Abstract
Our recent X-ray micro computer-tomographic (μCT) investigations of Prophaethon shrubsolei and Odontopteryx toliapica from the Lower Eocene London Clay Formation of England revealed the avian brain to have been essentially modern in form by 55 Ma, but that an important vision-related synapomorphy of living birds, the eminentia sagittalis of the telencephalon, was poorly developed. This evidence suggested that the feature probably appeared close to the end of the Mesozoic. Here we use μCT analysis to describe the endocranium of Halcyornis toliapicus, also from the London Clay Formation. The affinities of Halcyornis have been hotly debated, with the taxon referred to the Charadriiformes (Laridae), Coraciiformes (Alcedinidae, and its own family Halcyornithidae) and most recently that Halcyornithidae may be a possible senior synonym of Pseudasturidae (Pan-Psittaciformes). Unlike Prophaethon and Odontopteryx, the eminentia sagittalis of Halcyornis is strongly developed and comparable to that of living species. Like those London Clay taxa, the eminentia sagittalis occupies a rostral position on the telencephalon. The senses of Halcyornis appear to have been well developed. The length of the cochlear duct of the inner ear indicates a hearing sensitivity within the upper range of living species, and enlarged olfactory lobes suggest a reasonable reliance on sense of smell. The optic nerves were especially well developed which, together with the strong development of the eminentia sagittalis, indicates a high degree of visual specialization in Halcyornis. The advanced development of the eminentia sagittalis further supports a Mesozoic age for the appearance of this structure and associated neural architectural complexity found in extant Aves. The eminentia sagittalis of living Psittaciformes is situated caudally on the telencephalon, making a Pan-Psittaciformes relationship unlikely for Halcyornis.
Introduction
The brains of birds and mammals fit the endocranium so tightly that the internal surface of the braincase retains a reasonably accurate record of the morphology and size of the organ (CitationIwaniuk & Nelson 2002; CitationStriedter 2005). With the advent of X-ray and synchrotron micro computer-tomographic (μCT) analysis, these endocranial features can now be examined in the absence of natural endocasts or damaged specimens that expose the endocranial surface (CitationWitmer et al. 2008). However, uncrushed avian cranial remains suitable for μCT analysis are very rare in the fossil record.
The Lower Eocene (∼55 Ma) London Clay Formation of south-east England is famous for its three-dimensionally preserved bird fossils, which include articulated or associated remains and cranial material. During the 1970s and 1980s Cyril Walker and Colin Harrison worked intensively on these fossils, erecting numerous new taxa and revising many of the groups represented in the formation (e.g. CitationHarrison & Walker 1977). These bird fossils are important for a variety of reasons, not least of which is their deposition in a subtropical shallow shelf marine environment (CitationAllison 1988) only 10 Ma after the end-Cretaceous extinction event. This period also saw an explosive neornithine speciation event, in which the majority of extant avian clades made their earliest appearance in the fossil record (CitationMayr 2005; CitationDyke & van Tuinen 2004; Citationvan Tuinen et al. 2006). The in-the-round preservation of the London Clay bird remains means that these skulls may offer crucial information about neurological development at this pivotal period in avian evolution.
Figure 1 Endocranial casts of Eocene fossil birds in left lateral (top row) and dorsal (bottom row) views. A, B, Odontopteryx toliapica; C, D, Prophaethon shrubsolei; E, F, ‘Numenius’ gypsorum. A–D are virtual endocranial casts segmented from μCT scans. E and F are modified after CitationDechaseaux (1970).
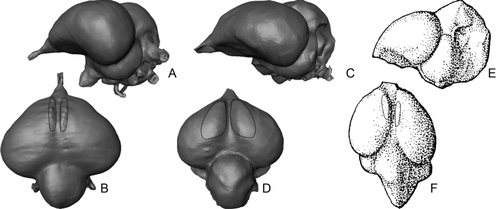
We have already investigated (CitationMilner & Walsh 2009) the brain and sensory development of two London Clay bird taxa, Odontopteryx toliapica CitationOwen, 1873 and Prophaethon shrubsolei CitationAndrews, 1899 (). These two taxa proved to have brains that were essentially modern in form, particularly with respect to telencephalon (forebrain) expansion and overall volume. Most importantly, both species possessed a feature called the eminentia sagittalis, which was not present in Archaeopteryx or any non-avian theropod, and therefore represents the earliest record of the structure (CitationMilner & Walsh 2009). The eminentia sagittalis of extant birds is derived from the hyperpallium (Jarvis et al. 2005) and occurs as a notable dorsal projection of the telencephalon. The region is mostly involved in integration and processing of the visual signal, and is largest in species with strong visual specializations, such as Strigiformes (CitationIwaniuk & Wylie 2006). Since it appears to be present in some form in all living bird species it may be regarded as a putative synapomorphy of Neornithes. If so, the differentiation of the hyperpallium that forms the eminentia sagittalis must have occurred by the likely time of origin of Neornithes in the Cretaceous (Clarke et al. 2005; Slack et al. 2006), although no dorsal expansion may have been evident at this time.
