Abstract
Until relatively recently, the gecko species Hemidactylus turcicus was believed to be the only member of the genus to occur in Jordan. Later taxonomic treatments confirmed the presence of another two species in the country – H. dawudazraqi and H. mindiae. It has been more than 10 years now since the last study on species of the genus Hemidactylus in Jordan was published and the distribution limits of the different species remain unknown. To shed light on the geographic and genetic boundaries between the species and different populations we collected material from across Jordan and sequenced each sample for two mitochondrial genes to provide identification by means of DNA barcoding. The results of phylogenetic analyses of species that occur in Jordan and neighbouring countries allowed us to assess the level of inter- and intraspecific genetic diversity of Hemidactylus in the region. Based on our results we confirm the presence of two species that were previously not known from the country – H. granosus and H. lavadeserticus. We also found another genetic lineage to be present in Jordan, implying that there may be yet another undescribed species of Hemidactylus present in Jordan. The results of this study will help to clarify the distribution of Hemidactylus species in Jordan and enable better delineation of the boundaries between the individual species.
Key words:
Introduction
The Levant region, a crossroad between continents and tectonic plates, supports one of the richest reptile diversity and endemism hotspots in the Western Palaearctic (Ficetola et al., Citation2018; Sindaco & Jeremčenko, Citation2008). The great diversity the region harbours is a result of a complex geomorphological and climatic history of the Neogene (Jandzik et al., Citation2018; Šmíd, Carranza, et al., Citation2013; Tamar et al., Citation2015). Lying at the southern edge of the Levant, Jordan with its over 90 species of squamate reptiles is a fitting example of a true biodiversity hotspot (Amr & Disi, Citation2011; Disi, Citation1996; Disi et al., Citation2001; Uetz et al., Citation2023).
Geckos (infraorder Gekkota) are represented in Jordan by nine genera in three families (Gekkonidae: Bunopus, Cyrtopodion, Hemidactylus, Mediodactylus, Stenodactylus, Trigonodactylus, and Tropiocolotes; Phyllodactylidae: Ptyodactylus; Sphaerodactylidae: Pristurus; Uetz et al., Citation2023). The most species-rich genera in Jordan are Hemidactylus with 2–3 species, Stenodactylus with 4 species, and Ptyodactylus with 3–4 species (Disi et al., Citation2001; Moravec et al., Citation2011; Nazarov et al., Citation2013). The exact diversity of species, however, remains unclear due to unresolved taxonomy and poor knowledge of their distribution and species limits (e.g., de Pous et al., Citation2016; Metallinou et al., Citation2015).
For instance, the genus Hemidactylus Goldfuss, 1820 had for long been represented in Jordan by a single and widely distributed species H. turcicus (Disi et al., Citation2001) until H. mindiae was discovered in the sandstone massifs of Wadi Ramm in the south of the country (Amr et al., Citation2007), and later on H. dawudazraqi was described from the western mountains and the neighbourhood of Azraq area (Moravec et al., Citation2011). The addition of these two species to the gecko fauna of Jordan resulted in the records of H. turcicus becoming uncertain, as it was not clear to which species they actually belong. Another species of a questionable status in Jordan is H. lavadeserticus that was described from the Syrian black basalt desert as a subspecies of H. turcicus and later on elevated to the species level (Moravec et al., Citation2011; Moravec & Böhme, Citation1997). The species is known only from its type locality in southern Syria, and although its occurrence in north-eastern Jordan is assumed (Amr et al., Citation2007, Citation2011; Disi et al., Citation2001, Citation2014; Moravec et al., Citation2011; Moravec & Böhme, Citation1997), it has never been formally confirmed. The basaltic outflows the species inhabits extend from Syria south to Jordan and northern Saudi Arabia, and its presence in these areas is thus fairly probable.
In this study we sampled Hemidactylus geckos broadly across Jordan to provide better understanding of the distribution of the individual species present in the country. We generated new genetic data of two mitochondrial genes for material originating from previously unsampled areas with the aim to infer their phylogenetic position within the genus and verify their species identification using methods of DNA barcoding. Our results allow detailed delineation of the distribution limits of the different species. Further, we confirm the presence of two species previously not recorded in Jordan and report the presence of a yet unnamed Hemidactylus species in southern Jordan.
Materials and methods
New material was collected during several fieldtrips carried out in Jordan by the authors in September 2019, and in October and November 2022. Additional material was donated by colleagues (see Acknowledgements). The specimens were captured, photographed, and released after a tiny portion of tail tip was removed for genetic analyses. The samples were stored in 96% ethanol. All captured specimens were tentatively identified in the field based on morphological characters detailed in previous taxonomic studies of the genus (Moravec et al., Citation2011; Moravec & Böhme, Citation1997; Šmíd, Moravec, et al., Citation2013).
