 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objective
COVID-19 is an ongoing pandemic and has been extensively studied. However, the effects of COVID-19 during pregnancy, particularly on placental function, have not been verified. In this study, we used blood oxygen level-dependent magnetic resonance imaging (BOLD-MRI) to evaluate whether COVID-19 incidence during pregnancy has any lasting effects with respect to placental oxygenation.
Methods
This is a case-control study, in which eight cases of singleton pregnancies before 30 weeks gestation with COVID-19 mothers were included. Placental oxygenation was evaluated using BOLD-MRI after 32 weeks of gestation. BOLD-MRI was consecutively performed under normoxia (21% O2), hyperoxia (100% O2), and normoxia for 4 min each. Individual placental time–activity curves were evaluated to calculate the peak score (peakΔR2*) and the time from the start of maternal oxygen administration to the time of peakΔR2* (time to peakΔR2*). Eighteen COVID-19-free normal pregnancies from a previous study were used as the control group.
Results
No significant differences were found between the two groups regarding maternal background, number of days of delivery, birth weight, and placental weight. The parameter peakΔR2* was significantly decreased in the COVID-19 group (8 ± 3 vs. 5 ± 1, p < .001); however, there was no significant difference in time to peakΔR2* (458 ± 74 s vs. 471 ± 33 s, p = .644).
Conclusions
In this study, BOLD-MRI was used to evaluate placental oxygenation during pregnancy in COVID-19-affected patients. COVID-19 during pregnancy decreased placental oxygenation even post-illness, but had no effect on fetal growth; further investigation of the possible effects of COVID-19 on the fetus and mother is warranted.
Introduction
The novel, highly transmissible coronavirus, severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), emerged at the end of 2019 and spread rapidly worldwide, reaching a pandemic status by March 2020 [Citation1]. SARS-CoV-2 infection can cause a wide range of illnesses, from asymptomatic to the acute respiratory condition known as coronavirus 2019 (COVID-19), which has a diverse and varied clinical course ranging from mild to fatal [Citation2]. COVID-19 morbidity during pregnancy is associated with a consistent and significant increase in severe maternal morbidity, mortality, and neonatal complications [Citation3]. However, the impact of COVID-19-affected pregnancies on fetal development and placental function has not been elucidated.
It has been reported that a higher percentage of babies who were delivered more than 2 weeks after COVID-19 infection in mothers were affected by fetal growth restriction (FGR) compared to those who were delivered within 2 weeks of maternal COVID-19 infection [Citation4]. Moreover, reports have suggested that COVID-19 morbidity may affect fetal development [Citation3]. In contrast, some studies report no significant effect on fetal development [Citation5,Citation6]. This difference is thought to be due to variable severity; however, no studies regarding abnormal fetal growth parameters or Doppler studies in pregnant women with COVID-19 during pregnancy have been reported [Citation6,Citation7].
Several studies have isolated the virus in the placenta of pregnant women who tested positive for the virus at or before delivery [Citation8–11]. Recent studies have reported an association between COVID-19 in pregnancy and the development of preeclampsia [Citation12–14]. In this context, it has been suggested that SARS-CoV-2 binds to the placental ACE2 receptor, causing several placental lesions and possibly vascular diseases such as preeclampsia. This can lead to incomplete perfusion and placental failure, and clinically to FGR and perinatal death [Citation15,Citation16].
However, it is unclear whether SARS-CoV-2 infection during pregnancy has similar placental effects in a non-acute settings. Furthermore, no reports have evaluated the effects of SARS-CoV-2 infection on placental function. An objective evaluation of the persistent effects on placental function after the disease is of pressing need for pregnant women affected by COVID-19.
Blood oxygen level-dependent magnetic resonance imaging (BOLD-MRI) has received much attention as a method of assessing fetal blood oxygenation, which depends on placental oxygen diffusion [Citation17,Citation18]. BOLD-MRI exploits the differences in the magnetic properties of hemoglobin and deoxyhemoglobin; deoxyhemoglobin causes local magnetic field inhomogeneities, leading to a decrease in the BOLD-MRI signals.
