Abstract
Objective
To describe international surveillance and treatment strategies for managing anti-SSA/Ro autoantibody positive pregnancies.
Study Design
An electronic REDCap questionnaire was distributed to Fetal Heart Society and North American Fetal Therapy Network members which queried institution-based risk stratification, surveillance methods/frequency, conduction abnormality treatments, and postnatal anti-SSA/Ro pregnancy assessment.
Results
101 responses from 59 centers (59% US, 17% international) were collected. Most (79%) do not risk stratify pregnancies by anti-SSA/Ro titer; those that do use varied cutoff values. Many pregnant rheumatology patients are monitored for cardiac abnormalities regardless of maternal anti-SSA/Ro status. Surveillance strategies were based on maternal factors (anti-SSA/Ro status 85%, titer 25%, prior affected child 79%) and monitoring durations varied. Most respondents treat 2° and 3° fetal atrioventricular block, commonly with dexamethasone and/or IVIG.
Conclusions
Wide variation exists in current fetal cardiac surveillance and treatment for anti-SSA/Ro autoantibody positive pregnancies, highlighting the need for evidence-based protocols to optimize care.
Introduction
Fetal 3° atrioventricular block (AVB), identified in the second trimester of pregnancy in an otherwise normally developing heart, is almost universally associated with maternal anti-SSA/Ro autoantibodies which include reactivity to both the 60kD and 52kD SSA/Ro antigens in most cases and results in death in nearly 20% of cases [Citation1–4]. According to the 2014 American Heart Association Scientific Statement on the Diagnosis and Treatment of Fetal Cardiac Disease, fetal echocardiographic screening of anti-SSA/Ro positive pregnancies is recommended either weekly or biweekly to surveil for development of anti-SSA/Ro-mediated fetal heart disease [Citation5]. While risk stratification based on maternal autoantibody titer levels and home surveillance with fetal handheld Doppler monitors are now increasingly used by select physicians caring for anti-SSA/Ro positive pregnancies [Citation3,Citation6–9], we and others have recently demonstrated using both commercially available and research testing that having low titers of both anti-SSA/Ro52 and SSA/Ro60 confer a lower risk of fetal heart disease [Citation2,Citation3,Citation10]. No universal evidence-based guidelines exist for risk stratification, management, or treatment of these patients. A previous survey published in the field of rheumatology further highlighted the lack of official guidelines and variations in management [Citation11].
Due to the paucity of data guiding clinical management of anti-SSA/Ro autoantibody positive pregnancies, we hypothesized that wide practice variations exist in both the approach to prenatal surveillance and management of detected fetal AVB and extranodal fetal cardiac disease, collectively referred to as cardiac neonatal lupus. To evaluate this hypothesis, we developed an electronic survey to collect data on current worldwide monitoring protocols and treatment strategies employed by pediatric cardiologists and maternal-fetal medicine specialists.
Materials and methods
The electronic survey study was distributed through the Fetal Heart Society to its members as well as to the North American Fetal Therapy Network membership and maternal-fetal medicine colleagues of the study team. This study was designated as quality improvement and granted a not research determination by the Children’s Minnesota Institutional Review Board. Completion of the electronic survey was voluntary and anonymous.
Study data were collected and managed using a REDCap (Research Electronic Data Capture) survey hosted at the University of Colorado [Citation12,Citation13]. Survey questions were developed collaboratively by the author working group during a series of virtual preparatory meetings. The created survey queried institution-based patient risk stratification methods, frequency and modes of fetal cardiac surveillance, treatment of various conduction system and extranodal abnormalities, and postnatal management of offspring of anti-SSA/Ro autoantibody positive pregnancies prior to initiation of the NIH-sponsored STOP-BLOQ (Surveillance and Treatment to Prevent Fetal Atrioventricular Block Likely to Occur Quickly) clinical trial. Survey responses are described in aggregate and summarized as frequencies and percentages.