The eminentia sagittalis of the Early Eocene P. shrubsolei was poorly developed compared with living relatives (e.g. Phaethon rubricauda), and that of O. toliapica was, at best, rudimentary. The Late Eocene bird ‘Numenius’ gypsorum () from the Paris Basin also possessed an eminentia sagittalis as poorly developed as that of O. toliapica (CitationMilner & Walsh 2009). Based on this evidence, we concluded that the structure was probably in an early stage of evolution in the Palaeogene, and presumably appeared either towards the end of the Cretaceous or at the beginning of the Cenozoic. However, by the lower Miocene eminentia sagittalis development was no different to living species (CitationMilner & Walsh 2009).
The eminentia sagittalis of P. shrubsolei, O. toliapica and N. gypsorum was positioned rostrally on the dorsal surface of the telencephalon (), but in living species its position and shape are highly variable. In some species it is positioned rostrally; in others it may be situated caudally or even occupy all of the dorsal and part of the ventral surface of the telencephalon. With some notable exceptions (e.g. traditional Pelecaniformes, in which both rostral and caudal forms occur; CitationStingelin 1957) the form of the eminentia sagittalis is generally stable within clades. CitationStingelin (1957, p. 78) hypothesized a ‘Grundtypus’ in which the eminentia sagittalis was short, rostrally positioned and caudally divergent from the interhemispheric fissure. Interestingly, the form we observed in the three Eocene species confirms this view, although in each case the medial margin of the development abuts the interhemispheric fissure closely along its length. Nothing is presently known of how this ‘Grundtypus’ developed over time into the diversity of forms found in living species. The possibility exists that a basic caudal form may have arisen independently, rather than as a development of the rostral form. The London Clay Formation avian assemblage has the potential to provide critical information about the diversity in eminentia sagittalis form—and that of the brain as a whole—during this early development of the structure.
Here we use μCT to visualize and describe the brain morphology of another London Clay Formation taxon, Halcyornis toliapicus CitationKoenig, 1825. This specimen provides new evidence that avian neural architecture was more advanced in the Mesozoic than previously supposed.
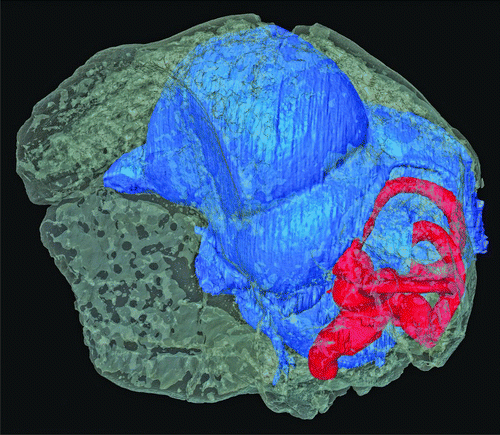
Figure 2 μCT visualization of the skull of Halcyornis toliapicus (NHMUK A130) rendered semi-transparent to reveal the virtual endocranial cast (blue) and inner ear (red). The skull is shown in ‘alert’ posture, based on the position of the horizontal semicircular canal (sensu CitationWitmer et al., 2003; CitationMilner & Walsh 2009).
Taxonomic status of Halcyornis toliapicus
Despite the early description of the specimen almost 200 years ago, its relative incompleteness has led to divided interpretations of the affinities of Halcyornis toliapicus. CitationKoenig (1825) regarded it as a gull, naming it Larus toliapicus (Laridae; Charadriiformes), while CitationOwen (1846) considered it to be an early kingfisher and erected Halcyornis (Alcedinidae; Coraciiformes) to replace ‘Larus’. Later, an affinity with gulls was again suggested by CitationLydekker (1891). CitationHarrison & Walker (1972) revised the taxon, believing it to be close to the rollers (Coraciidae; Coraciiformes), and erected a new family, Halcyornithidae. Most recently CitationMayr (2007) suggested that the Halcyornithidae might be a senior synonym of the Pseudasturidae, potentially making Halcyornis a stem-group psittaciform (Pan-Psittaciformes).
Material and methods
The type specimen of Halcyornis toliapicus in the Natural History Museum (NHM), London (NHMUK A130) comprises an incomplete cranium lacking the rostral portion of the skull from a fracture line part way along the frontals. The palatal region is abraded and the skull roof is damaged, such that part of the endocranial cast is visible where fossil bone is missing.