In this study, we assessed placental oxygenation using BOLD-MRI after 32 weeks of gestation, when symptoms of COVID-19-infected mothers had abated, to evaluate the association between COVID-19 disease during pregnancy and placental function.
Materials and methods
Collection of maternal, fetal, and placental data
Pregnant women with COVID-19 from 1 December 2020 to August 2021 in Mie Prefecture were included. Individuals who continued to manage their pregnancies in Mie Prefecture, met the inclusion criteria listed below, and were able to give written consent for placental MRI imaging after 32 weeks of gestation in Mie University were included: a total of eight singleton pregnant women with COVID-19. The inclusion criteria were as follows: (1) pregnant women aged >20 years; (2) COVID-19 diagnosis based on positive SARS-CoV-2 PCR test result during pregnancy, but must have a negative SARS-CoV-2 PCR test or no symptoms for 2 weeks after the positive test at the time of the MRI imaging; (3) gestational age >32 weeks at the time of MRI; (4) singleton pregnancy.
To the extent that ultrasound pregnancy examinations allowed, abnormalities of the umbilical cord attachment site, excessive torsion of the umbilical cord, and other abnormalities of the umbilical cord were excluded. The placental weight, including the umbilical cord, was measured postpartum. Fetal ultrasound was performed within three days of the MRI. The estimated fetal weight (EFW) and umbilical artery pulsatility index (PI) were evaluated.
Our previous study evaluated placental oxygenation using BOLD-MRI after 32 weeks of gestation in a maternal cohort of normally developing fetuses [Citation18]. The group with normal pregnancies in a previous report was compared with the subjects of this study as a control group.
COVID-19 management
Pregnancies complicated by COVID-19 were managed at the hospital at the time of diagnosis and differed between facilities. All patients in this study were hospitalized. The SpO2 values were obtained and recorded at the time of the visit. The management policy after hospitalization was as follows: (1) oxygen to be administered to patients with SpO2 <95%; (2) vital signs, including maternal body temperature, SpO2, and blood pressure, measured at least three times a day; (3) acetaminophen for antipyretic purposes should not be used in routine care.
In addition to the above strategies, anticoagulants, antivirals, and steroids were administered at some facilities. Treatment methods were inconsistent among facilities, and most patients were hospitalized for bed rest, although oxygen, steroids, anticoagulants, monoclonal antibodies, and antivirals were administered on a case-to-case basis.
MRI protocol
MR images were acquired using a 3.0-T scanner (Ingenia 3.0 Ω, Philips Healthcare, Best, The Netherlands). During the MRI scan, the pregnant women were placed in the left lateral decubitus position to minimize aortocaval compression, and a dS torso coil was placed over the abdomen to cover the entire uterus. T2-weighted images were acquired in the uterus’s long- and short-axis planes to assess the placental position and morphology.
Next, a 12-min dynamic BOLD-MRI was performed using a fast field echo–echo planar imaging sequence with the following parameters: repetition time, 5000 ms; echo time, 35 ms; field of view, 400 × 400 mm; matrix, 128 × 128; fat saturation using spectral presaturation with inversion recovery; sensitivity encoding factor, 1.8; and in-plane resolution, 3.1 × 3.1 mm. Short-axis slices of the uterus covering the entire placenta were obtained using a slice thickness of 5 mm and a slice gap of 1 mm. For the 12-min BOLD-MRI scan, each slice image was repeated every 5 s, resulting in approximately 144 frames for each slice. Using a non-rebreathing facial mask, the oxygen supply to the mother was changed during this scan over three consecutive 4-min intervals from room air (fraction of inspired oxygen (FiO2) 21%) to oxygen (flow rate 10 L/min, FiO2 100%) and back to room air. The total scan time was approximately 20 min, which the pregnant women tolerated well.
The patients were instructed to trigger an alert when they became aware of fetal movements and uterine contractions during the MRI imaging. If the alert was triggered before oxygen was administered, the imaging was repeated; if the alert was triggered after oxygen was administered, the observation was terminated and the data were excluded from the analyses.
MRI analysis
MR images were analyzed using in-house developed software written in MATLAB (MathWorks Inc., Natick, MA). The acquired images were registered to mitigate motion using a 3D rigid transformation approach using an affine transformation designed for BOLD images.
