Abstract
Objective
Postpartum hemorrhage is a leading cause of maternal mortality and morbidity around the globe. The novel low-suction vacuum hemorrhage device (VHD) provides an alternative treatment option for cases of postpartum hemorrhage when first-line uterotonic agents fail. This systematic review aims to review current data evaluating the overall efficacy and safety of VHDs in treating postpartum hemorrhage.
Methods
We searched CINAHL Ultimate, Academic Search Premier, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, MEDLINE with Full Text, and PubMed and reference lists of retrieved studies for eligible studies that included outcomes of effectiveness, efficacy, or safety. Two independent reviewers used Covidence.org to screen Titles and Abstracts for 69 studies of which six were included in the analysis. Secondary outcomes measured across studies included time to bleeding control, total device deployment time, and adverse effects.
Results
Six nonrandomized trials (N = 1018 participants) included studies conducted in Indonesia, the United States, Switzerland, and Canada. The VHDs were found to have 90% effectiveness in achieving bleeding control across the studies. For most patients, this was achieved in <5 min and required a total device deployment time of 3 h. Reported adverse events were not considered life-threatening, including endometritis in 11 patients and red blood cell transfusions in 38% of patients.
Conclusion
VHDs have the potential to be used as a rapidly effective means for mechanical intervention of postpartum hemorrhage. The efficacy and safety of VHDs must be further studied at the randomized controlled trial level to determine their clinical usage.
Introduction
The World Health Organization (WHO) reports approximately 800 people worldwide die per day from preventable pregnancy and childbirth-related causes, with a quarter due to postpartum hemorrhage [Citation1]. Uterine atony, a lack of uterine muscle tone, contributes to 80% of cases of PPH and can occur in both vaginal and cesarean births. Certain predisposing factors for PPH include bleeding disorders, placenta previa or accreta, multiple gestation, grand multiparity, uterine leiomyomas, operative delivery, or personal history of PPH [Citation2,Citation3].
The United States (US) has the highest maternal mortality ratio of developed nations, with the National Vital Statistics System (NVSS) reporting an average of 32.9 maternal deaths per 100,000 live births, making PPH the third leading cause of maternal death [Citation4,Citation5]. Despite efforts to improve early identification and intervention in cases of PPH, rates have continued to increase from 2.7% in 2009 to 4.3% of US births in 2019 [Citation6].
The treatment for PPH must begin immediately and includes bimanual uterine compression and the use of uterotonic agents [Citation2,Citation3,Citation7]. Intrauterine pack and balloon devices are indicated as adjunctive therapy when uterotonic medications prove to be insufficient or as alternative therapy if medications are contraindicated. In most cases, uterine balloon tamponade is deployed along with continued medications to aid in controlling hemorrhage, measuring blood loss, and allowing for stabilization and reevaluation [Citation2,Citation7]. Balloon tamponade has proven more successful in minimizing blood loss when compared with uterine packing in a 2022 systematic review and meta-analysis of observational studies [Citation8].
Balloon tamponades (e.g. the Bakri balloon) are 87.1% effective in controlling atonic postpartum hemorrhage [Citation9] and have been described previously [Citation2,Citation10]. The Bakri balloon was introduced in 1999, with several devices (e.g. Rüsch hydrostatic balloon catheter, hydrostatic condom catheter) following suit [Citation11,Citation12], and has since been studied in both randomized and nonrandomized settings to evaluate its efficacy and safety as described in a recent comprehensive systematic review [Citation9]. However, based on available data, it is unlikely these devices are effective when used alone, and the World Health Organization (WHO) released an updated recommendation stating balloon tamponade should only be used in clinical sites where other supportive interventions are available [Citation13].