Table 1 Measurements taken from virtual endocast of Halcyornis toliapicus (NHMUK A130). All measurements and proportions are rounded to the nearest integer. Note that the brain region volumes total less than 100% because this method does not include space inside the endocranial cast beyond the limits of the external expression of the region. Similarly, the brain region surface area total is less than 100% because beyond the absolute external limits of some regions there are poorly differentiated areas (e.g. diencephalon) that were not included.
μCT scanning and analysis
The specimen was scanned without filtering at 225 kV and 138 mA using the NHM Nikon Metrology HMX ST CT with a Perkin Elmer flat panel detector. The scan was optimized for 3142 projections, and reconstructed as 1868 slices with voxel dimensions of 14 × 14 × 14 μm. The endocranial cavity and endosseous inner ear were segmented from the scan slice data using Visage Amira 5.0 without down-sampling.
In living species comparisons of the relative size of specific brain regions provide a great deal of useful information regarding behaviour and ecology (‘cerebrotype’ analysis; CitationIwaniuk & Hurd 2005). However, despite the advent of μCT approaches, investigation of brain development in extinct birds is limited, as only the gross external morphology and overall volume of the brain can be determined from an endocranium. Here we use a new technique for estimating volume and surface area of brain regions as a percentage of total brain volume. This method is an extension of that used by CitationWalsh et al. (2009) for endosseous cochlear duct analysis, and involves digitally isolating the external expression of a particular region to allow surface area and volume measurements of that region to be collected. In the same way that regional dissections of Nissl-stained brains do not include undifferentiated tissue (CitationIwaniuk & Hurd 2005), this approach does not take into account the internal volume of undifferentiated regions, and may also include volume that was sinusoidal rather than neural tissue in life. The measurements recovered are consequently best regarded as ‘partial’ volumes and surface areas. Despite this relative crudeness, the partial volumes approach offers the only currently available quantitative estimate of the size of brain regions relative to overall brain volume/surface area for comparison of fossil species.
The partial volumes method involves converting segmentations of the endocranial cavity from voxel data to point cloud data format. Most CT visualization software packages are capable of exporting segmentations as point cloud files, and several suitable file formats (e.g. STL, PLY, VRML) are available. Here, polygon mesh stereolithograph (STL) models were used due to their wide compatibility with mesh editing software such as Inus Technologies RapidForm XOR, Geomagic Studio and Konica-Minolta Polygon Editing Tool (PET). Such packages are used to separate the individual brain regions from the whole model using the junction between the base of the region and that of adjacent regions as a delimiting feature. For instance, the external expression of the mesencephalon is delimited dorsally by the telencephalon, and ventrally by the rhombencephalon. Here, separations of the telencephalon (and eminentia sagittalis sub-region), olfactory lobes, mesencephalon, cerebellum and rhombencephalon were performed using Konica-Minolta PET, and saved as separate individual STL files. The mesh editing software was then used to fill holes in the mesh and calculate volume and surface area values from these separated regions.
Anatomical abbreviations
Common Anglicized anatomical terms are used throughout, except for crus commune, diencephalon, eminentia sagittalis, rhombencephalon, sacculus and telencephalon, which follow CitationBaumel et al. (1993). Abbreviations: c, cerebellum; ca, caudal ampulla; cc, crus commune; cd, cochlear duct; cf, cerebellar fissure; csc, caudal semicircular canal; d, diencephalon; dcv, dorso-caudal cerebral vein; es, eminentia sagittalis; fl, flocculus; fm, position of foramen magnum; ha, horizontal ampulla; hsc, horizontal semicircular canal; II, optic nerve; III, occulomotor nerve; isv, impression of the semicircular vein; IV, trochlear nerve; IX, glossopharyngeal nerve; m, mesencephalon; ol, olfactory lobes; os, occipital sinus; ow, oval window; p, pituitary; pca, path of the carotid artery; r, rhombencephalon; ra, rostral ampulla; rsc, rostral semicircular canal; rw, round window; s, sulcus; sa, sacculus; t, telencephalon; V, trigeminal nerve; VI, abducens nerve; VII, facial nerve; VIII, vestibulocochlear nerve; X, vagus nerve; XII, hypoglossal nerve.
Description
The scan resulted in slices with good contrast between bone and matrix, although the pixel brightness values of bone were close to those of the surrounding air. Nonetheless, the entire endocranial cast of Halcyornis except the medulla and brain stem caudal of the foramen magnum could be reconstructed digitally (). This virtual endocranial cast includes the pathways of all major cranial nerves and some venous and arterial blood vessels where these form part of the endocranial space. No evidence of incomplete fusion of cranial bones is evident, and full ossification of the dorsum sellae (CitationWingstrand 1951) demonstrates that the type specimen of Halcyornis toliapicus was adult at time of death. The endosseous labyrinths of the inner ear were possible to reconstruct despite slight damage to the otic capsule on both sides. Neither columella is present in this specimen. The reconstructed endocranial cast is clearly fully modern in form, with a highly dorsally and laterally expanded telencephalon relative to the rest of the brain (see ). The brain fitted so closely into the braincase that even the paths of blood vessels are impressed into the endocranial surface.