Three consecutive slices at the center of the placenta were selected for measurement. Placental regions of interest (ROIs) in the initial frame were manually delineated so that each ROI covered the placenta in each slice and propagated to all time frames. The location of the ROI was manually corrected in each time frame if required. Time–signal intensity curves of the placenta were obtained for each 12-min BOLD-MRI scan with minimal manual ROI correction, regardless of maternal oxygenation status. All ROIs were placed by a single observer (SM). Manual ROI correction cannot correct for coarse fetal movements but was implemented when motion correction was not sufficient for some fetal/placental movements. BOLD-MRI imaging was T2* (=1/R2*)-weighted; therefore, when the oxygenation level increased during a BOLD-MRI scan, the deoxygenated hemoglobin concentration decreased, thereby increasing the signal and decreasing the R2*. ΔR2*, the relative change in R2* from the baseline and an indicator of the change in oxygen saturation levels, can be calculated from the time-signal intensity curves of the placenta in the BOLD images, as follows [Citation19]:
where S(t) and Sbaseline represent the signal intensity at a certain time point, and baseline, respectively. TE represents the BOLD-MRI echo time. The baseline value of the placental time–signal intensity curve from BOLD-MRI was determined as the mean signal intensity during the first interval of oxygenation with room air. The time–ΔR2* curves were generated using the software, and the corresponding peakΔR2* and time to peakΔR2* values during oxygenation were automatically determined by the software. The mean values of three slices of peakΔR2* values, and the time from starting maternal oxygen administration to peakΔR2* during oxygenation per case, were treated as the values for that case.
Statistical methods
The Mann–Whitney U-test was used to compare peakΔR2* and time to peakΔR2* values between the control and COVID-19 groups. The same test was performed to compare background factors, such as maternal variables: age, gravity, MRI (gestational days); neonatal variables: delivery (gestational days) and birth weight (g, percentile); and placental variables (placental weight (g)) between the two groups.
In all tests, statistical significance was set at p < .05. Data were analyzed using SPSS version 25 (IBM Corporation, Armonk, NY).
Ethical approval
This study was conducted in accordance with the principles of the Declaration of Helsinki and approved by the Institutional Review Board of Mie University Hospital (no. 3172, 19 June 2017). All participants provided written informed consent before participating in the study.
Results
shows the demographics of the mothers recruited in the COVID-19 group. Of the eight cases, two were vaccinated, but both patients became symptomatic within five days of vaccination. The post-hospitalization treatment, course, and number of vaccinations in the eight cases are described in the Supplementary table.
Table 1. Maternal demographics.
shows fetal, neonatal, and placental information at the time of the MRI. compares the maternal background of the COVID-19 group with that of the control group. Maternal age was the parameter that showed a significant difference between the groups (p = .047), with no significant differences in other factors. Maternal pre-pregnancy body mass index values ranged from 16 to 25 kg/m2. There were no pregnancy-related complications other than those related to COVID-19 that required medical intervention or affected fetal growth. A comparison of the BOLD-MRI parameters between the groups is shown in and . ΔR2* values were significantly lower in the COVID-19 group than in the normal pregnancy group (p < .001). None of the mothers recruited for the study developed hypertensive disorders of pregnancy, and only one case had a small for gestational age infant at birth.
Figure 1. (A, B) Normal pregnancies and (C, D) pregnancies with a history of COVID-19 disease. (A, C) Diagrams of placental area designation. (B, D) Changes in ΔR2* values with maternal oxygen administration. Maternal oxygen is administered between 240 and 480 s, indicating increased ΔR2* values.
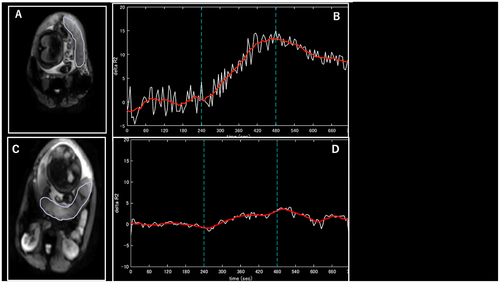
Table 2. Fetal, neonatal, and placental demographics.