We gathered available distribution records for all Jordanian Hemidactylus species from the published literature, museum catalogues, and our own and colleagues’ field observations. Localities were in most cases georeferenced using gazetteers in the primary literature. Localities that lacked geographic coordinates were georeferenced using the Geographic Names database (http://www.geographic.org/geographic_names/). The map was generated in ArcGIS 10.7 (ESRI 2011).
Genomic DNA was extracted from ethanol-preserved tail tip tissue samples using the DNeasy® Blood & Tissue Kit (Qiagen, Germany). We PCR-amplified two mitochondrial (mtDNA) genes 12S rRNA (12S) and cytochrome b (cytb) with the primers 12Sa and 12Sb for 12S, and L14910 and H16064 for cytb. More details on the primers, their sequences, original references, and PCR conditions are shown in Šmíd, Carranza, et al. (Citation2013). PCR products were then visualized on electrophoresis and bidirectionally Sanger-sequenced in Macrogen (the Netherlands). For this study, we generated 87 new sequences for 50 samples. Geneious R11 (Kearse et al., Citation2012) was used to inspect the raw sequence files, to assemble contigs, generate consensus sequences and concatenate alignments. An additional 78 sequences that were most similar to our newly generated ones based on BLAST searches and originating from the studies of Carranza and Arnold (Citation2006, Citation2012); Moravec et al. (Citation2011), and Šmíd et al. (Šmíd, Carranza, et al. Citation2013; Šmíd, Moravec, et al. Citation2013, Citation2023) were downloaded from GenBank. Based on the previously published phylogenies of the Hemidactylus geckos (references cited above) we used H. flaviviridis as an outgroup to root the tree.
Sequences of both genetic markers were aligned separately by MAFFT v.7 (Katoh et al., Citation2019). The Q-INS-I strategy that considers the secondary structure of the RNA was applied for the 12S, while the cytb was aligned using the default auto strategy. Sequences of cytb were translated into amino acids using the vertebrate mitochondrial genetic code and no stop codons were detected, indicating that no pseudogenes were amplified. All samples used in this study including information on their origin, GPS coordinates, and GenBank accession numbers are listed in Supplemental Appendix S1.
We performed a Maximum likelihood (ML) analysis using a concatenated dataset of the two mtDNA markers. The ML was carried out in IQ-TREE (Nguyen et al., Citation2015) using its online web interface W-IQ-TREE (Trifinopoulos et al., Citation2016). The concatenated alignment was partitioned by gene and the best substitution models were selected automatically for each gene by ModelFinder (Kalyaanamoorthy et al., Citation2017) as implemented in IQ-TREE. The best models of nucleotide substitution were identified as TIM2 + F + G4 for 12S and TIM + F + I + G4 for cytb. Branch support was assessed by the Shimodaira–Hasegawa-like approximate likelihood ratio test (SH-aLRT; Guindon et al., Citation2010) and the Ultrafast bootstrap approximation algorithm (UFBoot; Minh et al., Citation2013), both with 1000 replicates.
We also carried out a Bayesian inference (BI) using MrBayes v. 3.2.1 (Ronquist et al., Citation2012) with the same partitioning strategy as for the ML analysis. Character state frequencies, GTR substitution rates and Gamma shape parameters were unlinked for the partitions. We ran three independent runs for 10 million generations with sampling frequency every 10,000 generations. Stationarity was determined by the sequentially calculated standard deviations of the split frequencies being lower than 0.01. After inspecting that the runs had converged, we discarded as burn-in 10% of posterior trees from each run. A 50% majority-rule consensus tree was then produced from all post-burnin posterior trees. Nodes that received UFBoot ≥95, SH-aLRT ≥80, and Bayesian posterior probability (pp) ≥0.95 were considered strongly supported. Inter- and intraspecific uncorrected p-distances with pairwise deletion were estimated using MEGA X (Kumar et al., Citation2018).