Results
The questionnaire was completed by 101 respondents (), 73.3% pediatric cardiologists and 26.7% maternal-fetal medicine physicians. More than half of respondents reported >10 years’ post-training clinical experience in their medical field. The majority of respondents identified their institution as academic (79.2%), with 12.9% in private practice. The survey captured 59 unique institutions (18 duplicates, 24 did not report their center); the geographical distribution of the respondent centers is shown in . Most centers (53.5%) self-reported estimated annual fetal echocardiogram volumes of 500–1500. Nearly two-thirds of respondents estimated their annual anti-SSA/Ro pregnancy referrals as between 5 and 20 (61.2%), with 17.8% reporting more than 20 cases per year.
Figure 1. Geographical distribution of 59 unique respondent centers: 74.6% US centers, 8.5% Canadian, and 16.9% other international.
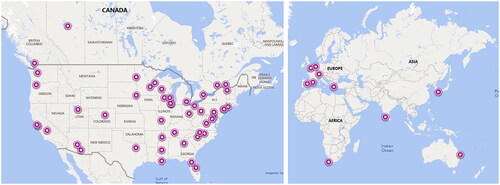
Table 1. Survey respondents’ training and practice center volumes.
Pregnancy risk stratification
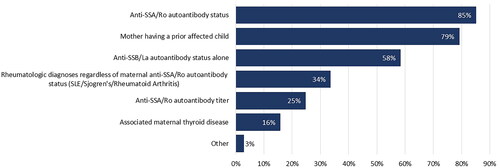
Monitoring strategies for patients with anti-SSA/Ro positive pregnancies were based on a spectrum of maternal factors (), the most common being positive anti-SSA/Ro autoantibody status (85.2%) and patient history of a previously affected child (79.2%). Respondents reported that clinical experience (82.2%) and medical literature (11.9%), as well as guidance from fetal cardiology colleagues and other field experts, inform their pregnancy management decisions.
Although positive anti-SSA/Ro autoantibody status was a primary indication for fetal monitoring, the vast majority (79.2%) of respondents indicated that their practice does not risk-stratify individual patients by autoantibody titer level. Those that considered anti-SSA/Ro titer levels for risk stratification reported a variety of thresholds for low and high cutoffs. Approximately half (44.6%) of respondents shared that their institutional lab provides test results for anti-Ro/SSA antibodies as positive if > 1 antibody index (AI) with values between 1 and 8 AI and > 8 AI (BioPlex assay) with no further titering. Only 20.8% reported the option for testing at laboratories with a broader ranges of positive values.
In addition, 33.7% of respondents reported routine fetal cardiac surveillance for pregnant patients with rheumatologic diagnoses (systemic lupus erythematosus, Sjögren’s disease, rheumatoid arthritis) regardless of anti-SSA/Ro autoantibody status. Surprisingly, 58.4% reported surveilling pregnancies with only anti-SSB/La positivity in the absence of anti-SSA/Ro.
Methods of pregnancy surveillance
Fetal echocardiography
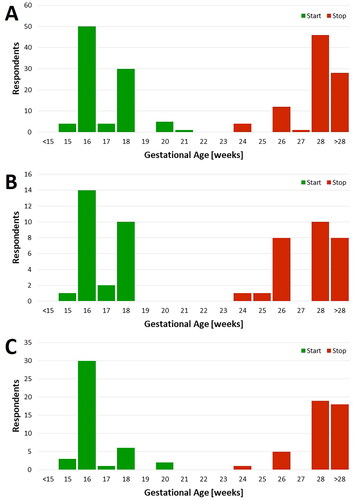
Fetal echocardiography was reported as the primary tool for fetal surveillance (94.1%), with monitoring frequencies of weekly (31.6% of respondents), every-other-week (51.6%), monthly (11.6%), and less than monthly (5.3%). Fetal echocardiography assessment included: fetal heart rate (98.9%), atrioventricular (AV) interval (94.7%), heart rhythm (94.7%), pericardial effusion (92.6%), AV valve regurgitation (91.6%), general cardiac function (89.5%), and evaluation for endocardial fibroelastosis (83.2%). The gestational age (GA) distributions of echo surveillance are shown in with most respondents reporting surveillance initiation between 16 and 18 weeks and discontinuation after 28 weeks. Many respondents apply a uniform monitoring protocol that is occasionally modified based on observed individual patient risk, but the majority do not modify their monitoring protocol for perceived high-titer patients (63.2%). Of those that do, some increased echo frequency (11.6%), lengthen surveillance duration (3.2%), or both (3.2%). Additionally, most respondents do not modify their echo monitoring protocols for patients with a previously affected child (52.6%), while a minority increase echo frequency (28.4%), lengthen surveillance duration (5.3%), or both (11.6%). For findings of fetal valvar regurgitation or increased myocardial echogenicity without changes in the rhythm/mechanical PR interval, 64.2% of respondents would increase the frequency of echo surveillance.
