Abstract
Feto-fetal hemorrhage (FFH) through placental vascular anastomoses is believed to be responsible for the death or damage of a “second twin” after the demise of a “first twin (co-twin)” in monochorionic twin pregnancies. However, the timing of FFH has been difficult to determine. The resulting anemia in the surviving twin can be suspected by the finding of an elevated middle cerebral artery peak-systolic velocity (MCA-PSV), but this elevation may lag for at least 4 h after the demise of the first twin. Knowledge of the timing of FFH may have important clinical implications, as it may dictate if and when attempts to prevent death or damage to the second twin by delivery or intrauterine fetal transfusion would be warranted. We present a case that supports the notion that FFH occurs before the actual demise of the first twin. A review of the literature was also conducted.
Introduction
Feto-fetal hemorrhage (FFH) through placental vascular anastomoses is believed to be responsible for the death or damage of a “second twin” after the demise of a “first twin (co-twin)” in monochorionic twin pregnancies [Citation1,Citation2]. Although the timing of FFH has been difficult to determine, it has been suspected to occur before the actual demise of the first twin [Citation3]. The resulting anemia in the surviving twin can be suspected by the finding of an elevated middle cerebral artery peak-systolic velocity (MCA-PSV), but this elevation may lag for at least 4 h after the demise of the first twin [Citation4]. Knowledge of the timing of FFH may have important clinical implications, as it may dictate if and when attempts to prevent death or damage to the second twin either by delivery [Citation5] or by intrauterine fetal transfusion [Citation6] would be warranted. We present a case that supports the notion that FFH occurs before the actual demise of the first twin. The study was approved by WCGIRB protocol number 20225542, and the subject gave written informed consent. A review of the literature is discussed.
Case report
The patient was a 26-year-old, gravida 4, para 2, referred at 17 1/7 weeks with a monochorionic diamniotic twin gestation with type II SIUGR. The pregnancy was the result of spontaneous conception. Medical history was significant only for Rh-negative, for which she had received Rhogam in all prior pregnancies. An ultrasound performed (GE Voluson E10 bt20) showed normal anatomy in both twins, but the bladder was not visible in twin B. Twin B had oligohydramnios, with a maximum vertical pocket (MVP) of 1.6 cm, but twin A did not have polyhydramnios (MVP 6.1 cm). Twin A had an EFW of 173 g at the 33rd percentile, while B had an EFW of 137 g, at <3rd percentile, for a 21% discordance. Doppler studies in twin A were normal, including a middle-cerebral artery peak systolic velocity (MCA-PSV) at 1.02 MOM. Doppler studies in twin B showed persistent absent end-diastolic velocity (AEDV) in the umbilical artery, pulsatile flow in the umbilical vein, and reverse flow in the ductus venous. The MCA-PSV of twin B was at 1.1 MOM. The fetal heart rate (FHR) of twin A was 145 bpm. Twin B had a FHR of 146 bpm, but showed frequent episodes of bradycardia. The patient was diagnosed as having type II SIUGR of twin B [Citation7]. The ultrasound findings did not meet strict criteria for twin-twin transfusion syndrome (TTTS) [Citation8], nor for twin-anemia-polycythemia sequence (TAPS) [Citation9]. In addition to being type II SIUGR, venous Dopplers in twin B were abnormal.