Results
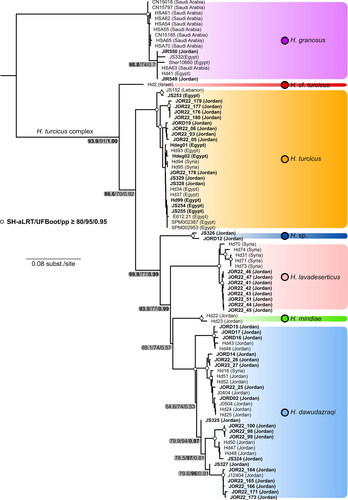
The final concatenated alignment of the two mtDNA markers included 92 samples. The total length was 1537 base pairs (bp) − 418 bp of 12S and 1137 bp of cytb. Both phylogenetic analyses resulted in an identical topology at deep nodes with a slight difference in the support values (see below). According to the results (), two samples from Aqaba in south-western Jordan (JIR549 and JIR550; ) belong to H. granosus (SH-aLRT = 96.3/UFBoot = 98/pp = 1.00, support values are given in this order hereafter) and are genetically close to other samples of that species from the Red Sea coast in Sinai (Egypt) and Saudi Arabia with strong nodal support (98.8/74/0.7). The clade of H. turcicus complex (sensu Moravec et al., Citation2011) consisted of six species and was fairly strongly supported (93.9/91/1.00); all species within the complex were also strongly supported. A species referred to as H. cf. turcicus in accordance with previous studies (Moravec et al., Citation2011; Šmíd, Carranza, et al., Citation2013) represented by a single sample from north-eastern Israel was recovered to be sister to the remaining species, but without convincing support (86.6/70/0.92). Hemidactylus turcicus represented by samples from north-western Jordan, Lebanon, Sinai (Egypt) and Syria was inferred to branch off next (99.9/77/0.99). The clade of Hemidactylus sp. with two samples from south-western Jordan was inferred as sister to the remaining three species (93.5/77/0.99). The remaining species H. lavadeserticus from the black lava desert of Syria and north-eastern Jordan, H. mindiae from Wadi Ramm in southern Jordan, and H. dawudazraqi from southern Syria and Jordan formed a clade (93.5/77/0.99), but the relationships between them remained unresolved. Hemidactylus dawudazraqi is genetically differentiated into geographically disparate subclades.
Fig. 1. Distribution of the genus Hemidactylus in Jordan and neighbouring countries. Large colourful symbols indicate the origin of the DNA-barcoded material. The light green symbols show records of H. mindiae that were not included in this study. Stars denote type localities. Black dots show literature records from Jordan that were referred to as H. turcicus and which need to be re-examined either morphologically or genetically. Colours correspond to those in .
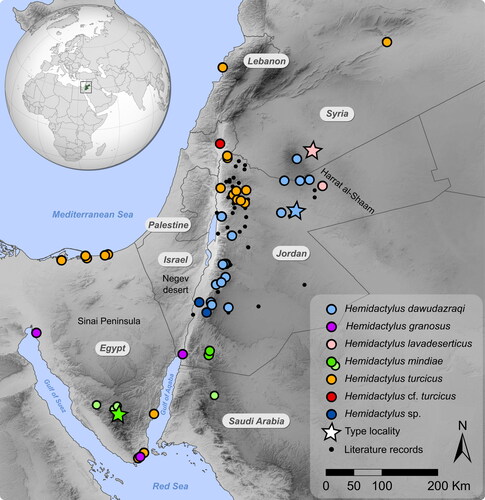
Fig. 2. Phylogenetic positions of the newly analysed samples from Jordan and neighbouring countries within the Hemidactylus phylogeny. The tree is a result of the ML analysis based on the concatenated mitochondrial dataset of 12S and cytb. Support values are indicated below nodes. White dots at nodes represent SH-aLRT ≥80, UFBoot ≥95 and Bayesian posterior probabilities ≥0.95. New samples are highlighted in bold. Colours correspond to those in . Note that H. granosus is not the sister lineage to the H. turcicus complex but is part of another clade within the Hemidactylus phylogeny (Šmíd, Moravec, et al., Citation2013, Citation2020, Citation2023). It was included here to show the position of the new samples from Aqaba.
Inter- and intraspecific uncorrected p-distances based on both 12S and cytb genes are summarized in . The genetic distances between the new samples of H. lavadeserticus from Jordan and those from Syria ranged between 0–0.3% in the 12S and 2.7–2.9% in the cytb. Two new samples of H. granosus differed from other samples from the rest of its range by 0–1.4% in the 12S and 0–1.3% in the cytb. Notable interspecific distances in the cytb were found between the putative new species from south-western Jordan and the remaining species (see ), and ranged between 9.9% (to H. cf. turcicus) and 11.7% (to H. lavadeserticus). Interspecific distances in the 12S were considerably lower and ranged between 2.6% (to H. mindiae) and 6.4% (to H. cf. turcicus). The highest intraspecific genetic variability was found in H. dawudazraqi (1.6% in the 12S and 4.6% in the cytb), and corresponds to the geographic structuring of the species.