Recent innovations in medical device engineering, such as vacuum hemorrhage control, have mechanisms of action that promote clinical PPH management while complimenting the body’s physiologic response [Citation10]. Current prospective research on the vacuum hemorrhage control device known as the JadaⓇ System manufactured by Organon (Jersey City, NJ), included a first-in-human feasibility study in Indonesia followed by a large-scale, multicenter study conducted in the US, the success of which led to FDA approval in 2020 [Citation10,Citation14]. The system utilizes a novel, low-suction vacuum technology to control bleeding in PPH [Citation10,Citation15]. Through its novel design, it effectively empties the uterine cavity of blood into a collection container and collapses the uterus using negative pressure. The device stays in place for a minimum of 1.5 h and up to 24 h, depending on patient status and provider discretion [Citation10]. The features of the JadaⓇ System safeguard against drawbacks of preceding devices, such as the risk of perforation with curettage, while encouraging the uterus to work in tandem with the hemorrhage control device, unlike balloon or packing tamponade.
While existing research is mainly limited to observational cohort studies, to the best of our knowledge, this is the first systematic review to analyze current data evaluating the effectiveness and safety of vacuum hemorrhage control in the management of PPH.
Materials and methods
This protocol was registered with the International Platform of Registered Systematic Review and Meta-Analysis Protocols (INPLASY) on 17 October 2023 and is available at doi: 10.37766/inplasy2023/10.0058 [Citation16]. An ethics statement is not applicable because this study is based exclusively on published literature.
Search strategy
The authors used EBSCOHost to simultaneously search CINAHL Ultimate, Academic Search Premier, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, and MEDLINE. PubMed was searched separately using the following Boolean search string, which was developed with assistance from a health-science librarian: (“postpartum” or “postnatal”) AND (“hemorrhage” or “hemorrhage” or “hemorrhaging” or “haemorrhaging”) AND (“vacuum device” or “Jada”). Search results were limited to peer-reviewed studies and included literature from inception until October 4, 2023. Two independent reviewers (LMC & AVK) used Covidence to screen and confirm eligibility [Citation17]. For eligible studies, data were independently extracted, and conflicts were resolved by a consensus-based discussion or arbitration by a third reviewer (SJR). The reference lists of included studies were manually searched to identify additional qualifying studies.
Study selection and eligibility criteria
Eligible studies included Randomized controlled trials (RCTs), nonrandomized studies of interventions, and case studies that reported quantitative data on efficacy, effectiveness, or safety profiles. Additional inclusion criteria applied were patients diagnosed with postpartum hemorrhage and studies written in English. Studies were excluded if they did not use a vacuum or suction device to manage postpartum hemorrhage, as well as nonquantitative and nonhuman studies.
Data extraction and risk of bias assessment
All data used in this systematic review were aggregated using Microsoft Excel (Microsoft, Inc. Redmond, WA, USA). The risk of bias of included nonrandomized studies and case series was independently assessed by two independent reviewers (LMC & AVK) according to the Joanna Briggs Institute (JBI) critical appraisal checklist for cohort studies and case series tools (Supplemental Table 1 and 2) [Citation18,Citation19].
Data processing
For this review, efficacy (treatment success) was defined as hemorrhage control without the need for escalatory interventions. Data were collected and reported as efficacy rates. Studies that did not directly report the efficacy rate but included the rate of escalatory intervention were used to estimate an indirect efficacy rate by subtracting the number of patients receiving escalatory intervention from the treated population. Weighted means were calculated to determine average overall efficacy rates and adjusted for comparison. The categorical data reported as time for bleeding control were assigned categories of <1 min, 1–5 min, or ≥5 min for means of comparison.
Results
Study selection
The search strategy resulted in 81 relevant citations. After screening the titles and abstracts, eight articles remained and underwent full-text review. Consensus was reached on six studies which were retained in the present systematic review [Citation14,Citation20–24]. This study selection process is represented in the PRISMA Flow Diagram (Supplemental Figure 1) [Citation25]. One study was excluded because its data on VHD success is duplicated from another included study [Citation23] for research question expansion and the other excluded for missing data. Four of the included studies demonstrate moderate (n = 3) [Citation14,Citation20,Citation22] to high (n = 1) [Citation23] risk of bias (Supplemental Table 1 and 2).