Telencephalon
In dorsal view () the telencephalon forms a rounded triangle broader than long (16.7 mm mediolaterally, 11.3 mm rostrocaudally), and fully occludes the hemispheres of the mesencephalon (tectum mesencephali). In lateral view the telencephalon is almost as tall as it is long (9.8 mm dorsoventrally). The olfactory lobes are situated directly at the rostral apex of the telencephalon, and the olfactory nerves (I) are directed rostrally (), as in most species with an approximately straight skull axis and unlike the ventral direction seen in species with strong axial flexion (e.g. Scolopax, Psittaciformes). Unlike most living species, the olfactory lobes are large compared with the size of the telencephalon and the brain as a whole, and are similar in relative proportions to that of many seabirds (e.g. Diomedea spp.). The network of the dorso-caudal cerebral vein is impressed on the caudal surface.
Figure 3 Virtual endocranial cast of Halcyornis toliapicus (NHMUK A130) in A, dorsal; B, ventral; C, caudal; D, rostral; E, left lateral views. See text for list of abbreviations. Scale bar = 5 mm.
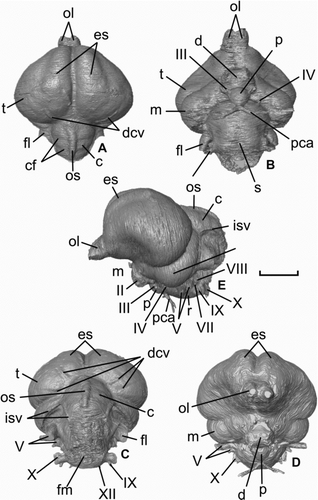
The large size of the eminentia sagittalis is particularly notable, especially compared with the poorer development of the two other species from the London Clay and ‘Numenius’ gypsorum. Like those species the feature is rostrally positioned (), but covers more than 21% of the total surface area of the telencephalon, and extends caudally more than half the length of this region. The dorsal and lateral expansion is well within the range of living species ().
Diencephalon
The optic nerves (II; ) entered the orbit through well-defined foramina in the interorbital septum, making their diameter measurable, and as such the morphology of the foramina conforms to the Type 1 optic nerve exit of CitationHall et al. (2009). At almost 2.0 mm in diameter the foramina indicate that the nerves were relatively thick. The pituitary gland was small and spherical (), and the two paths of the cartotid artery converged only slightly ventral of the sella turcica (). No impression of the pineal organ is apparent on the endocranium ().
Mesencephalon
The visible portion of the mesencephalon (tectum mesencephali) is lunate in outline () and, in terms of partial volume, moderately small relative to the telencephalon (11%) and endocranial cast as a whole (5%). The semicircular vein was not enclosed in a bony tunnel, and its rostral part is deeply impressed on the part of the endocranium that housed the caudal surface of the mesencephalon, extending caudo-ventrally almost as far as the exit for the trigeminal nerve (V).
Figure 4 Virtual rendering of the left endosseous inner ear labyrinth of Halcyornis toliapicus (NHMUK A130) in: A, rostral; B, lateral; C, caudal; D, medial; E, dorsal views. See text for list of abbreviations. Scale bar = 5 mm.
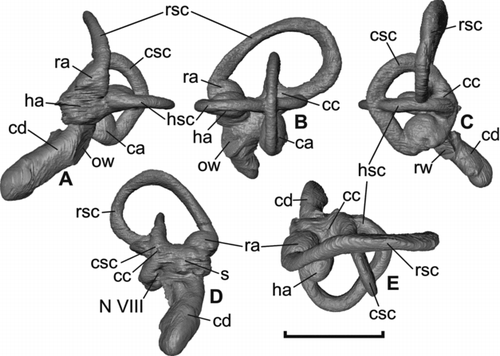
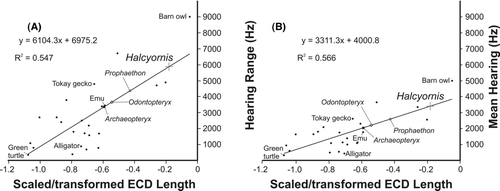
Figure 5 Hearing sensitivity of Halcyornis toliapicus (A, hearing range; B, mean hearing) based on regressions calculated from data derived from living bird and reptile species (see CitationWalsh et al. 2009; reproduced with permission of Royal Society Publishing).