Table 1. Mean genetic distances (uncorrected p-distances) between the Hemidactylus species included in this study based on the 12S (above the diagonal) and cytb (below the diagonal) mitochondrial genes. Intraspecific distances are shown on the diagonal in bold for cytb and 12S, respectively.
Discussion
Our study builds on previous studies focusing on Hemidactylus geckos in the Levant region (Šmíd, Carranza, et al., Citation2013; Šmíd, Moravec, et al., Citation2013) and in Jordan in particular (Amr et al., Citation2007; Moravec et al., Citation2011). It significantly expands the geographic sampling of Hemidactylus across Jordan and fills in many gaps in previously genetically unsampled areas (). It should be noted that the primary aim of this study was to provide detailed geographic delineation of the species present in Jordan and focus on species identification by means of DNA barcoding rather than resolving their phylogenetic relationships. Since we only analysed two mitochondrial markers it is not surprising that the phylogenetic relationships obtained in this study differ from those already published (e.g., the position of Hemidactylus cf. turcicus from north-eastern Israel, Moravec et al., Citation2011; Šmíd, Carranza, et al., Citation2013, Citation2020). The results of our phylogenetic analyses indicate that there are more species of Hemidactylus geckos in Jordan than previously thought. We confirm H. turcicus to be present in Jordan and for the first time we also report the presence of H. lavadeserticus and H. granosus.
After the taxonomic revision of Moravec et al. (Citation2011), the status of the presence of H. turcicus in Jordan was unclear as in that study the species was represented only by samples from Egypt, Syria and some other circum-Mediterranean countries. According to our results, H. turcicus seems to be restricted to the north-western part of Jordan, which matches the distribution of other Mediterranean elements in the country (Amr & Disi, Citation2011; Disi et al., Citation2001). The limiting factors that prevent H. turcicus to extend further south remain questionable. The north-west of the country receives on average the highest annual precipitation (Disi, Citation1996; Disi et al., Citation2001), and therefore one of the possible explanations could be that H. turcicus is less arid-adapted than H. dawudazraqi (as assumed from its known distribution). Our field observations also confirm its synanthropic lifestyle known from other parts of the species’ range (Baha El Din, Citation2006; Bar et al., Citation2021; Handal et al., Citation2016).
We herein also confirm the presence of H. lavadeserticus in Jordan. Until now, the species was known only from the type locality in Ar´Raqiyeh in southern Syria (Moravec & Böhme, Citation1997) and although it has been assumed to occur in Jordan as well (Amr et al., Citation2007, Citation2011; Moravec et al., Citation2011) there was no solid evidence for it. Our samples from the black lava desert of the Harrat Ash Shaam in the eastern Badia region of eastern Jordan correspond both genetically and morphologically to H. lavadeserticus from the type locality in Syria, which finally enables the conclusion to be drawn that the species is also present in Jordan. The new locality lies 11 km northeast of Safawi and about 65 km south-south-east from the species’ type locality. The general habitat of the area included several small wadis (up to 40 m wide) running in a north-to-south orientation. These wadis are surrounded by flat fields of basaltic rocks and boulders with a sparse vegetation cover (). All individuals were found shortly after sunset (after 18:10) moving actively on the ground among the basaltic rocks. Other reptile species found in syntopy with H. lavadeserticus were Ptyodactylus puiseuxi by night and Mesalina sp. 4 (sensu Sindaco et al., Citation2018), Pseudotrapelus sinaitus and Uromastyx aegyptia by day. The closest published records of Hemidactylus in the area are from Safawi and Buqayawiyah and are documented by two specimens housed at the collection of Jordan University Museum in Amman (Disi et al., Citation1999). These specimens, JUM 2257 (Safawi) and JUM 2258 (Buqayawiyah), show head scale arrangements (postmentals not touching second lower labial) typical for H. dawudazraqi and H. turcicus and thus do not pre-date the first records of H. lavadeserticus in Jordan presented here. Whether H. lavadeserticus extends across Jordan also into northern Saudi Arabia remains to be answered by further field surveys. Recent herpetofaunal inventories of the Turaif region and the Harat Al Harrah Protected Area did not confirm the presence of any Hemidactylus species (Aloufi et al., Citation2022; Al-Sadoon et al., Citation2016). However, the north of Saudi Arabia is rather empty in terms of Hemidactylus distribution records (see Sindaco & Jeremčenko, Citation2008; Šmíd et al., Citation2021) and the presence of the genus there cannot be completely ruled out. A continuous distribution along the black lava desert from Syria to Saudi Arabia is known for many other species (e.g., Stenodactylus grandiceps, Pseudotrapelus sinaitus, Trapelus agnetae, Pseudocerastes fieldi, Ptyodactylus puiseuxi) and it would not be surprising if the range of H. lavadeserticus followed the entire extent of the lava outflows as well.