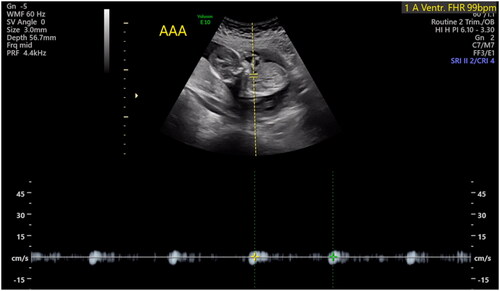
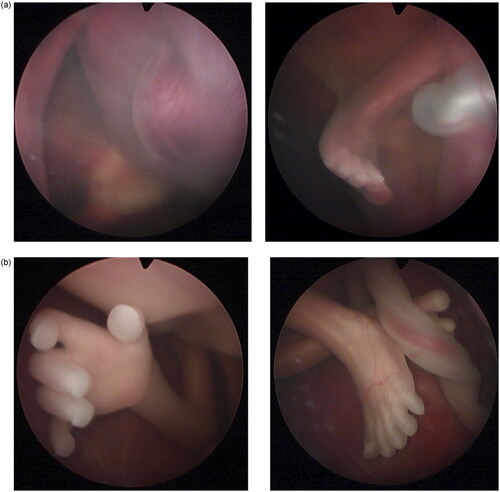
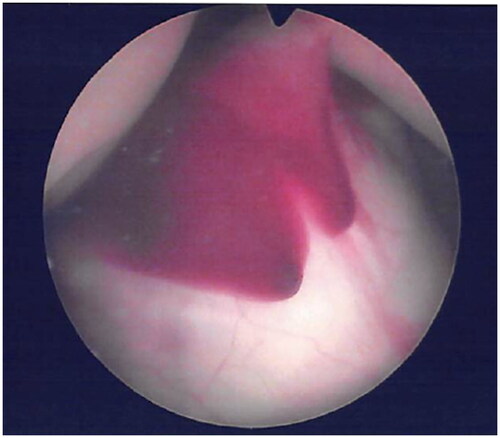
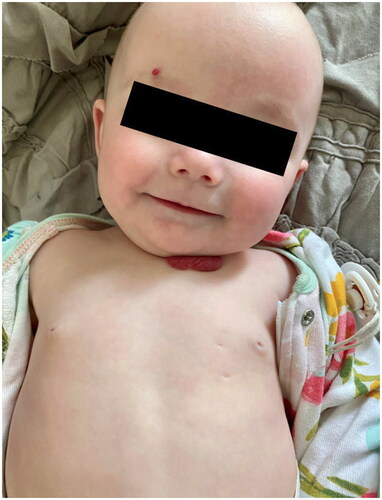
The patient was counseled, and she agreed to proceed with surgery to separate the circulations of the twins, preferably via selective laser photocoagulation of communicating vessels (SLPCV) [Citation8], or, as a last resort, umbilical cord occlusion of twin B [Citation10]. Ultrasound in the operating room showed a FHR in twin B of 99 bpm and decreasing (). The FHR of twin A was 129 bpm. The maternal heart rate was 67 bpm. Under local anesthesia, a 3.5 mm trocar was inserted into the amniotic cavity of twin A, and the fetuses were examined using a 3.3 mm diagnostic endoscope. Twin B was noted to be plethoric, and twin A was pale (). A quick endoscopic assessment of the placenta disclosed approximately five arterio-venous anastomoses. The SLPCV procedure was started with ablation of 3 of the 5 anastomoses (arteriovenous, from the smaller to the larger twin). However, some of the anastomoses were not readily accessible for laser occlusion, as they were behind a fold of the dividing membrane in the vascular equator. Meanwhile, ultrasound showed an agonal FHR for twin B with concomitant bradycardia of 109 bpm in twin A. Further attempts to complete the SLPCV were abandoned. Instead, the umbilical cord of twin B was occluded with Diode-laser energy using a 600-micron fiber through an operating endoscope. The FHR of twin A remained at 107–109 bpm, with poor contractility. In view of the fetoscopic appearance of fetal anemia in twin A and the presence of fetal bradycardia, the decision was made to transfuse twin A. An intravascular transfusion into the umbilical vein of twin A was not deemed practical, given the posterior location of the umbilical cord, the small diameter of the umbilical vein (approximately 2 mm), and the time-constrained situation. Therefore, the decision was made to perform a cardiocentesis, with an intracardiac injection of 5.5 cc of O-Rh negative blood. A fetal blood sample could not be obtained before or after the cardiocentesis. The procedure required two separate entries into the fetal heart due to a change in the fetal position. The FHR of twin A recovered progressively to 130 bpm. Endoscopy showed the cardiocenteses sites (). The entire surgery from skin incision, endoscopy, SLPCV, cord occlusion, and fetal transfusion lasted 48 min. The laser aspect of the surgery, including occlusion of vascular anastomoses and umbilical cord, lasted 23 min. The patient was hospitalized for observation, with supportive care. On post-operative day 1 (POD1), the FHR of twin A was 154 bpm. MCA-PSV could not be obtained due to fetal position. Twin B was a demise, as expected. On POD2, the FHR of twin A was 149 bpm with no signs of fetal anemia (MCA-PSV of 1.4 MOM). Minimal chorioamniotic separation was noted. The patient was discharged home. She underwent weekly ultrasounds at her referring physician’s office. At 26 weeks, the patient was admitted with spontaneous premature rupture of membranes. She received antenatal corticosteroids and antibiotics and remained hospitalized until 32.1 weeks when she was delivered by cesarean section for a non-reassuring FHR tracing. The surviving twin weighed 1729 g at the 54th percentile, and had Apgar scores of 7 and 8 at 1 and 5 min, respectively. At age 20 months, the child is healthy and is achieving all milestones. A picture of the infant at 8 months of age shows the two sites of the cardiocenteses ().