Table 3. Comparison between the normal pregnancy group and the COVID-19 group.
Table 4. Results of BOLD-MRI.
Discussion
The results of this study revealed that patients who experienced COVID-19 during pregnancy had a lower ΔR2* compared to healthy controls. The women in this study were infected with COVID-19 in their first and second trimesters of pregnancy, and placental function was assessed using BOLD-MRI after 32 weeks of gestation. This study was designed to determine the impact of the disease during pregnancy and after healing from COVID-19.
Currently, there is no method for assessing placental function in real-time. Indirect methods for evaluating placental function include the evaluation of EFW and placental weight. However, EFW has limitations, such as significant inter-assessor errors, and placental weight shows no correlation between birth weight and placental weight in cases of FGR [Citation20]. Attempts have also been made to evaluate placental insufficiency from the vascular resistance of umbilical arteries using ultrasound Doppler imaging; however, the results are not highly reproducible [Citation21]. In a study evaluating the detection of FGR associated with placental insufficiency, only 46% of FGR cases showed a significant increase (>90%) in the PI, suggesting that PI is unreliable for evaluating placental vascular resistance [Citation22].
It has also been reported that, in general, Doppler flow remains normal in late-onset placental dysfunction [Citation23]. Currently, it is difficult to accurately evaluate placental function in real-time or even placental function as a cause of FGR. In a previous study, our group first evaluated BOLD-MRI in late pregnancy of normal singleton cases and showed that BOLD-MRI parameters were increased by maternal oxygenation in all cases of normal pregnancy and could be a reference index of placental oxygenation in normal pregnancy after 32 weeks of gestation [Citation18]. Next, our group performed BOLD-MRI in cases of FGR and suggested this method is capable in assessing decreased placental function [Citation24]. In this study, we applied this method to pregnant patients with a history of COVID-19.
SARS-CoV-2 is a viral infection that has been reported to induce inflammation, unlike cytomegalovirus and Zika virus infections [Citation25–27]. Furthermore, there have been no reports showing an association of placental pathology with the obvious characteristics of infected viruses [Citation28,Citation29]. There are some reports of vascular malformations and thrombosis affecting the placenta in COVID-19 cases. Vascular malformations include maternal vascular malperfusion (MVM) and fetal vascular malperfusion (FVM), which are recognized patterns of placental damage associated with abnormal and detrimental spiral artery and stromal blood flow due to infection or inflammation [Citation30,Citation31].
A literature review showed MVM in 30–40% of pathological placentas from COVID-19 complicated pregnancies, suggesting the influence of inflammation on COVID-19 morbidity [Citation32,Citation33]. Reports on non-acute placental pathology have also confirmed the presence of MVM and FVM [Citation34,Citation35].
In contrast, there is currently no unanimous consensus as to whether thrombus formation is promoted in COVID-19-affected patients. More precisely, it is difficult to assess whether COVID-19 disease itself specifically promotes thrombus formation. In previous reports, systematic reviews have suggested an increased risk of intraplacental thrombus in COVID-19-affected cases compared to uninfected cases, although the existence of observational bias cannot be ruled out [Citation33]. There have been only a few reports of intraplacental thrombosis in COVID-19-affected patients.
Histological changes, such as fibrin deposition, inflammatory changes, and thrombus formation in the intervillous and perivillous space, have been reported to be greater with higher viral loads [Citation36]. This may simply be a finding obtained due to the intensity of the inflammation or infection to which such patients were exposed. However, it is unclear whether COVID-19 is of greater intensity than other infections and inflammations. Reports of placentitis (SARS-CoV-2 placentitis) characteristic of COVID-19 have also been published [Citation37]. The information available is mainly related to histiocytic intervillositis, with inflammatory infiltration of CD68-positive macrophages. Interestingly, COVID-19 cases with histiocytic intervillositis may be at high risk for the infection transmitted from the mother to the newborn infant [Citation38,Citation39]. However, histiocytic intervillositis is not specific for COVID-19 infection, the virus has been shown to be directly demonstrated within the syncytiotrophoblast by either in situ hybridization or immunohistochemistry, suggesting that it is a placental modification associated with COVID-19 [Citation40–42].
