal right subclavian artery originates from the innominate artery, which is the first branch of the aorta, and ARSA is defined as an abnormal right subclavian artery originating from the beginning of the descending aorta, as the fourth branch of the aortic arch, bypassing the posterior aspect of the trachea and proceeding toward the right shoulder [Citation5]. The prevalence of ARSA in the normal population has thus been reported to be approximately 0.4%–2% [Citation6–9]. In our study, the prenatal detection rate of ARSA was 1.11%, which is consistent with the results of other studies. While variations in sex distribution have been noted in different studies, some studies have pointed out that the incidence of ARSA in females is higher than that in males [Citation10,Citation11], while others have pointed out that the sex distribution of ARSA is equal [Citation9]. Nonetheless, our findings support a female-biased incidence for ARSA, noting a probable relationship between ARSA and fetal sex, with females being at a higher risk than males.
In recent years, ARSA has become prominent as an ultrasonographic marker of trisomy 21 syndrome. There have been many relevant studies on the prenatal ultrasonography of ARSA and its prevalence and correlation with trisomy 21 syndrome. Due to the risk of miscarriage with invasive chromosomal testing with methods such as amniocentesis [Citation12], for iARSA, the choice of chromosomal screening for fetuses remains a controversial issue.
In our study, the incidence of chromosomal abnormalities was significantly higher in the niARSA group than in the iARSA group. Chromosomal abnormalities in the iARSA group were microdeletions, microduplications, and sex chromosome abnormalities, among others. Loss-of-heterozygosity (LOH) events was observed in one case, with chromosomes 1, 6, and 16 being largely affected, which may be related to blood-related parents. There were no fetuses with trisomy 21 in the iARSA group. Morlando et al. [Citation13] and Borenstein et al. [Citation14] each reported one case of an iARSA fetus combined with trisomy 21. However, De León-Luis et al. [Citation15] noted that the sensitivity and specificity of ARSA as a soft marker of trisomy 21 were 31.8% and 99.3%, respectively; the overall positive likelihood ratio of ARSA was 52.6, and the positive likelihood ratios of iARSA and niARSA were 0 and 199. Another study reported that the positive and negative likelihood ratios of iARSA fetal prediction for trisomy 21 were 0.00 and 1.005, respectively [Citation16]. Borenstein et al. reported that [Citation17] in the first trimester of pregnancy effective screening for trisomy 21 is provided by a combination of fetal NT thickness and maternal serum free β-hCG and PAPP-A,with a detection rate of 90% for a false-positive rate of 5%, suggesting that ARSA in the first trimester is unlikely to be a useful marker of trisomy 21.Given the weak correlation between iARSA and trisomy 21 and the fact that trisomy 21 was not found in the iARSA fetuses in our study, iARSA was not recommended as an independent risk factor for invasive fetal chromosomal testing in the absence of a combination of advanced maternal age, abnormal serologic tests, or other abnormal clinical profiles. Options such as noninvasive genetic testing may be recommended depending on the financial situation of the pregnant woman to minimize missed serological screening.
There were 4 cases of trisomy 21 in the niARSA group in this study, with all 4 cases combined with an abnormal nasal bone (absent or hypoplastic nasal bone) and 2 cases combined with NT thickening. ARSA, nasal bone abnormalities, and NT thickening are the three most powerful independent ultrasound indicators of trisomy 21 [Citation8]. Therefore, for fetuses with niARSA, especially those with nasal bone abnormalities or NT/NF thickening, additional clinical attention should be given, and karyotyping is recommended.
In a large autopsy study, Zapata et al. [Citation9] reported that the prevalence of ARSA was 2.9% in patients with congenital heart disease (CHD) and 0.1% in patients without CHD. Moreover, several studies have suggested that the presence of ARSA may be associated with an increased incidence of intracardiac malformations [Citation14,Citation18]. Additionally, some studies have suggested that there is no significant correlation between ARSA and intracardiac malformations [Citation8,Citation19,Citation20]. In the present study, the most frequent structural abnormality coexisting with ARSA was cardiac malformations (38.81%), whose frequency was significantly higher than that of other structural malformations, and ventricular septal defects (14/26) were the most common. Therefore, the presence of other intra- and extracardiac malformations should be strongly considered when ARSA is detected, and for complex multiple cardiac malformations, careful scanning should be performed to prevent missed ARSA.
In the present study, there was one niARSA case complicated by chromosome 22q11 micro-deletion. Clinically, 22q11 microdeletion is present in the majority of patients with DiGeorge or velo-cardio-facial syndrome [Citation4]. The clinical features associated with 22q11.2 deletion include CHD, thymic hypoplasia, hypocalcemia, cleft palate, and other facial appearance. The most common CHD was conotruncal anomalies. The associated cardiac anomalies in our study were pulmonary atresia and ventricular septal defect. Barrea et al. [Citation21] suggested that prenatal thymus examination can be a valuable tool for the detection of 22q11 microdeletion. In fact, most but definitely not all cases of 22q11 microdeletion are associated with thymic defects [Citation22]. We believe that more prospective studies are needed to confirm the association between 22q11.2 micro-deletion and iARSA. For patients with ARSA combined with other intracardiac abnormalities, especially conotruncal abnormalities, evaluation of the fetal thymus is helpful for risk stratification of related chromosomal abnormalities and genetic counseling.