At-home handheld Doppler monitoring
Home handheld fetal heart rate monitoring (FHRM) is used by 27.7% of respondents to monitor anti-SSA/Ro pregnancies. Of those respondents who utilize home FHRM, Doppler monitors are provided to patients who are anti-SSA/Ro autoantibody positive (71.4%), have had a previously affected child (57.1%), and/or have a perceived high anti-SSA/Ro autoantibody titer (42.9%); 92.9% of respondents that utilize handheld FHRM for surveillance have a group 24-h on-call cardiologist to respond to abnormal FHRM calls. The gestational age range for handheld FHRM surveillance initiation and completion is shown in . Similar to the reported echo surveillance windows, most respondents using FHRM recommend initiating home monitoring between 16 and 18 weeks and discontinuing after 26 weeks.
In-office fetal heart rate monitoring
Nearly half (45.5%) of respondents report using weekly or biweekly in-office FHRM using either Doptone or ultrasound to surveil anti-SSA/Ro autoantibody pregnancies for development of 3° AVB. Respondents reported commencing the in-office monitoring around 16 weeks and continuing until ≥ 28 weeks ().
Maternal lab assessment
Only a small proportion of respondents (18.8%) recommended routine laboratory electrolyte testing and, if needed, replenishing maternal 25(OH) Vitamin D, calcium, and magnesium levels. Nearly 40% of respondents said their practice recommended checking thyroid studies (TSH/free T4) and anti-thyroglobulin antibodies if these have not already been obtained.
Detection and treatment of affected pregnancies
Sixty-three percent of respondents reported that their current surveillance protocols have successfully detected incomplete (1° or 2°) AVB. On the other hand, 75.2% of respondents reported that they have had patients who were not undergoing routine surveillance present with fetal 1°, 2°, or 3° AVB. Once AVB is detected, the respondents reported a wide variety of medical treatment strategies, outlined in .
Table 2. Variation in treatment of fetal atrioventricular block.
Routine postnatal care for uneventful anti-SSA/Ro positive pregnancies also varied for respondents, with 77.2% reporting routine postnatal ECGs, 36.6% obtaining postnatal echocardiograms, 18.8% conducting neonatal complete blood count and liver enzyme assessment, and 6.9% recommending minimizing sun exposure in the first 6 months of life.
Sub-specialty practice differences
No major difference in risk-stratification by titer, monitoring strategy, or use of echocardiography were found between cardiologists and maternal-fetal medicine physicians. Maternal-fetal medicine respondents monitored with echocardiography slightly more frequently than cardiology practitioners (44% vs 23% weekly, 37 vs. 53% bi-weekly, 7% vs. 20% monthly or less).
Discussion
Our survey results demonstrate that wide practice variation currently exists worldwide in patient risk stratification, surveillance, and treatment approaches for affected offspring of anti-SSA/Ro autoantibody positive pregnancies, in spite of scientific statements that provide care recommendations [Citation5]. Recent publications by the maternal-fetal medicine and fetal cardiology communities continue to highlight the ongoing controversies and dearth of evidence-based guidelines regarding optimal prenatal surveillance and management for this disease [Citation14–16]. Based on our survey results, most physicians rely on individual clinical experience, rather than on medical literature, to drive decision-making.