Fig. 3. Individuals of Hemidactylus species and their habitats from Jordan. (3.1) Adult individual of H. dawudazraqi, with the ventral side of the head in the inset, from the vicinity of Qasr Amra, Jordan. (3.2) Habitat in the vicinity of Qasr Amra, Jordan. (3.3) Adult individual of H. granosus from Aqaba, Jordan. (3.4) Habitat at the Ancient Islamic City of Ayla in Aqaba, Jordan. (3.5) Adult individual of H. lavadeserticus from Jordan, with the ventral side of the head in the inset, from 11 km northeast of Safawi, Jordan. (3.6) Habitat 11 km northeast of Safawi, Jordan. Photos taken by the authors if not stated otherwise.

Hemidactylus granosus is another species that we formally report to occur in Jordan in this study. Two individuals were sampled in the Ancient Islamic City of Ayla in Aqaba. Both individuals were found during daytime under dry palm leaves (). Other species found in the same habitat were Cyrtopodion scabrum, Ptyodactylus cf. hasselquistii, and Chalcides ocellatus. The present record lies approx. 194 km from the nearest verified record of H. granosus in Sharm el-Sheikh at the tip of the Sinai Peninsula (Šmíd, Moravec, et al., Citation2013). The distribution of H. granosus covers most of north-western Arabia and extends along the Red Sea coasts to Egypt. Given the propensity of the species to live in close association with humans (Šmíd, Moravec, et al., Citation2013), its presence in Aqaba, a major port city of southern Jordan, is not that surprising. Curiously, the herpetology collection of the Museum of Comparative Zoology, Harvard, houses six Hemidactylus specimens collected by J.C. Philips and W.M. Mann in Aqaba in 1914 (collection accessions MCZ R9661-9666; Barbour, Citation1914). It would be of interest to examine these specimens to confirm their identification and possibly shed light on the timeline of Hemidactylus dispersal along the Red Sea. A sympatric occurrence of H. turcicus and H. granosus has already been reported from Sharm el-Sheikh (Šmíd, Moravec, et al., Citation2013) and therefore cannot be ruled out in Aqaba as well. What must be kept in mind though, is that the topology of the tree inferred here does not reflect the latest view on the relationships between the species as H. granosus belongs to a different part of the Hemidactylus phylogeny (Šmíd, Moravec, et al., Citation2013, Citation2020, Citation2023) and was included here solely to show the position of the new samples from Aqaba within the species.
Fig. 4. Individuals of Hemidactylus species and their habitats from Jordan. 4.1, Adult individual of H. mindiae from Wadi Ramm, Jordan (photo: David Modrý). 4.2, Habitat at Wadi Ramm, Jordan. 4.3, Adult individual of H. turcicus, with the ventral side of the head in the inset, from 20 km north of Amman, Jordan. 4.4, Habitat 20 km north of Amman, Jordan. Photos taken by the authors if not stated otherwise.

The results of our phylogenetic analyses () suggest the possible existence of a yet unnamed species of Hemidactylus in south-western Jordan. The phylogenetic results indicate that the species belongs to a clade together with H. lavadeserticus, H. mindiae, and H. dawudazraqi. It must however be born in mind that our results are based only on two mitochondrial genes and that a broader sampling of loci will be necessary to infer the position of this lineage within the genus with confidence. Our sampling of this lineage was very limited as it comprised only two tissue samples from Wadi Araba and Ar Rajiv, two localities separated by approx. 21 km, which along with the lack of voucher specimens for morphological examination prevents us from drawing taxonomic conclusions. In terms of genetic distances, the species’ differentiation from the other Hemidactylus species is at a similar level to differences observed between other species pairs of the H. turcicus complex (), implying that a species-level status may be warranted. This will however require a more thorough taxonomic revision with more material examined and more lines of evidence laid out. Whether this species is restricted to Wadi Araba only or if it reaches the adjoining Negev Desert and extends further west into Sinai in Egypt remains to be answered with a broader sampling from the neighbouring countries where H. turcicus is also widespread (Baha El Din, Citation2006; Bar et al., Citation2021; Handal et al., Citation2016; Werner, Citation2016).