Rhombencephalon
The cerebellum was broader (6.8 mm) than long (5.6 mm) and tapered caudally (). Compared with many extant taxa (e.g. Passeriformes, Strigiformes, Psittaciformes) the cerebellum is large (7% of total endocranial cast volume). Three distinct folia are present, along with an impression of the occipital sinus, which is deep and narrow (). The impression of the semicircular vein preserved over the mesencephalon also extends over the lateral surfaces of the cerebellum. The exit of these vessels in the caudal region of the skull is not possible to determine due to damage in this region (). The flocculus was relatively large, rostrocaudally compressed and directed strongly caudolaterally. The medulla was globose and bore a wide and shallow sulcus on the ventral surface. The cranial nerves V–XII are relatively easy to trace, although the vestibulocochlear nerve (VIII) is only fully traceable on the left side. As in other bird species, the trigeminal nerve (V) has the largest diameter (measurements of the diameter of each nerve at its exit from the endocranium are given in ).
Endosseous labyrinth of the inner ear
The semicircular canals of the labyrinth () have a relatively short span, although as is normal for living species the rostral semicircular canal is the most expanded. Unlike Prophaethon and Odontopteryx the canals are all strongly elliptical in cross section (). Many living species possess semicircular canals that are fully circular in section, but a continuum of variation exists between the compression seen in Halcyornis and full circularity (SW pers. obs.). Cross-sectional morphology as strongly elliptical as that observed here is seen in Anseriformes (e.g. Cygnus, Aythya) and some palaeognaths (e.g. Struthio, although the sectional shape is more circular in Dromaius). The rostral, horizontal and caudal semicircular canals share their caudal origin at the caudal surface of a short and broad crus commune, also like Cygnus and Aythya. As in those anseriform taxa, the expansion of the three canals is far less pronounced than that observed in most Passeriformes (e.g. Corvus). For instance, the rostral semicircular canal is rostrocaudally wider than its dorsoventral length; in Odontopteryx and Prophaethon this canal is dorsoventrally longer than rostrocaudally wide.
Table 2 Dimensions and intersections of the endosseous inner ear labyrinth of Halcyornis toliapicus.
The endosseous cochlear duct is slightly curved and relatively long, with a mean length of almost 5.0 mm measured from the base of the pars vestibularis. Using the methods of CitationWalsh et al. (2009) to estimate mean hearing range and sensitivity from the scaled and transformed endosseous cochlear duct length, we calculate that Halcyornis would have had a mean hearing range of approximately 5900 Hz and a mean hearing sensitivity of approximately 3400 Hz (). These values put the hearing sensitivity of Halcyornis within the upper range for living species.
Discussion
The brain of Halcyornis toliapicus demonstrates a level of neural development well within the range of living bird species, particularly with respect to the development of the eminentia sagittalis. The dorsal and lateral expansion of this structure is more advanced than previously observed in Odontopteryx toliapica, Prophaethon shrubsolei and ‘Numenius’ gypsorum (CitationMilner & Walsh 2009), and indicates that those species were not fully representative of avian neurological development in the Eocene. Most importantly, the advanced morphology of the eminentia sagittalis in Halcyornis makes a Cretaceous age for the appearance of the structure and its associated cognitive abilities far more likely.
Considering its role in visual cognition, the large size of the eminentia sagittalis is consistent with the apparent diameter of the optic nerve, and suggests that Halcyornis had a well developed visual sense. The moderate size of the tectum mesencephali does not contradict this prediction, as many visually specialized species (e.g. Strigiformes) have a far smaller tectum mesencephali relative to overall brain size (SW pers. obs.). Much of the avian optic nerve bundle consists of axons that connect photoreceptor cells in the retina to centres in the diencephalon and mesencephalon, and its thickness (in fossils estimated from optic foramen diameter as a maximum thickness) relative to orbit or inner sclerotic ring diameter can be used to estimate diurnal or nocturnal activity patterns in extinct species (CitationHall et al. 2009; see also CitationHall & Ross 2007; CitationHall 2008). However, whether the vision of Halcyornis was optimized for acuity or light gathering is probably impossible to estimate as the sclerotic ring was not preserved, and the orbit is too incomplete for confident measurement of its diameter.