We were surprised to find that approximately half of survey respondents routinely surveilled pregnant patients afflicted with rheumatologic diagnoses in the absence of anti-SSA/Ro autoantibodies. It is well accepted that the presence of maternal anti-La/SSA autoantibodies in the absence of anti-SSA/Ro is uncommon. A previous study by Brito-Zeron et al. found that fetal 3° AVB resulting from isolated anti-La/SSA autoantibodies is exceptionally rare with <1% of reported cases in the published literature [Citation17].
Routine screening for anti-SSA/Ro and anti-SSB/La antibodies among pregnant women is important, as in addition to the impact on the fetus, anti-SSA/Ro antibodies in asymptomatic mothers have also been associated with a new-onset atrioventricular conduction disturbance without a medical history of neonatal lupus [Citation18]. Further study is needed to optimize screening recommendations in anti-SSA/Ro autoantibody positive pregnancies to not only standardize practice, but to minimize potential cost burden as surveillance may not be necessary in pregnancies with titers below a certain antibody threshold [Citation2].
Currently, two studies are underway that will lead to necessary evidence-based guidelines. The first is the ongoing prospective AVB study STOP BLOQ (Surveillance and Treatment to Prevent Fetal Atrioventricular Block Likely to Occur Quickly; ClinicalTrials.gov identifier: NCT04474223). The STOP BLOQ study risk stratifies pregnancies by anti-SSA/Ro autoantibody titer (low risk, <1,000 ELISA units [EU] and high risk, >1,000 EU), employs thrice-daily home Doppler monitoring in high-titer pregnancies, and investigates the efficacy of rapid initiation of steroid and IVIG treatment on fetal 2° AVB to restore normal rhythm or prevent progression to 3° AVB and/or extranodal disease [Citation10]. The second study is the Slow Heart Registry of Fetal Immune-mediated High Degree Heart Block (ClinicalTrials.gov identifier: NCT04559425), which prospectively compares morbidity and mortality between treated and untreated fetuses with AVB through the first 2 years of life.
The primary limitation of this study is the limited responses received, of which the vast majority of respondents were from medical practices in the US. The results of this study primarily reflect the experience of physicians in developed economies; there were only a few respondents from developing economies including Asia, South America, or Africa, and we therefore cannot report how physicians in these areas manage these pregnancies with more limited resources. In addition, since the majority of responses were from academic medical groups, it is difficult to draw conclusions on management differences that may occur in private practice maternal fetal medicine and pediatric cardiology groups.
Although a rare disease, affecting only ∼2–5% of pregnancies complicated by anti-SSA/Ro autoantibodies, cardiac neonatal lupus is associated with significant morbidity and mortality, including lifelong permanent pacing, dilated cardiomyopathy, heart failure, heart transplantation, and/or premature death [Citation15,Citation16]. Without consistent and collaborative surveillance and management of pregnancies complicated by anti-SSA/Ro autoantibodies, patients may receive inequitable and inadequate surveillance and/or treatment. Evidenced-based clinical practice guidelines are needed to detect disease in time for treatment and to assure physicians that the risk benefit ratio favors surveillance and in most cases, in utero treatment.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Izmirly PM, Saxena A, Kim MY, et al. Maternal and fetal factors associated with mortality and morbidity in a multi-racial/ethnic registry of anti-SSA/Ro-associated cardiac neonatal lupus. Circulation. 2011;124(18):1–7. doi: 10.1161/CIRCULATIONAHA.111.033894.
- Jaeggi E, Kulasingam V, Chen J, et al. Maternal anti-Ro antibody titers obtained with commercially available immunoassays are strongly associated with immune-mediated fetal heart disease. Arthritis Rheumatol. 2023;75(9):1556–1565. doi: 10.1002/art.42513.