The findings presented here indicate that the diversity of Hemidactylus geckos in Jordan is higher than was believed. At the moment, there are five formally described and one yet unnamed species of Hemidactylus documented for the country. Although the results of the mtDNA barcoding gave us a better insight into the distribution of the different species across Jordan, many gaps still remain to be filled. For example, samples from the southeast (Amr et al., Citation1994) and extreme northeast of the country were not available to us. These regions are still poorly explored with regards to their herpetofauna (Amr & Disi, Citation2011; Disi et al., Citation2001). Despite their remoteness, poor infrastructure and accessibility, more attention should be given to these regions as they have high potential for new discoveries. Along with many other studies, our results demonstrate that mtDNA barcoding can be used as a powerful tool to discover cryptic species and support species identification based on other types of data.
Associate Editor: Dr Susan Tsang
Supplemental Material
Download PDF (176.9 KB)Acknowledgements
We would like to thank the Royal Society for the Conservation of Nature (RSCN, Jordan) for granting us permission for sample collection. We would like to thank Roberto Sindaco, Petr Benda and Antonín Reiter for donating several samples originating from Egypt and Jordan. KMP and all Czech authors would like to express their gratitude and thanks to the kind and always helpful people of Jordan, who facilitated the fieldwork in many ways. We also thank two anonymous reviewers for constructive comments on the first version of the manuscript.
Supplemental material
Supplemental material for this article can be accessed here: http://dx.doi.org/10.1080/14772000.2023.2237033.
Additional information
Funding
References
- Aloufi, A. A., Amr, Z. S., & Abu Baker, M. A. (2022). Reptiles from ‘Uruq Bani Ma’arid and Harat al Harrah protected areas in Saudi Arabia. Herpetology Notes, 15, 483–491.
- Al-Sadoon, M. K., Paray, B. A., & Al-Otaibi, H. S. (2016). Survey of the reptilian fauna of the Kingdom of Saudi Arabia. V. The lizard fauna of Turaif region. Saudi Journal of Biological Sciences, 23, 642–648. https://doi.org/10.1016/j.sjbs.2016.04.005
- Amr, Z. S., Al-Oran, R., & Disi, A. M. (1994). Reptiles of southern Jordan. Snake, 26, 41–49.
- Amr, Z. S., & Disi, A. M. (2011). Systematics, distribution and ecology of the snakes of Jordan. Vertebrate Zoology, 61, 179–266. https://doi.org/10.3897/vz.61.e31150
- Amr, Z. S., Modrý, D., Abu Baker, M., Qarqas, M., Al Zaidanyen, J., & Moravec, J. (2007). First record of Hemidactylus mindiae Baha El Din 2005 from Jordan. Herpetozoa, 20, 73–75.
- Amr, Z. S., Modrý, D., & Al Shudiefat, M. F. (2011). Badia: The living desert. Al Rai Printing Press.
- Baha El Din, S. (2006). A guide to reptiles and amphibians of Egypt. The American University in Cairo Press.
- Bar, A., Haimovitch, G., & Meiri, S. (2021). Field guide to the amphibians and reptiles of Israel. Edition Chimaira.
- Barbour, T. (1914). Notes on some reptiles from Sinai and Syria. Proceedings of the New England Zoölogical Club, 5, 73–92.
- Carranza, S., & Arnold, E. N. (2006). Systematics, biogeography, and evolution of Hemidactylus geckos (Reptilia: Gekkonidae) elucidated using mitochondrial DNA sequences. Molecular Phylogenetics and Evolution, 38, 531–545. https://doi.org/10.1016/j.ympev.2005.07.012
- Carranza, S., & Arnold, E. N. (2012). A review of the geckos of the genus Hemidactylus (Squamata: Gekkonidae) from Oman based on morphology, mitochondrial and nuclear data, with descriptions of eight new species. Zootaxa, 3378, 1–95. https://doi.org/10.11646/zootaxa.3378.1.1
- de Pous, P., Machado, L., Metallinou, M., Červenka, J., Kratochvíl, L., Paschou, N., Mazuch, T., Šmíd, J., Simó-Riudalbas, M., Sanuy, D., & Carranza, S. (2016). Taxonomy and biogeography of Bunopus spatalurus (Reptilia; Gekkonidae) from the Arabian Peninsula. Journal of Zoological Systematics and Evolutionary Research, 54, 67–81. https://doi.org/10.1111/jzs.12107
- Disi, A. M. (1996). A contribution to the knowledge of the herpetofauna of Jordan. VI. The Jordanian herpetofauna as a Zoogeographic indicator. Herpetozoa, 9, 71–81.