Although not specific to COVID-19-affected patients, various reports have described an association between inflammation and the placenta. Studies using an inflammatory model in which rats were injected with a single high dose of the endotoxin lipopolysaccharide showed that abnormal maternal inflammation was associated with altered utero-placental perfusion and placental hypoxia [Citation43,Citation44]. It has also been reported that the more advanced the maternal exposure to inflammation, the greater the spectrum of effects, which result in an increased morbidity rate [Citation45,Citation46]. Although these evaluations directly assessed placental tissue in animal models, animal models of acute placental injury associated with inflammation have shown reduced perfusion on functional MRI [Citation47]. These studies were evaluated during inflammation exposure and not strictly during the non-acute phase of cases with pre-existing inflammation, as is being evaluated in the present study. The spectrum of effects increases with increasing exposure to inflammation, suggesting that such inflammation may leave irreversible damage to the placenta [Citation48,Citation49]. Reports have suggested that decreased BOLD values are associated with COVID-19 disease and that microcirculatory disturbances may occur because of COVID-19 [Citation50]. Although the cause of the microcirculatory defects has not been determined, these changes may be related to the present study’s findings of decreased oxygenation of the placenta. The results of this study suggest that the strong systemic response of the mother to COVID-19 may also affect the placenta, possibly resulting in abnormal placental organization.
The results of our study suggest that the strong systemic response to maternal COVID-19 may also affect the placenta, and that abnormal placental oxygenation could be due to tissue abnormalities, mainly MVM, associated with microthrombus formation. Interestingly, despite maternal exposure to inflammation, a poor association between COVID-19 and FGR has been reported [Citation6]. This is consistent with our findings that acute maternal inflammation due to COVID-19 differs from inflammatory exposure associated with abnormal trophoblastic growth and hypoxia associated with placental abnormalities, such as hypertensive disorders of pregnancy, which impair placental oxygenation but does not result in FGR. It is possible that relatively brief exposure to inflammation did not cause irreversible or severe changes that would result in FGR.
Limitations
A limitation of this study was that no placental pathology was observed. The patients were admitted to a general hospital during COVID-19 infection; however, after symptom improvement, the pregnant patients often delivered the infant at a facility that did not have a specialized pathologist. Thus, placental pathology data were not submitted. However, even when the placenta was subject to pathology, certain problems could not be resolved. The lack of uniform standards for the evaluation of placental pathology, including inflammatory findings and trophoblastic development, is related to the number of weeks of gestation.
Another important limitation is the evaluation of a specific part of the placenta only. In this study, we evaluated the placental area with the greatest thickness. If the area affected by placental oxygenation due to inflammation is localized, a small cohort, such as that in this study, may lead to a bias at the site of the lesion. Moreover, the effects of abnormal placental oxygenation on the developing child cannot be elucidated until long-term follow-up in the neonatal period is conducted.
Conclusions
In this study, we assessed placental oxygenation through BOLD-MRI analysis in pregnant women who were affected by COVID-19 with resolved symptoms. The results showed a decrease in ΔR2*, suggesting an inflammatory response in pregnancy during COVID-19 exposure. There are many issues to be addressed in the future. There is an urgent need to create a system that allows placental pathology to be evaluated regardless of the delivery facility, and to propose and implement a consistent evaluation method regarding pathology sample sites, examination methods, and observation items. It is also necessary to follow up on the neurodevelopment of infants.
Supplemental Material
Download MS Word (14.5 KB)Acknowledgements
We express our sincere gratitude to Shinichi Takase for performing BOLD-MRI and to Ryohei Nakayama for the development of software for BOLD-MRI analysis.
Disclosure statement
The authors report there are no competing interests to declare.
Data availability statement
The data that support the findings of this study are available on request because the data contain potentially identifying or sensitive patient information. This restriction is imposed by the Institutional Review Board (Contact; Data Manager: [email protected]).
Additional information
Funding
References
- Hu B, Guo H, Zhou P, et al. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021;19(3):141–154. doi: 10.1038/s41579-020-00459-7.
- Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782–793. doi: 10.1001/jama.2020.12839.