Discussion
Our case adds support to the hypothesis that FFH occurs while both twins are still alive during the final stages of decompensation of the first twin [Citation3]. As the first twin deteriorates, the second twin continues to transfuse the first twin through the placental vascular anastomoses. FFH may also continue after demise of the first twin [Citation11], most likely for a short period of time until the blood pressure of the second twin equalizes and is unable to transfer any further blood across the placenta and into the demised first twin. Depending on the type, size and number of the placental vascular anastomoses, the magnitude of the FFH may result in death, intact survival, or survival with neurological damage of the second twin [Citation6].
A recent systematic review and meta-analysis showed that the death of a first monochorionic twin was associated with a 41% likelihood of fetal demise or 27% neonatal death of the second twin, or 28.5% risk of neurodevelopmental morbidity if the second twin survived [Citation12]. In 2002, our group reported the first case of fetoscopic demonstration of perimortem FFH in a patient with TTTS at 17 3/7 weeks [Citation13]. Both fetuses had been alive 3 h before surgery, though the donor twin had shown bradycardia down to 90 bpm. At the time of surgery, the donor twin had already died and appeared plethoric, while the recipient twin was still alive, but pale [Citation13]. Five arteriovenous anastomoses were found and were lasered in case FFH was still ongoing. The surviving twin did not require any transfusion. An elevated MCA-PSV of 54 cm/s, consistent with approximately 2.08 MOM was noted the following day. The patient miscarried 3 days later, and surgical pathology confirmed the fetoscopic findings, including occlusion of all 5 arteriovenous anastomoses identified during surgery. While our case report supported the hypothesis that fetal anemia and the potential adverse consequences in a surviving monochorionic twin from the demise of a first co-twin result from FFH rather than from embolic phenomena [Citation14], it was unable to establish the timing of the development of such anemia.
In the current case, the characteristic fetoscopic findings of a plethoric agonal first twin and an anemic surviving second twin were seen, yet both fetuses were still alive. Neither twin had shown an elevated MCA-PSV before surgery nor evidence of TAPS. Because the MCA-PSV is known not to increase until several hours after the demise of the first twin [Citation4,Citation15], the diagnosis of fetal anemia in the second twin, and thus the degree of FFH, could not be suspected before the fetoscopic observation. Parrott et al. reported ultrasound evidence of FFH in a patient with TTTS diagnosed with a moribund donor twin at 21 3/7 weeks. In that case, ultrasound showed reverse arterial perfusion in the agonal twin. That patient underwent ultrasound-guided umbilical cord occlusion of the moribund twin. Upon leaving the operating room, the FHR of the surviving twin was 125 bpm. However, the FHR deteriorated in the next 10 min, down to 85 bpm. Intracardiac adrenalin was administered, and the FHR recovered. An intravascular transfusion was performed 3.5 h later, when the MCA-PSV rose from 0.93 MOM (25.26 cm/s) to 2.19 MOM (52.29 cm/s), although the fetal hematocrit had remained unchanged between the two blood sampling procedures at approximately 18% [Citation4]. The patient delivered at 24 weeks, and the second twin died after birth. In our case, a similar deterioration in the FHR of the second twin occurred. However, the fetoscopic image allowed us to conclude that the second twin was anemic prior to any elevation of the MCA-PSV. This helped us make the decision to transfuse the second twin at more quickly and decrease the risk of demise or injury of this fetus from perimortem FFH [Citation16].