- Disi, A. M., Amr, Z. S., & Hamidan, N. (2014). Diversity, threats, and conservation, of the terrestrial and freshwater herpetofauna of Jordan. Russian Journal of Herpetology, 21, 221–233.
- Disi, A. M., Modrý, D., Bunian, F., Al-Oran, R. M., & Amr, Z. S. (1999). Amphibians and reptiles of the Badia region of Jordan. Herpetozoa, 12, 135–146.
- Disi, A. M., Modrý, D., Nečas, P., & Rifai, L. (2001). Amphibians and reptiles of the Hashemite Kingdom of Jordan. An atlas and field guide. Edition Chimaira.
- Ficetola, G. F., Falaschi, M., Bonardi, A., Padoa-Schioppa, E., & Sindaco, R. (2018). Biogeographical structure and endemism pattern in reptiles of the Western Palearctic. Progress in Physical Geography: Earth and Environment, 42, 220–236. https://doi.org/10.1177/0309133318765084
- Guindon, S., Dufayard, J.-F., Lefort, V., Anisimova, M., Hordijk, W., & Gascuel, O. (2010). New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Systematic Biology, 59, 307–321. https://doi.org/10.1093/sysbio/syq010
- Handal, E. N., Amr, Z. S., & Qumsiyeh, M. B. (2016). Some records of reptiles from the Palestinian territories. Russian Journal of Herpetology, 23, 261–270. https://doi.org/10.30906/1026-2296-2016-23-4-261-270
- Jandzik, D., Jablonski, D., Zinenko, O., Kukushkin, O. V., Moravec, J., & Gvoždík, V. (2018). Pleistocene extinctions and recent expansions in an anguid lizard of the genus Pseudopus. Zoologica Scripta, 47, 21–32. https://doi.org/10.1111/zsc.12256
- Kalyaanamoorthy, S., Minh, B. Q., Wong, T. K. F., von Haeseler, A., & Jermiin, L. S. (2017). ModelFinder: Fast model selection for accurate phylogenetic estimates. Nature Methods, 14, 587–589. https://doi.org/10.1038/nmeth.4285
- Katoh, K., Rozewicki, J., & Yamada, K. D. (2019). MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Briefings in Bioinformatics, 20, 1160–1166. https://doi.org/10.1093/bib/bbx108
- Kearse, M., Moir, R., Wilson, A., Stones-Havas, S., Cheung, M., Sturrock, S., Buxton, S., Cooper, A., Markowitz, S., Duran, C., Thierer, T., Ashton, B., Meintjes, P., & Drummond, A. (2012). Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics (Oxford, England), 28, 1647–1649. https://doi.org/10.1093/bioinformatics/bts199
- Kumar, S., Stecher, G., Li, M., Knyaz, C., & Tamura, K. (2018). MEGA X: Molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution, 35, 1547–1549. https://doi.org/10.1093/molbev/msy096
- Metallinou, M., Červenka, J., Crochet, P.-A., Kratochvíl, L., Wilms, T., Geniez, P., Shobrak, M. Y., Brito, J. C., & Carranza, S. (2015). Species on the rocks: Systematics and biogeography of the rock-dwelling Ptyodactylus geckos (Squamata: Phyllodactylidae) in North Africa and Arabia. Molecular Phylogenetics and Evolution, 85, 208–220. https://doi.org/10.1016/j.ympev.2015.02.010
- Minh, B. Q., Nguyen, M. A. T., & von Haeseler, A. (2013). Ultrafast approximation for phylogenetic bootstrap. Molecular Biology and Evolution, 30, 1188–1195. https://doi.org/10.1093/molbev/mst024
- Moravec, J., & Böhme, W. (1997). A new subspecies of the Mediterranean Gecko, Hemidactylus turcicus from the Syrian lava desert (Squamata: Sauria: Gekkonidae). Herpetozoa, 10, 121–128.