Study characteristics
The characteristics of the included studies are summarized in . Study locations included the United States [Citation20,Citation21,Citation23], Indonesia [Citation14], Switzerland [Citation24], and Canada [Citation22], and ranged from 2016 to 2023 with a total population of 1,018. Of the six reviewed texts, three were retrospective [Citation21–23], and three were prospective in design, including one proof-of-concept and two observational cohort studies [Citation14,Citation20,Citation24]. Of the six studies, four included patients with PPH due to atony [Citation14,Citation20,Citation22,Citation23], with one study including atony and atony plus other causes (retained placenta, coagulopathy, vaginal laceration, placental abruption, cervical laceration, and placenta accreta) [Citation21], and another including PPH due to atony or non-specified placental pathology [Citation24].
Table 1. Characteristics of the included studies.
The vacuum hemorrhage device (VHD) used in five of the six analyzed studies was the FDA-approved JadaⓇ System [Citation14,Citation20–23]. The sixth study included in the review uniquely utilized a modified Bakri balloon uterine balloon tamponade device inflated to a much smaller volume than typically indicated and connected to a vacuum suction [Citation24]. The studies utilizing the JadaⓇ System deployed the vacuum suction at a pressure of 80 mmHg ±10 mmHg [Citation14,Citation20,Citation22,Citation23], versus the modified Bakri balloon system to produce uterine balloon tamponade which used an intrauterine pressure of 60–70 kPa or 450–525 mmHg [Citation24]. The reasons for device deployment were similar amongst studies reviewed and included a combination of first line uterotonic agent failure and estimated blood loss ≥500 ml [Citation14,Citation20,Citation22,Citation24]. Data collection time points ranged from at the time of intervention, only [Citation14,Citation22–24] through hospital discharge [Citation21] and 6-weeks status post [Citation20,Citation21].
Efficacy of VHD
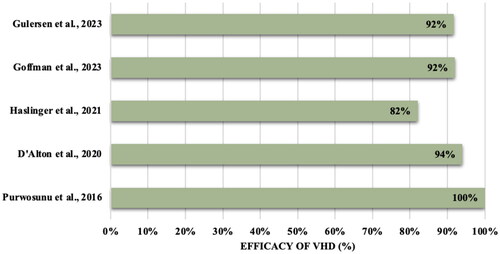
The main outcome of interest was the efficacy of VHD in the treatment of primary PPH. In the overall efficacy analysis, five studies demonstrated patients with postpartum hemorrhage who failed first line therapies and subsequently treated with a VHD achieved a 90% success rate in achieving bleeding control and preventing escalatory intervention (). The same studies included stratified data for patients with atonic PPH (n = 723) who were exposed to the same interventions and measures, demonstrating a 92% treatment success rate [Citation14,Citation20–22,Citation24]. When considering the studies that specifically used the JadaⓇ System, there was also a 92% success rate [Citation14,Citation20–22].
Figure 1. Efficacy (%) of vacuum hemorrhage device (VHD) in treating PPH across five observational cohort studies (N = 1018).
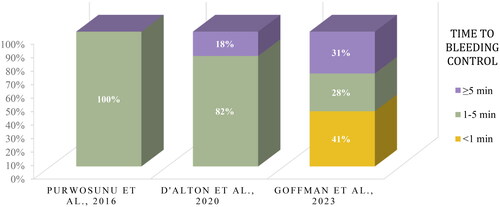
Time to bleeding control was assessed in three of the six included studies [Citation14,Citation20,Citation21]. After combining reported data into groups by time (<1 min., 1–5 min., or ≥5 min.) for comparison, most patients in this dataset achieved hemorrhage control in less than five minutes (). Nearly half (47%) of the patients described in this dataset achieved bleeding control within three minutes. Across four of the included studies, the device was deployed for an average of 3 h (2.5 h [Citation24], 3.18 h [Citation23], and 3.85 hours [Citation14,Citation20,Citation24]).