Given that FFH does appear to occur before the actual demise of the first twin, treatment options to prevent the death or damage of the second twin are limited. Neurological damage has been reported to occur in the surviving twin with an interval of only 30 min between the death of a co-twin and delivery [Citation5]. Alternatively, intrauterine rescue transfusions of an anemic surviving twin have been performed 8–24 h or more after the diagnosis of the demise of the first twin [Citation6,Citation17–19]. shows a review of the literature of patients that have undergone a rescue transfusion after the demise of a monochorionic twin. Overall, nineteen out of 34 patients (55.9%) had a favorable outcome, whereas 44.1% had an adverse outcome, either from fetal or neonatal demise (8/34, 23.5%) or from neurological damage by antenatal ultrasound (7/34, 20.6%). Twenty-one of these 34 patients (61.7%) had spontaneous demise of the first twin, whereas the remaining 13 patients had either laser therapy or cord occlusion prior to the demise of the first twin. There is a tendency for a worse outcome in cases of spontaneous demise than in those with a prior vasoocclusive procedure (12/21, 57% vs. 3/13, 23%, p = .055, spontaneous vs. vasoocclusive, respectively). Thus, rescue intravascular fetal transfusion after laser treatment or cord occlusion may be more successful than in patients with spontaneous fetal demise. It is conceivable that the rate or the degree of FFH may differ between the two groups (spontaneous vs. vasoocclusive), as all vascular communications are patent in the spontaneous demise group, whereas only a few anastomoses, if any, could be patent after laser therapy. Using data on the interval between demise of the first twin and the hemoglobin of the second twin, patients with spontaneous fetal demise show an expected worsening fetal hemoglobin value with time (). shows the fetal hemoglobin of the second twin relative to the interval of death of the first twin after a vasoocclusive procedure. The paradoxical higher fetal hemoglobin values over time in this group may suggest the contribution of a different mechanism for blood loss from the second twin. Indeed, depending on the sequence in which the placental vascular anastomoses are lasered, temporary fetal anemia in one twin may result despite complete obliteration of all of the placental vascular anastomoses [Citation20,Citation21]. Regardless, the level of the fetal hemoglobin prior to transfusion does not appear to be predictive of an adverse outcome, whether the demise of the first twin occurred spontaneously (mean 5.9 g/dL vs. 5.6 g/dL, p = .68, non-adverse vs. adverse outcome, respectively) or the demise of the first twin occurred after a vasoocclusive procedure (mean 6.5 g/dL vs. 7.4 g/dL, p = .55, non-adverse vs. adverse outcome, respectively). Neither gestational age (22.7 vs. 23.7 weeks, p = .39) nor donor/recipient status (p = .56) appear to be predictive of outcome either. Therefore, counseling of the patient either to deliver (if viable) or to offer a rescue transfusion in the second twin after the demise of a first twin should consider that the ideal time for intervention may have already passed. If the first twin is still alive albeit not deemed salvageable, or if the demise of the first twin has already occurred but reverse blood flow can still be demonstrated in its umbilical cord [Citation11], emergency occlusion of all of the vascular anastomoses or the umbilical cord of the first twin could be considered to prevent further FFH, in addition to transfusing the second twin. Because the MCA-PSV may not be elevated at the time, it should not be used to guide the decision on whether to perform a cordocentesis or an intrauterine transfusion. If an endoscopic procedure is performed, visualization of a plethoric and a pale fetus, as in the current case, should serve as supportive evidence to decide transfusing the second twin immediately, without waiting for sonographic suggestion of fetal anemia.
Figure 5. Spontaneous demise of the first twin. Fetal hemoglobin of the second twin relative to the interval after spontaneous demise of the first twin and perinatal outcome (data extracted from ).
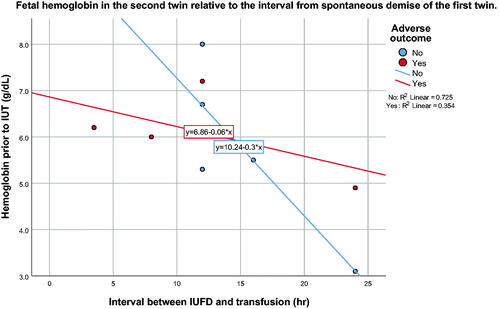
Figure 6. Demise of the first twin after a vasoocclusive procedure. Fetal hemoglobin of the second twin relative to the interval after demise of the first twin from a vasoocclusive procedure and perinatal outcome (data extracted from ).
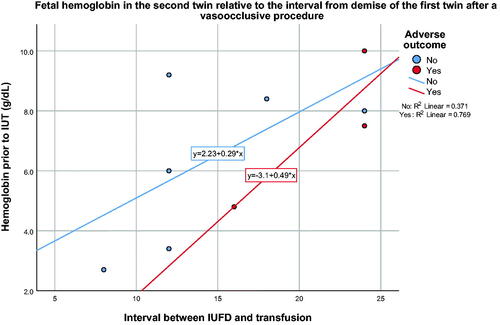
Table 1. Intravascular rescue transfusion after spontaneous demise of a monochorionic twin.
Authors contributions
Dr. Kontopoulos and Dr. Quintero participated in the writing and editing of this manuscript.
Acknowledgments
We would like to thank Dr. Antonio González Ruiz for the kind referral.
Disclosure statement
The authors report there are no competing interests to declare.