The olfactory lobes of most living birds are small, although all species appear to employ olfaction in some form (CitationRoper 1999). The largest olfactory bulbs are found in Apteryx, carrion feeders such as vultures, and seabirds (CitationStager 1967; CitationPearson 1972; CitationCunningham et al. 2007; CitationCorfield et al. 2008). The relatively large olfactory lobes of Halcyornis suggest that this species had a reasonable reliance on its sense of smell. The heightened sensory capabilities of Halcyornis are further illustrated by our estimates for hearing sensitivity, which are far higher than the values calculated by CitationWalsh et al. (2009) for Odontopteryx and Prophaethon. Since the vocalization range of most birds falls within the lower half of their hearing sensitivity (CitationKonishi 1970), Halcyornis is likely to have had a relatively wide and high vocalization frequency range. From these observations it appears that, despite the age of this taxon, Halcyornis had sensory adaptations that matched and even exceeded those of most living birds. It seems likely that similar adaptations had evolved in other coeval avian taxa by the Palaeocene, and were probably already present in Mesozoic Neornithes.
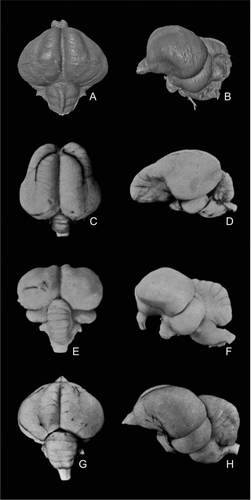
Figure 6 Comparison of the virtual endocranial cast of Halcyornis toliapicus (NHMUK A130) with fresh brain material of taxa previously suggested to be closely related, in dorsal (left column) and left lateral (right column) views. A, B, Halcyornis toliapicus; C, D, Psittacus erithacus; E, F, Coracius garrulus; G, H, Larus argentatus. Note that the semicircular vein seen in A and B is an impression on the endocranium and is therefore not visible in D, F or H. C–H modified after CitationStingelin (1957).
Compared with living birds that are regarded as aerobatic flyers (CitationHadžiselimović & Savković 1964; CitationSipla 2007) the semicircular canals of Halcyornis are not well expanded. The horizontal semicircular canal in particular is normally well expanded in manoeuvrable fliers, with the expansion being achieved by a medial origin of the canal at the crus commune (CitationSipla 2007). The caudal origin of the canal in Halcyornis and the general form of the canals and crus commune are very similar to that of ducks and swans, which are generally adapted for fast straight line flight, sometimes over long distances and at altitude. The strong oval section of the canals is also a feature found in Anseriformes, and is also apparent in flightless palaeognaths. We have, however, observed less severe ovality in other species (e.g. Buteo buteo). The low angle (81°) between the horizontal and rostral semicircular canals is another characteristic consistent with relatively poor manoeuvrability (CitationHadžiselimović & Savković 1964), and this combined evidence seems to support the likelihood that Halcyornis was not a particularly acrobatic flier. However, without data on the body mass of Halcyornis for scaling purposes it is difficult to draw firm conclusions at this time.
Although it remains possible that a caudally-positioned eminentia sagittalis may have given rise independently to the caudal forms seen today, Halcyornis clearly exhibits the rostral type, and evidence that a caudal form existed at the same time is still lacking. It is notable that the other three Eocene taxa for which the feature is known (Odontopteryx toliapica, Prophaethon shrubsolei and ‘Numenius’ gypsorum) also had a rostrally positioned eminentia sagittalis. The lateral expansion of the eminentia sagittalis in Halcyornis is consistent with the development originally predicted by CitationStingelin (1957) for his basic ‘Grundtypus’. Assuming all extant bird species inherited the structure from a common ancestor that lived in the Late Cretaceous, transitional forms may yet be found in the early Cenozoic. The London Clay Formation avifaunal assemblage may hold vital information for investigating this possibility.
The rostral position of the eminentia sagittalis does provide some new data on the taxonomic position of Halcyornis. Living psittaciforms have a caudally-positioned eminentia sagittalis, making a Pan-Psittaciformes affinity unlikely. However, the brain of psittaciform species is unusual among birds in possessing a distinctively long telencephalon in which there appears to have been selective expansion of the rostralmost portions (). If so, the apparent caudal position in this group may be a relict, although developmental work would be required to test this. shows a comparison of the brain of Halcyornis with the two other taxa previously suggested to be most closely related. Several features of the coraciid brain differ greatly from Halcyornis, including the dorsal outline of the telencephalon (), narrow and poorly developed eminentia sagittalis (), relatively larger cerebellum () and marked ventral deflecture of the rostral portion of the telencephalon and olfactory bulbs (). By comparison, the larid brain () is a close match in morphology with the virtual endocranial cast of Halcyornis, even in the branching of the dorso-caudal cerebral vein (). Differences are relatively small and include a larger cerebellum and less well developed eminentia sagittalis in Larus. Thus it seems that the original identification of CitationKoenig (1825) may have been largely correct.