- Villar J, Ariff S, Gunier RB, et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: the INTERCOVID Multinational Cohort Study. JAMA Pediatr. 2021;175(8):817–826. doi: 10.1001/jamapediatrics.2021.1050.
- Mullins E, Perry A, Banerjee J, et al. Pregnancy and neonatal outcomes of COVID-19: the PAN-COVID study. Eur J Obstet Gynecol Reprod Biol. 2022;276:161–167. doi: 10.1016/j.ejogrb.2022.07.010.
- Mullins E, Hudak ML, Banerjee J, et al. Pregnancy and neonatal outcomes of COVID-19: coreporting of common outcomes from PAN-COVID and AAP SONPM Registries. Ultrasound Obstet Gynecol. 2021;57(4):573–581. doi: 10.1002/uog.23619.
- Rizzo G, Mappa I, Maqina P, et al. Effect of SARS-CoV-2 infection during the second half of pregnancy on fetal growth and hemodynamics: a prospective study. Acta Obstet Gynecol Scand. 2021;100(6):1034–1039. doi: 10.1111/aogs.14130.
- Soto-Torres E, Hernandez-Andrade E, Huntley E, et al. Ultrasound and Doppler findings in pregnant women with SARS-CoV-2 infection. Ultrasound Obstet Gynecol. 2021;58(1):111–120. doi: 10.1002/uog.23642.
- Hecht JL, Quade B, Deshpande V, et al. SARS-CoV-2 can infect the placenta and is not associated with specific placental histopathology: a series of 19 placentas from COVID-19-positive mothers. Mod Pathol. 2020;33(11):2092–2103. doi: 10.1038/s41379-020-0639-4.
- Lu-Culligan A, Chavan AR, Vijayakumar P, et al. Maternal respiratory SARS-CoV-2 infection in pregnancy is associated with a robust inflammatory response at the maternal–fetal interface. Med. 2021;2(5):591–610.e10. doi: 10.1016/j.medj.2021.04.016.
- Debelenko L, Katsyv I, Chong AM, et al. Trophoblast damage with acute and chronic intervillositis: disruption of the placental barrier by severe acute respiratory syndrome coronavirus 2. Hum Pathol. 2021;109:69–79. doi: 10.1016/j.humpath.2020.12.004.
- Argueta LB, Lacko LA, Bram Y, et al. Inflammatory responses in the placenta upon SARS-CoV-2 infection late in pregnancy. iScience. 2022;25(5):104223. doi: 10.1016/j.isci.2022.104223.
- Papageorghiou AT, Deruelle P, Gunier RB, et al. Preeclampsia and COVID-19: results from the INTERCOVID prospective longitudinal study. Am J Obstet Gynecol. 2021;225(3):289.e1–289.e17. doi: 10.1016/j.ajog.2021.05.014.
- Conde-Agudelo A, Romero R. SARS-CoV-2 infection during pregnancy and risk of preeclampsia: a systematic review and meta-analysis. Am J Obstet Gynecol. 2022;226(1):68–89.e3. doi: 10.1016/j.ajog.2021.07.009.
- Marchand G, Patil AS, Masoud AT, et al. Systematic review and meta-analysis of COVID-19 maternal and neonatal clinical features and pregnancy outcomes up to June 3, 2021. AJOG Glob Rep. 2022;2(1):100049. doi: 10.1016/j.xagr.2021.100049.
- Seethy AA, Singh S, Mukherjee I, et al. Potential SARS-CoV-2 interactions with proteins involved in trophoblast functions – an in-silico study. Placenta. 2021;103:141–151. doi: 10.1016/j.placenta.2020.10.027.
- Schwartz DA, Avvad-Portari E, Babál P, et al. Placental tissue destruction and insufficiency from COVID-19 causes stillbirth and neonatal death from hypoxic–ischemic injury. Arch Pathol Lab Med. 2022;146(6):660–676. doi: 10.5858/arpa.2022-0029-SA.
- Sørensen A, Sinding M, Peters DA, et al. Placental oxygen transport estimated by the hyperoxic placental BOLD MRI response. Physiol Rep. 2015;3(10):e12582. doi: 10.14814/phy2.12582.