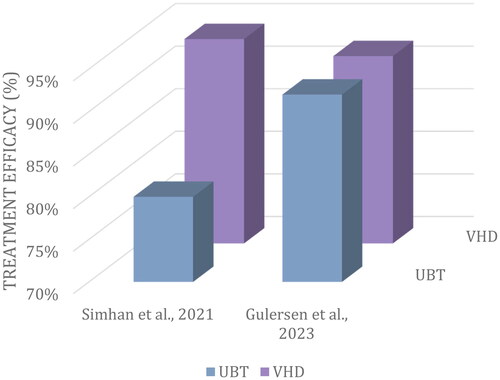
Two studies sought to compare intervention outcomes between VHD (n = 178) and uterine balloon tamponade (UBT) (n = 142) [Citation22,Citation23]. Patients treated with VHD benefited from an equal, if not greater, success rate as compared with the UBT treatment group (). The need for red blood cell (RBC) transfusions was reported in 38% of VHD participants versus 60% of participants in the UBT group [Citation22,Citation23].
Figure 3. Average efficacy (%) of UBT (n = 178) when compared to VHD (n = 142) intervention in primary PPH.
Safety outcomes related to the VHD included endometritis in 11 reported cases [Citation20,Citation21], disruption of vaginal laceration repair, bacterial vaginosis and vaginal candidiasis (each n = 1) [Citation20] and hemorrhagic shock (n = 1) [Citation21]. When treatment success with VHD was not obtained, routine escalation of care was initiated including additional surgical or nonsurgical treatments. Of the three studies that reported on treatment failure (n = 942), 0.2% of patients required B-lynch compression suture, 2.2% required hysterectomy, 35.4% required RBC transfusion, 6.7% required ICU admission, and 0.3% required uterine artery embolization [Citation20–22].
Discussion
The overall efficacy analysis conducted in this systematic review included five studies indicating a 90% success rate when VHD was used to treat PPH [Citation14,Citation20–22,Citation24]. For clinical context, a systematic review and meta-analysis on the efficacy and safety of the UBT device demonstrated an overall pooled success rate of 85.9% and 87.1% in cases of uterine atony only where our results demonstrated a 92% success rate for cases of uterine atony [Citation9]. In those cases where success was not achieved, patients were treated with surgical or other nonsurgical interventions, further emphasizing that not all PPH can be treated with first and second-line therapies [Citation26].
The device also achieved bleeding control within minutes for most patients in the dataset. In comparison, the Bakri balloon took an average of nine minutes to achieve hemostasis in patients with atonic PPH [Citation27]. While there has yet to be an RCT directly comparing the two devices, it is promising to observe rapid hemorrhage control with VHD treatment. The incorporation of an additional, effective medical device can improve the ability of providers and healthcare systems to manage postpartum hemorrhage better and prevent escalatory measures that have permanent effects, such as hysterectomy [Citation28]. However, despite the reported efficacy rate of VHDs, the lack of data on safety and adverse event outcomes may limit its ability to be incorporated into clinical practice.
The results of this systematic review show comparable efficacy between a VHD and a UBT device, which suggests the novel VHD may have the potential to become an equivalent option to the much-used UBT in the management of PPH. This result draws from two studies in the review, totaling 320 participants, limiting its generalizability [Citation22,Citation23]. Of note, in a recently published abstract on a retrospective study including 380 patients with PPH, rates of complications were similar between the two devices, concluding that in the setting where both devices are available, the rapid use of either device is likely to be more important than the device choice [Citation29].
A limitation of this review is the novelty of the device and the lack of data available on its use. The combined sample size for this systematic review is just above 1,000 participants, lending to concern about its overall generalizability. Although a safety profile was an intended outcome of this review, only two published studies reported on this measure, limiting the confidence of our recommendation. However, the safety profile was consistent with those expected risks in the setting of PPH and safety events were resolved without fatal complications, suggesting an acceptable risk-benefit profile [Citation20,Citation21].
Four of the included studies demonstrate moderate (n = 3) [Citation14,Citation20,Citation22] to high (n = 1) [Citation23] risk of bias, requiring their results and the overall results of this review to be interpreted with caution. The main limitation of this review is that it does not include any RCTs to examine the efficacy and safety of VHD for the treatment of PPH and, therefore, does not present a high level of evidence.