Additional information
Funding
References
- Fusi L, McParland P, Fisk N, et al. Acute twin-twin transfusion: a possible mechanism for brain-damaged survivors after intrauterine death of a monochorionic twin. Obstet Gynecol. 1991;78(3 Pt 2):517–520.
- Okamura K, Murotsuki J, Tanigawara S, et al. Funipuncture for evaluation of hematologic and coagulation indices in the surviving twin following co-twin’s death. Obstet Gynecol. 1994;83(6):975–978.
- Nicolini U, Poblete A. Single intrauterine death in monochorionic twin pregnancies [editorial. Ultrasound Obstet Gynecol. 1999;14(5):297–301.
- Parrott J, Schwartz T, Cowles T, et al. Perimortem demonstration and treatment of recipient-to-Donor transfusion in monochorionic-diamniotic twin gestation. Fetal Diagn Ther. 2017;42(3):232–235.
- Karageyim Karsidag AY, Kars B, Dansuk R, et al. Brain damage to the survivor within 30 min of co-twin demise in monochorionic twins. Fetal Diagn Ther. 2005;20(2):91–95.
- Tanawattanacharoen S, Taylor MJ, Letsky EA, et al. Intrauterine rescue transfusion in monochorionic multiple pregnancies with recent single intrauterine death. Prenat Diagn. 2001;21(4):274–278.
- Gratacos E, Lewi L, Munoz B, et al. A classification system for selective intrauterine growth restriction in monochorionic pregnancies according to umbilical artery doppler flow in the smaller twin. Ultrasound Obstet Gynecol. 2007;30(1):28–34.
- Quintero R. Selective laser photocoagulation of communicating vessels in twin-twin transfusion syndrome. In: Quintero R, editor. Diagnostic and operative fetoscopy. New York: the Parthenon Publishing Group; 2002. p. 43–54.
- Lopriore E, Middeldorp JM, Oepkes D, et al. Twin anemia-polycythemia sequence in two monochorionic twin pairs without oligo-polyhydramnios sequence. Placenta. 2007;28(1):47–51.
- Quintero RA, Chmait RH, Murakoshi T, et al. Surgical management of twin reversed arterial perfusion sequence. Am J Obstet Gynecol. 2006;194(4):982–991.
- Jou HJ, Ng KY, Teng RJ, et al. Doppler sonographic detection of reverse twin-twin transfusion after intrauterine death of the donor. J Ultrasound Med. 1993;12(5):307–309.
- Mackie FL, Rigby A, Morris RK, et al. Prognosis of the co-twin following spontaneous single intrauterine fetal death in twin pregnancies: a systematic review and meta-analysis. BJOG. 2019;126(5):569–578.
- Quintero RA, Martinez JM, Bermudez C, et al. Fetoscopic demonstration of perimortem feto-fetal hemorrhage in twin-twin transfusion syndrome. Ultrasound Obstet Gynecol. 2002;20(6):638–639.
- Benirschke K. Intrauterine death of a twin: mechanisms, implications for surviving twin, and placental pathology. Semin Diagn Pathol. 1993;10(3):222–231.
- Nakata M, Sumie M, Murata S, et al. A case of monochorionic twin pregnancy complicated with intrauterine single fetal death with successful treatment of intrauterine blood transfusion in the surviving fetus. Fetal Diagn Ther. 2007;22(1):7–9.
- Ong SS, Zamora J, Khan KS, et al. Prognosis for the co-twin following single-twin death: a systematic review. BJOG. 2006;113(9):992–998.
- Ohkuchi A, Minakami H, Shiraishi H, et al. Intrauterine death of one twin, with rescue of the other, in twin-twin transfusion syndrome. Ultrasound Obstet Gynecol. 2002;19(3):293–296.
- Quarello E, Stirnemann J, Nassar M, et al. Outcome of anaemic monochorionic single survivors following early intrauterine rescue transfusion in cases of feto-fetal transfusion syndrome. Bjog. 2008;115(5):595–601.
- Senat MV, Bernard JP, Loizeau S, et al. Management of single fetal death in twin-to-twin transfusion syndrome: a role for fetal blood sampling. Ultrasound Obstet Gynecol. 2002;20(4):360–363.
- Quintero RA, Ishii K, Chmait RH, et al. Sequential selective laser photocoagulation of communicating vessels in twin-twin transfusion syndrome. J Matern Fetal Neonatal Med. 2007;20(10):763–768.
- Chmait RH, Kontopoulos EV, Quintero RA. Sequential laser surgery for twin-twin transfusion syndrome. Am J Perinatol. 2014;31(Suppl 1):S13–S8.