- Magawa S, Nii M, Ishida M, et al. Evaluation of the placental oxygenation index using blood oxygen level-dependent magnetic resonance imaging (BOLD-MRI) during normal late pregnancy. J Matern Fetal Neonatal Med. 2022;35(25):5274–5281. doi: 10.1080/14767058.2021.1878140.
- Luo J, Abaci Turk E, Bibbo C, et al. In vivo quantification of placental insufficiency by BOLD MRI: a human study. Sci Rep. 2017;7(1):3713. doi: 10.1038/s41598-017-03450-0.
- Jakó M, Surányi A, Kaizer L, et al. Maternal hematological parameters and placental and umbilical cord histopathology in intrauterine growth restriction. Med Princ Pract. 2019;28(2):101–108. doi: 10.1159/000497240.
- Albaiges G, Missfelder-Lobos H, Parra M, et al. Comparison of color Doppler uterine artery indices in a population at high risk for adverse outcome at 24 weeks’ gestation. Ultrasound Obstet Gynecol. 2003;21(2):170–173. doi: 10.1002/uog.30.
- Unterscheider J, Daly S, Geary MP, et al. Optimizing the definition of intrauterine growth restriction: the multicenter prospective Porto study. Am J Obstet Gynecol. 2013;208(4):290.e1–290.e6. doi: 10.1016/j.ajog.2013.02.007.
- Parra-Saavedra M, Crovetto F, Triunfo S, et al. Placental findings in late-onset SGA births without Doppler signs of placental insufficiency. Placenta. 2013;34(12):1136–1141. doi: 10.1016/j.placenta.2013.09.018.
- Magawa S, Nii M, Enomoto N, et al. Evaluation of placental oxygenation in fetal growth restriction using blood oxygen level-dependent magnetic resonance imaging. Placenta. 2022;126:40–45. doi: 10.1016/j.placenta.2022.06.005.
- Garcia AG, Fonseca EF, Marques RL, et al. Placental morphology in cytomegalovirus infection. Placenta. 1989;10:1–18.
- Martines RB, Bhatnagar J, Keating MK, et al. Notes from the field: evidence of zika virus infection in brain and placental tissues from two congenitally infected newborns and two fetal losses – Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65(6):159–160. doi: 10.15585/mmwr.mm6506e1.
- Martines RB, Bhatnagar J, Ramos AM, et al. Pathology of congenital zika syndrome in Brazil: a case series. Lancet. 2016;388(10047):898–904. doi: 10.1016/S0140-6736(16)30883-2.
- Tasca C, Rossi RS, Corti S, et al. Placental pathology in COVID-19 affected pregnant women: a prospective case-control study. Placenta. 2021;110:9–15. doi: 10.1016/j.placenta.2021.04.002.
- Zhang P, Salafia C, Heyman T, et al. Detection of severe acute respiratory syndrome coronavirus 2 in placentas with pathology and vertical transmission. Am J Obstet Gynecol MFM. 2020;2(4):100197. doi: 10.1016/j.ajogmf.2020.100197.
- Ernst LM. Maternal vascular malperfusion of the placental bed. APMIS. 2018;126(7):551–560. doi: 10.1111/apm.12833.
- Shanes ED, Mithal LB, Otero S, et al. Placental pathology in COVID-19. Am J Clin Pathol. 2020;154(1):23–32. doi: 10.1093/ajcp/aqaa089.
- Sharps MC, Hayes DJL, Lee S, et al. A structured review of placental morphology and histopathological lesions associated with SARS-CoV-2 infection. Placenta. 2020;101:13–29. doi: 10.1016/j.placenta.2020.08.018.
- Di Girolamo R, Khalil A, Alameddine S, et al. Placental histopathology after SARS-CoV-2 infection in pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol MFM. 2021;3(6):100468. doi: 10.1016/j.ajogmf.2021.100468.
- Gulersen M, Prasannan L, Tam HT, et al. Histopathologic evaluation of placentas after diagnosis of maternal severe acute respiratory syndrome coronavirus 2 infection. Am J Obstet Gynecol MFM. 2020;2(4):100211. doi: 10.1016/j.ajogmf.2020.100211.