- Kaizer AM, Lindblade C, Clancy R, et al. Reducing the burden of surveillance in pregnant women with no history of fetal atrioventricular block using the negative predictive value of anti-Ro/SSA antibody titers. Am J Obstet Gynecol. 2022;227(5):761.e1. doi: 10.1016/j.ajog.2022.05.071.
- Reed JH, Clancy RM, Lee KH, et al. Umbilical cord blood levels of maternal antibodies reactive with p200 and full-length Ro 52 in the assessment of risk for cardiac manifestations of neonatal lupus. Arthritis Care Res (Hoboken). 2012;64(9):1373–1381. doi: 10.1002/acr.21704.
- Donofrio MT, Moon-Grady AJ, Hornberger LK, et al. Diagnosis and treatment of fetal cardiac disease: a scientific statement from the American Heart Association. Circulation. 2014;129(21):2183–2242. doi: 10.1161/01.cir.0000437597.44550.5d.
- Cuneo BF, Moon-Grady AJ, Sonesson SE, et al. Heart sounds at home: feasibility of an ambulatory fetal heart rhythm surveillance program for anti-SSA-positive pregnancies. J Perinatol. 2017;37(3):226–230. doi: 10.1038/jp.2016.220.
- Cuneo BF, Sonesson SE, Levasseur S, et al. Home monitoring for fetal heart rhythm during anti-Ro pregnancies. J Am Coll Cardiol. 2018;72(16):1940–1951. doi: 10.1016/j.jacc.2018.07.076.
- Kaplinski M, Cuneo BF. Novel approaches to the surveillance and management of fetuses at risk for anti-Ro/SSA mediated atrioventricular block. Semin Perinatol. 2022;46(4):151585. doi: 10.1016/j.semperi.2022.151585.
- Jaeggi E, Laskin C, Hamilton R, et al. The importance of the level of maternal anti-Ro/SSA antibodies as a prognostic marker of the development of cardiac neonatal lupus erythematosus a prospective study of 186 antibody-exposed fetuses and infants. J Am Coll Cardiol. 2010;55(24):2778–2784. doi: 10.1016/j.jacc.2010.02.042.
- Buyon JP, Masson M, Izmirly CG, et al. Prospective evaluation of high titer autoantibodies and fetal home monitoring in the detection of atrioventricular block among anti-SSA/Ro pregnancies. Arthritis Rheumatol. 2024;76(3):411–420.
- Clowse ME, Eudy AM, Kiernan E, et al. The prevention, screening and treatment of congenital heart block from neonatal lupus: a survey of provider practices. Rheumatology (Oxford). 2018;57(suppl_5):v9–v17. doi: 10.1093/rheumatology/key141.
- Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208.
- Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010.
- For Maternal-Fetal Medicine. Electronic Address Pso S, Silver R, Craigo S, et al. Society for Maternal-Fetal Medicine Consult Series #64: systemic lupus erythematosus in pregnancy. Am J Obstet Gynecol. 2023;228(3):B41–B60.
- Cuneo BF, Buyon JP, Sammaritano L, et al. Knowledge is power: regarding SMFM consult series #64: systemic lupus erythematosus in pregnancy. Am J Obstet Gynecol. 2023;229(4):361–363. doi: 10.1016/j.ajog.2023.06.040.
- Osmundson SS, Grobman W, Silver R, et al. Society for maternal-fetal medicine response to Cuneo et al. Am J Obstet Gynecol. 2023;229(4):364–365. doi: 10.1016/j.ajog.2023.06.039.
- Brito-Zerón P, Izmirly PM, Ramos-Casals M, et al. The clinical spectrum of autoimmune congenital heart block. Nat Rev Rheumatol. 2015;11(5):301–312. doi: 10.1038/nrrheum.2015.29.
- Lazzerini PE, Capecchi PL, Laghi-Pasini F. Isolated atrioventricular block of unknown origin in adults and anti-Ro/SSA antibodies: clinical evidence, putative mechanisms, and therapeutic implications. Heart Rhythm. 2015;12(2):449–454. doi: 10.1016/j.hrthm.2014.10.031.