A larid affinity is further supported by the Type 1 morphology of the optic foramen; according to CitationHall et al. (2009) the Type 1 foramen is found in Laridae, but within Coraciiformes is not present in Coraciidae, Leptosomidae and Momotidae, which have Type 3 foramina. Type 1 foramina are, however, found in Upupidae, and sometimes Bucerotidae and Phoeniculidae (CitationHall et al. 2009). Consistent with CitationMayr's (2007) Pan-Psittaciform hypothesis, Type 1 foramina are also a characteristic of living Psittaciformes (CitationHall et al. 2009). Further work is currently needed to test the reliability of endocranial morphology in cladistic analysis, but these data may eventually help to clarify the systematic position of Halcyornis and provide an important new source of phylogenetic information for the classification of avian clades as a whole.
Acknowledgements
We thank Richie Abel (NHM) for making the μCT scanner available and for discussion of scanning and visualization techniques. Monja Knoll (Portsmouth University, UK), Larry Witmer (Ohio University, USA), Gerald Mayr (Senkenberg Institute, Germany) and Gareth Dyke (University College Dublin, Eire) are thanked for useful discussion on brain form and function and the taxonomy of Halcyornis. We are especially grateful to Paul Barrett for allowing virtual endosseous inner ear labyrinths generated as part of NERC grant NE/E008380/1 to be used for comparative purposes. This manuscript was greatly improved by comments from Larry Witmer and Estelle Bourdon (American Museum of Natural History, USA).
References
- Allison , P. A. 1988 . Taphonomy of the Eocene London Clay biota . Palaeontology , 31 : 1079 – 1100 .
- Andrews , C. A. 1899 . On the remains of a new bird from the London Clay of Sheppey . Proceedings of the Zoological Society of London , : 776 – 785 .
- Baumel , J. J. , King , A. S. , Breazile , J. E. , Evans , H. E. and Vanden Berge , J. C. 1993 . Nomina anatomica avium: handbook of avian anatomy , 2nd edition , 779 Cambridge : The Nuttall Ornithological Club .
- Clarke , J. A. , Tambussi , C. P. , Noriega , J. I. , Erickson , G. M. and Ketcham , R. A. 2005 . Definitive fossil evidence for the extant avian radiation in the Cretaceous . Nature , 433 : 305 – 308 .
- Corfield , J. R. , Wild , J. M. , Hauber , M. E. , Parsons , S. and Kubke , M. F. 2008 . Evolution of brain size in the palaeognath lineage, with an emphasis on New Zealand ratites . Brain, Behavior and Evolution , 71 : 87 – 99 .
- Cunningham , S. , Castro , I. and Alley , M. 2007 . A new prey-detection mechanism for kiwi (Apteryx spp.) suggests convergent evolution between paleognathous and neognathous birds . Journal of Anatomy , 211 : 493 – 502 .
- Dechaseaux , C. 1970 . Moulages endocraniens d’oiseaux de l’Éocène Supérieur du Bassin de Paris . Annales de Paléontologie , 56 : 69 – 72 .
- Dyke , G. J. and van Tuinen , M. 2004 . The evolutionary radiation of modern birds (Neornithes): reconciling molecules, morphology and the fossil record . Zoological Journal of the Linnean Society , 141 : 153 – 177 .
- Hadžiselimović , H. and Savković , L. 1964 . Appearance of semicircular canals in birds in relation to mode of life . Acta Anatomica , 57 : 306 – 315 .
- Hall , M. I. 2008 . The anatomical relationships between the avian eye, orbit and sclerotic ring: implications for inferring activity patterns in extinct birds . Journal of Anatomy , 212 : 781 – 794 .
- Hall , M. I. and Ross , C. F. 2007 . Eye shape and activity pattern in birds . Journal of Zoology , 271 : 437 – 444 .
- Hall , M. I. , Iwaniuk , A. N. and Gutiérrez-Ibáñez , C. 2009 . Optic foramen morphology and activity pattern in birds . The Anatomical Record: Advances in Integrative Anatomy and Evolutionary Biology , 292 : 1827 – 1845 .
- Harrison , C. J. O. and Walker , C. A. 1972 . The affinities of Halcyornis from the Lower Eocene . Bulletin of the British Museum (Natural History), Geology Series , 21 : 153 – 170 .
- Harrison , C. J. O. and Walker , C. A. 1977 . Birds of the British Lower Eocene . Tertiary Research Special Paper , 3 : 1 – 52 .
- Iwaniuk , A. N. and Hurd , P. L. 2005 . The evolution of cerebrotypes in birds . Brain, Behavior and Evolution , 65 : 215 – 230 .
- Iwaniuk , A. N and Nelson , J. 2002 . Can endocranial volume be used as an estimate of brain size in birds? . Canadian Journal of Zoology , 80 : 16 – 23 .