- Patberg ET, Adams T, Rekawek P, et al. Coronavirus disease 2019 infection and placental histopathology in women who delivering at term. Am J Obstet Gynecol. 2021;224(4):382.e1–382.e18. doi: 10.1016/j.ajog.2020.10.020.
- Kreis NN, Ritter A, Louwen F, et al. A message from the human placenta: structural and immunomodulatory defense against SARS-CoV-2. Cells. 2020;9(8):1777. doi: 10.3390/cells9081777.
- Linehan L, O'Donoghue K, Dineen S, et al. SARS-CoV-2 placentitis: an uncommon complication of maternal COVID-19. Placenta. 2021;104:261–266. doi: 10.1016/j.placenta.2021.01.012.
- Patanè L, Morotti D, Giunta MR, et al. Vertical transmission of coronavirus disease 2019: severe acute respiratory syndrome coronavirus 2 RNA on the fetal side of the placenta in pregnancies with coronavirus disease 2019 – positive mothers and neonates at birth. Am J Obstet Gynecol MFM. 2020;2(3):100145. doi: 10.1016/j.ajogmf.2020.100145.
- Kirtsman M, Diambomba Y, Poutanen SM, et al. Probable congenital SARS-CoV-2 infection in a neonate born to a woman with active SARS-CoV-2 infection. CMAJ. 2020;192(24):E647–E650. doi: 10.1503/cmaj.200821.
- Vivanti A, Vauloup-Fellous C, Prevot S, et al. Transplacental transmission of SARS-CoV-2 infection. Nat Commun. 2020;11(1):3572. doi: 10.1038/s41467-020-17436-6.
- Smithgall MC, Liu-Jarin X, Hamele-Bena D, et al. Third trimester placentas of SARS-CoV-2-positive women: histomorphology, including viral immunohistochemistry and in situ hybridization. Histopathology. 2020;77(6):994–999. doi: 10.1111/his.14215.
- Hosier H, Farhadian SF, Morotti RA, et al. SARS-CoV-2 infection of the placenta. J Clin Invest. 2020;130(9):4947–4953. doi: 10.1172/JCI139569.
- Renaud SJ, Cotechini T, Quirt JS, et al. Spontaneous pregnancy loss mediated by abnormal maternal inflammation in rats is linked to deficient uteroplacental perfusion. J Immunol. 2011;186(3):1799–1808. doi: 10.4049/jimmunol.1002679.
- Cotechini T, Komisarenko M, Sperou A, et al. Inflammation in rat pregnancy inhibits spiral artery remodeling leading to fetal growth restriction and features of preeclampsia. J Exp Med. 2014;211(1):165–179. doi: 10.1084/jem.20130295.
- Borzychowski AM, Sargent IL, Redman CW. Inflammation and pre-eclampsia. Semin Fetal Neonatal Med. 2006;11(5):309–316. doi: 10.1016/j.siny.2006.04.001.
- Cotechini T, Graham CH. Aberrant maternal inflammation as a cause of pregnancy complications: a potential therapeutic target? Placenta. 2015;36(8):960–966. doi: 10.1016/j.placenta.2015.05.016.
- Wu D, Lei J, Jia B, et al. In vivo assessment of the placental anatomy and perfusion in a mouse model of intrauterine inflammation. J Magn Reson Imaging. 2018;47(5):1260–1267. doi: 10.1002/jmri.25867.
- Ward EJ, Bert S, Fanti S, et al. Placental inflammation leads to abnormal embryonic heart development. Circulation. 2023;147(12):956–972. doi: 10.1161/CIRCULATIONAHA.122.061934.
- Goldstein JA, Gallagher K, Beck C, et al. Maternal–fetal inflammation in the placenta and the developmental origins of health and disease. Front Immunol. 2020;11:531543. doi: 10.3389/fimmu.2020.531543.
- Andescavage NN, Yuan L, Barnett S, et al. 1116 microstructural & functional changes in the placenta during the COVID-19 pandemic. Am J Obstet Gynecol. 2021;224(2):S687–S688. doi: 10.1016/j.ajog.2020.12.1140.