- Moravec, J., Kratochvíl, L., Amr, Z. S., Jandzik, D., Šmíd, J., & Gvoždík, V. (2011). High genetic differentiation within the Hemidactylus turcicus complex (Reptilia: Gekkonidae) in the Levant, with comments on the phylogeny and systematics of the genus. Zootaxa, 2894, 21–38. https://doi.org/10.11646/zootaxa.2894.1.2
- Nazarov, R., Melnikov, D., & Melnikova, E. (2013). Three new species of Ptyodactylus (Reptilia; Squamata; Phyllodactylidae) from the Middle East. Russian Journal of Herpetology, 20, 147–162. https://doi.org/10.13140/2.1.3072.5767
- Nguyen, L.-T., Schmidt, H. A., von Haeseler, A., & Minh, B. Q. (2015). IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Molecular Biology and Evolution, 32, 268–274. https://doi.org/10.1093/molbev/msu300
- Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D. L., Darling, A., Höhna, S., Larget, B., Liu, L., Suchard, M. A., & Huelsenbeck, J. P. (2012). MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology, 61, 539–542. https://doi.org/10.1093/sysbio/sys029
- Sindaco, R., & Jeremčenko, V. K. (2008). The reptiles of the western Palearctic, volume 1: Annotated checklist and distributional atlas of the turtles, crocodiles, amphisbaenians and lizards of Europe, North Africa, Middle East and Central Asia. Edizioni Belvedere.
- Sindaco, R., Simó-Riudalbas, M., Sacchi, R., & Carranza, S. (2018). Systematics of the Mesalina guttulata species complex (Squamata: Lacertidae) from Arabia with the description of two new species. Zootaxa, 4429, 513–547. https://doi.org/10.11646/zootaxa.4429.3.4
- Šmíd, J., Carranza, S., Kratochvíl, L., Gvoždík, V., Nasher, A. K., & Moravec, J. (2013). Out of Arabia: A complex biogeographic history of multiple vicariance and dispersal events in the Gecko Genus Hemidactylus (Reptilia: Gekkonidae). PloS One, 8, e64018. https://doi.org/10.1371/journal.pone.0064018
- Šmíd, J., Mazuch, T., Nováková, L., Modrý, D., Malonza, P. K., Elmi, H. S. A., Carranza, S., & Moravec, J. (2020). Phylogeny and systematic revision of the Gecko Genus Hemidactylus from the horn of Africa (Squamata: Gekkonidae). Herpetological Monographs, 33, 26–47. https://doi.org/10.1655/HERPMONOGRAPHS-D-19-00010.1
- Šmíd, J., Moravec, J., Kratochvíl, L., Gvoždík, V., Nasher, A. K., Busais, S. M., Wilms, T., Shobrak, M. Y., & Carranza, S. (2013). Two newly recognized species of Hemidactylus (Squamata, Gekkonidae) from the Arabian Peninsula and Sinai, Egypt. ZooKeys, 355, 79–107. https://doi.org/10.3897/zookeys.355.6190
- Šmíd, J., Sindaco, R., Shobrak, M., Busais, S., Tamar, K., Aghová, T., Simó-Riudalbas, M., Tarroso, P., Geniez, P., Crochet, P.-A., Els, J., Burriel-Carranza, B., Tejero-Cicuéndez, H., & Carranza, S. (2021). Diversity patterns and evolutionary history of Arabian squamates. Journal of Biogeography, 48, 1183–1199. https://doi.org/10.1111/jbi.14070
- Šmíd, J., Uvizl, M., Shobrak, M., Busais, S., Salim, A. F. A., AlGethami, R. H. M., AlGethami, A. R., Alanazi, A. S. K., Alsubaie, S. D., Rovatsos, M., Nováková, L., Mazuch, T., & Carranza, S. (2023). Diversification of Hemidactylus geckos (Squamata: Gekkonidae) in coastal plains and islands of southwestern Arabia with descriptions and complete mitochondrial genomes of two endemic species to Saudi Arabia. Organisms Diversity & Evolution, 23, 185–207. https://doi.org/10.1007/s13127-022-00572-w
- Tamar, K., Carranza, S., In den Bosch, H., Sindaco, R., Moravec, J., & Meiri, S. (2015). Hidden relationships and genetic diversity: Molecular phylogeny and phylogeography of the Levantine lizards of the genus Phoenicolacerta (Squamata: Lacertidae). Molecular Phylogenetics and Evolution, 91, 86–97. https://doi.org/10.1016/j.ympev.2015.05.002
- Trifinopoulos, J., Nguyen, L.-T., von Haeseler, A., & Minh, B. Q. (2016). W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Research, 44, W232–W235. https://doi.org/10.1093/nar/gkw256
- Uetz, P., Freed, P, Aguilar, R., Reyes, F., & Hošek, J. (Eds.) (2023). The reptile database. Retrieved March 28, 2023, from http://www.reptile-database.org
- Werner, Y. L. (2016). Reptile life in the land of Israel. Edition Chimaira.