- Iwaniuk , A. N. and Wylie , D. R. W. 2006 . The evolution of steropsis and the Wulst in caprimulgiform birds: a comparative analysis . Journal of Comparative Physiology A , 192 : 1313 – 1326 .
- Jarvis , E. D. , Güntürkün , O. , Bruce , L. , Csillag , A. , Karten , H. , Kuenzel , W. , Medina , L. , Paxinos , G. , Perkel , D. J. , Shimizu , T. , Striedter , G. , Wild , J. M. , Ball , G. F. , Dugas-Ford , J. , Durand , S. E. , Hough , G. E. , Husband , S. , Kubikova , L. , Lee , D. W. , Mello , C. V. , Powers , A. , Siang , C. , Smulders , T. V. , Wada , K. , White , S. A. , Yamamoto , K. , Yu , J. , Reiner , A. and Butler , A. B. 2005 . Avian brains and a new understanding of vertebrate brain evolution . Nature Reviews Neuroscience , 6 : 151 – 159 .
- Koenig , E. 1825 . Icones fossilium sectiles. Centuria prima 44 London
- Konishi , M. 1970 . Comparative neurophysiological studies of hearing and vocalizations in songbirds . Journal of Comparative Physiology A , 66 : 257 – 272 .
- Lydekker , R. 1891 . Catalogue of the fossil birds in the British Museum (Natural History) , 368 London : British Museum (Natural History) .
- Mayr , G. 2005 . The Paleogene fossil record of birds in Europe . Biological Reviews , 80 : 515 – 542 .
- Mayr , G. 2007 . New specimens of Eocene stem-group psittaciform birds may shed light on the affinities of the first named fossil bird, Halcyornis toliapicus Koenig, 1825 . Neues Jahrbuch für Geologie und Paläontologie Abhandlungen , 244 : 207 – 213 .
- Milner , A. C. and Walsh , S. A. 2009 . Avian brain evolution: new data from Palaeogene birds (Lower Eocene) from England . Zoological Journal of the Linnean Society , 155 : 198 – 219 .
- Owen , R. 1846 . A History of British Fossil Mammals and Birds , 560 London : van Voorst .
- Owen , R. 1873 . Description of the skull of a dentigerous bird (Odontopteryx toliapica) from the London Clay of Sheppey . Quarterly Journal of the Geological Society of London , 29 : 511 – 522 .
- Pearson , R. 1972 . The Avian Brain , 658 London and New York : Academic Press .
- Roper , T. J. 1999 . Olfaction in birds . Advances in the Study of Behavior , 28 : 247 – 332 .
- Sipla , J. S. 2007 . The semicircular canals of birds and non-avian dinosaurs , Stony Brook University . Unpublished PhD thesis
- Slack , K. E. , Jones , C. M. , Ando , T. , Harrison , G. L. , Fordyce , R. E. , Arnason , U. and Penny , D. 2006 . Early penguin fossils, plus mitochondrial genomes, calibrate avian evolution . Molecular Biology and Evolution , 23 : 1144 – 1155 .
- Stager , K. E. 1967 . Avian olfaction . American Zoologist , 7 : 415 – 419 .
- Stingelin , W. 1957 . Vergleichend morphologische untersuchungen am vorderhirn der Vögel auf cytologischer und cytoarchitektonischer grundlage , 123 Basel : Verlag Helbing and Lichtenhahn .
- Striedter , G. 2005 . Principles of brain evolution , 363 Sunderland : Sinauer Associates .
- van Tuinen , M. , Stidham , T. A. and Hadly , E. A. 2006 . Tempo and mode of modern bird evolution observed with large-scale taxonomic sampling . Historical Biology , 18 : 205 – 221 .
- Walsh , S. A. , Barrett , P. M. , Milner , A. C. , Manley , G. and Witmer , L. M. 2009 . Inner ear anatomy is a proxy for deducing auditory capability and behaviour in reptiles and birds . Proceedings of the Royal Society, Series B , 276 : 1355 – 1360 .
- Wingstrand , K. G. 1951 . The Structure and Development of the Avian Pituitary , 316 Lund : Håkan Ohlssons Boktryckeri .
- Witmer , L. M. , Chatterjee , S. , Franzosa , J. and Rowe , T. 2003 . Neuroanatomy of flying reptiles and implications for flight, posture and behaviour . Nature , 425 : 950 – 953 .
- Witmer , L. M. , Ridgely , R. C. , Dufeau , D. L. and Semones , M. C. 2008 . “ Using CT to peer into the past: 3D visualisation of the brain and ear regions of birds, crocodiles and nonavian dinosaurs ” . In Anatomical Imaging: Towards a new morphology , Edited by: Endo , H. and Frey , R. 67 – 87 . Tokyo : Springer Verlag .