There is still a gap in knowledge regarding the safety and efficacy of VHDs for PPH, and the future implication of VHD being used in clinical practice would be benefited by more research in this area. More RCTs and prospective studies are needed to investigate incidence rates of adverse outcomes linked to using such a device to control PPH before it can be widely deployed. A higher level of evidence, such as will be generated from the ongoing RCT to be completed in 2027, will show data gathered compared to a control group and will allow for definitive conclusions for VHD characteristics [Citation28].
Conclusion
Given the high efficacy demonstrated when VHD is used for PPH, it is anticipated the utilization of VHDs is beneficial to help aid in PPH treatment. Subgroup analysis of our review suggests (1) that VHD can establish bleeding control for most of its participants in under five minutes. (2) Average device deployment time was 3 h. (3) VHD shows comparable efficacy to UBT devices.
Supplemental Material
Download MS Word (249.3 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data is freely available as it is based exclusively on published literature.
Additional information
Funding
References
- Maternal and perinatal health. Accessed October 13, 2023. https://www.who.int/teams/sexual-and-reproductive-health-and-research-(srh)/areas-of-work/maternal-and-perinatal-health.
- Poggi SH. Postpartum hemorrhage & the abnormal puerperium. In: DeCherney AH, Nathan L, Laufer N, Roman AS, editors. Current diagnosis & treatment: obstetrics & gynecology. 12th ed. McGraw Hill Education; 2019; p. 350–362. https://accessmedicine.mhmedical.com/content.aspx?bookid=2559§ionid=206960406.
- Gill P, Patel A, Van Hook JW. Uterine Atony. In: StatPearls. StatPearls Publishing; 2023. Accessed October 12, 2023. http://www.ncbi.nlm.nih.gov/books/NBK493238/.
- Pregnancy Mortality Surveillance System | Maternal and Infant Health | CDC. Published March 31, 2023. Accessed October 11, 2023. https://www.cdc.gov/reproductivehealth/maternal-mortality/pregnancy-mortality-surveillance-system.htm.
- Declercq E, Thoma M. Measuring US maternal mortality. JAMA. 2023;330(18):1731–1732. doi: 10.1001/jama.2023.19945.
- Corbetta-Rastelli CM, Friedman AM, Sobhani NC, et al. Postpartum hemorrhage trends and outcomes in the United States, 2000-2019. Obstet Gynecol. 2023;141(1):152–161. doi: 10.1097/AOG.0000000000004972.
- Belfort MA. Overview of post partum hemorrhage; [updated 2024 April 30]. Available from: https://www.uptodate.com/contents/overview-of-postpartum-hemorrhage?search=overview%20of%20post%20partum%20hemorrhage&source=search_result&selectedTitle=1%7E150&usage_type=default&display_rank=1.
- Abul A, Al-Naseem A, Althuwaini A, et al. Safety and efficacy of intrauterine balloon tamponade vs uterine gauze packing in managing postpartum hemorrhage: a systematic review and meta-analysis. AJOG Glob Rep. 2022;3(1):100135. doi: 10.1016/j.xagr.2022.100135.
- Suarez S, Conde-Agudelo A, Borovac-Pinheiro A, et al. Uterine balloon tamponade for the treatment of postpartum hemorrhage: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;222(4):293.e1–293.e52. doi: 10.1016/j.ajog.2019.11.1287.
- D’Alton M, Rood K, Simhan H, et al. Profile of the Jada® System: the vacuum-induced hemorrhage control device for treating abnormal postpartum uterine bleeding and postpartum hemorrhage. Expert Rev Med Devices. 2021;18(9):849–853. doi: 10.1080/17434440.2021.1962288.
- Bakri Y, B-Lynch C, Alouini S. Second generation of intrauterine balloon tamponade: new perspective. BMJ Innov. 2020;6(1):1–3. doi: 10.1136/bmjinnov-2019-000404.
- Danso D, Reginald P. Internal uterine tamponade. Postpartum Hemorrhage New Thoughts New Approaches Int Fed Obstet Gynecol. Published online January 1, 2006.
- Weeks AD, Akinola OI, Amorim M, et al. World Health Organization recommendation for using uterine balloon tamponade to treat postpartum hemorrhage. Obstet Gynecol. 2022;139(3):458–462. doi: 10.1097/AOG.0000000000004674.