In summary, because iARSA is rarely associated with chromosomal abnormalities and has a good clinical prognosis, unnecessary induction of labor and invasive chromosomal testing should be avoided. In niARSA, the risk of chromosomal abnormalities is significantly higher, and pregnant women should be advised to undergo karyotyping and regular ultrasound follow-ups.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Chaoui R, Heling KS, Sarioglu N, et al. Aberrant right subclavian artery as a new cardiac sign in second- and third-trimester fetuses with down syndrome Am J Obstet Gynecol. 2005;192(1):257–263.
- Rauch R, Rauch A, Koch A, et al. Laterality of the aortic arch and anomalies of the subclavian artery-reliable indicators for 22q11.2 deletion syndromes?. Eur J Pediatr. 2004;163(11):642–645.
- Lee SH, Jung JM, Song MS, et al. Evaluation of cardiovascular anomalies in patients with asymptomatic turner syndrome using multidetector computed tomography. J Korean Med Sci. 2013;28(8):1169–1173.
- Volpe P, Marasini M, Caruso G, et al. 22q11 deletions in fetuses with malformations of the outflow tracts or interruption of the aortic arch: impact of additional ultrasound signs. Prenat Diagn. 2003;23(9):752–757.
- De León-Luis J, Bravo C, Gámez F, et al. Coronal view as a complementary ultrasound approach for prenatal diagnosis of aberrant right subclavian artery. Ultrasound Obstet Gynecol. 2012;40(3):370–371.
- Song MJ, Han BH, Kim YH, et al. Prenatal diagnosis of aberrant right subclavian artery in an unselected population. Ultrasonography. 2017;36(3):278–283.
- Ranzini AC, Hyman F, Jamaer E, et al. Aberrant right subclavian artery: correlation between fetal and neonatal abnormalities and abnormal genetic screening or testing. J Ultrasound Med. 2017;36(4):785–790.
- Paladini D, Sglavo G, Pastore G, et al. Aberrant right subclavian artery: incidence and correlation with other markers of down syndrome in second-trimester fetuses. Ultrasound Obstet Gynecol. 2012;39(2):191–195.
- Zapata H, Edwards JE, Titus JL. Aberrant right subclavian artery with left aortic arch: associated cardiac anomalies. Pediatr Cardiol. 1993;14(3):159–161.
- Polguj M, Chrzanowski Ł, Kasprzak JD, et al. The aberrant right subclavian artery (arteria lusoria): the morphological and clinical aspects of one of the most important variations—a systematic study of 141 reports. Sci World J. 2014;2014:292734.
- Rembouskos G, Passamonti U, Robertis VD, et al. Aberrant right subclavian artery (ARSA) in unselected population at first and second trimester ultrasonography. Prenat Diagn. 2012;32(10):968–975.
- Hyett J, Mogra R, Sonek J. First trimester ultrasound assessment for fetal aneuploidy. Clin Obstet Gynecol. 2014;57(1):142–158.
- Morlando M, Morelli C, Gaizo FD, et al. Aberrant right subclavian artery: the association with chromosomal defects and the related post-natal outcomes in a third level referral Centre. J Obstet Gynaecol. 2021;43(5):1–5.
- Borenstein M, Minekawa R, Zidere V, et al. Aberrant right subclavian artery at 16 to 23 + 6 weeks of gestation: a marker for chromosomal abnormality. Ultrasound Obstet Gynecol. 2010;36(5):548–552.
- De León-Luis J, Gámez F, Bravo C, et al. Second-trimester fetal aberrant right subclavian artery: original study, systematic review and meta-analysis of performance in detection of down syndrome. Ultrasound Obstet Gynecol. 2014;44(2):147–153.
- Yazıcıoğlu HF, Sevket O, Akın H, et al. Aberrant right subclavian artery in down syndrome fetuses. Prenat Diagn. 2013;33(3):209–213.
- Borenstein M, Cavoretto P, Allan L, et al. Aberrant right subclavian artery at 11 + 0 to 13 + 6 weeks of gestation in chromosomally normal and abnormal fetuses. Ultrasound Obstet Gynecol. 2008;31(1):20–24.
- Gul A, Corbacioglu A, Bakirci IT, et al. Associated anomalies and outcome of fetal aberrant right subclavian artery. Arch Gynecol Obstet. 2012;285(1):27–30.
- Scala C, Leone Roberti Maggiore U, Candiani M, et al. Aberrant right subclavian artery in fetuses with down syndrome: a systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2015;46(3):266–276.
- Esmer AC, Gul A, Nehir A, et al. Detection rate of trisomy 21 in fetuses with isolated and non-isolated aberrant right subclavian artery. Fetal Diagn Ther. 2013;34(3):140–145.
- Barrea C, Yoo SJ, Chitayat D, et al. Assessment of the thymus at echocardiography in fetuses at risk for 22q11.2 deletion. Prenat Diagn. 2003;23(1):9–15.
- Cavoretto PI, Sotiriadis A, Girardelli S, et al. Postnatal outcome and associated anomalies of prenatally diagnosed right aortic arch with concomitant right ductal arch: a systematic review and meta-analysis. Diagnostics. 2020;10(10):831–844.