- Purwosunu Y, Sarkoen W, Arulkumaran S, et al. Control of postpartum hemorrhage using vacuum-induced uterine tamponade. Obstet Gynecol. 2016;128(1):33–36. doi: 10.1097/AOG.0000000000001473.
- Support and Resources for the JADA® System. Accessed October 17, 2023. https://www.thejadasystem.com/resources/.
- Card L, Klinkowski A, Rose S, Sacred Heart University, et al. Innovations in Postpartum Hemorrhage Care: a Systematic Review of Vacuum Hemorrhage Control Devices (Protocol). INPLASY - International Platform of Registered Systematic Review and Meta-analysis Protocols. 2023; doi: 10.37766/inplasy2023.10.0058.
- Veritas Health Innovation. Covidence systematic review software. Covidence. Published 2023. Accessed October 10, 2023. https://www.covidence.org/.
- Munn Z, Barker TH, Moola S, et al. Methodological quality of case series studies: an introduction to the JBI critical appraisal tool. JBI Evid Synth. 2020;18(10):2127–2133. doi: 10.11124/JBISRIR-D-19-00099.
- Moola S, Munn Z, Tufanaru C, et al. Systematic reviews of etiology and risk. Vol. 5. In: Joanna Briggs Institute reviewer’s manual. Adelaide, Australia: The Joanna Briggs Institute; 2017. p. 217–269.
- D’Alton ME, Rood KM, Smid MC, et al. Intrauterine vacuum-induced hemorrhage-control device for rapid treatment of postpartum hemorrhage. Obstet Gynecol. 2020;136(5):882–891. doi: 10.1097/AOG.0000000000004138.
- Goffman D, Rood KM, Bianco A, et al. Real-world utilization of an intrauterine, vacuum-induced, hemorrhage-control device. Obstet Gynecol. 2023;142(5):1006–1016. doi: 10.1097/AOG.0000000000005366.
- Gulersen M, Gerber RP, Rochelson B, et al. Vacuum-induced hemorrhage control versus uterine balloon tamponade for postpartum hemorrhage. J Obstet Gynaecol Can. 2023;45(4):267–272. doi: 10.1016/j.jogc.2023.02.017.
- Simhan H, Sakamoto S, Seasely AR, et al. Balloon tamponade versus a novel vacuum-induced hemorrhage control device for treatment of postpartum uterine bleeding. Am J Obstet Gynecol. 2021;224(2):S683. doi: 10.1016/j.ajog.2020.12.1132.
- Haslinger C, Weber K, Zimmermann R. Vacuum-induced tamponade for treatment of postpartum hemorrhage. Obstet Gynecol. 2021;138(3):361–365. doi: 10.1097/AOG.0000000000004510.
- Haddaway NR, Page MJ, Pritchard CC, et al. PRISMA2020: an R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and open synthesis. Campbell Syst Rev. 2022;18(2):e1230. doi: 10.1002/cl2.1230.
- Liu LY, Nathan L, Sheen JJ, et al. Review of current insights and therapeutic approaches for the treatment of refractory postpartum hemorrhage. Int J Womens Health. 2023;15:905–926. doi: 10.2147/IJWH.S366675.
- Darwish AM, Abdallah MM, Shaaban OM, et al. Bakri balloon versus condom-loaded Foley’s catheter for treatment of atonic postpartum hemorrhage secondary to vaginal delivery: a randomized controlled trial. J Matern Fetal Neonatal Med. 2018;31(6):747–753. doi: 10.1080/14767058.2017.1297407.
- Tuuli M. Novel vacuum-induced hemorrhage control for postpartum hemorrhage: a multicenter randomized trial. clinicaltrials.gov; 2023. Accessed December 31, 2022. https://clinicaltrials.gov/study/NCT05382403.
- Shields LE, Foster M, Klein C, et al. 68 Prospective multicenter trial comparing balloon versus suction hemorrhage control devices for postpartum hemorrhage. Am J Obstet Gynecol. 2024;230(1):S51–S52. doi: 10.1016/j.ajog.2023.11.090.