ABSTRACT
Introduction: Advances in our understanding about atherosclerotic evolution have enabled us to identify specific plaque characteristics that are associated with coronary plaque vulnerability and cardiovascular events. With constant improvements in signal and image processing an arsenal of invasive and non-invasive imaging modalities have been developed that are capable of identifying these features allowing in vivo assessment of plaque vulnerability.
Areas covered: This review article presents the available and emerging imaging modalities introduced to assess plaque morphology and biology, describes the evidence from the first large scale studies that evaluated the efficacy of invasive and non-invasive imaging in detecting lesions that are likely to progress and cause cardiovascular events and discusses the potential implications of the in vivo assessment of coronary artery pathology in the clinical setting.
Expert commentary: Invasive imaging, with its high resolution, and in particular hybrid intravascular imaging appears as the ideal approach to study the mechanisms regulating atherosclerotic disease progression; whereas non-invasive imaging is expected to enable complete assessment of coronary tree pathology, detection of high-risk lesions, more accurate risk stratification and thus to allow a personalized treatment of vulnerable patients.
1. Introduction
Coronary artery disease (CAD) is the main cause of death in the developing world [Citation1]. Given the heavy burden that it imposes on society and an individual’s health, it is imperative to optimize and tailor the treatment of patients with CAD based on their risk profile, prognosis, and comorbidities. Over the last few years, a considerable effort has been made to identify new treatments that have led to improvements in outcomes in this population [Citation2–Citation4]. Despite these advances, the incidence of recurrent events remains high [Citation5]. To address the unmet need for an optimal management of these high-risk patients, new therapies have recently been developed and are currently undergoing preclinical or clinical evaluation that aim to reduce atherosclerotic disease progression and the risk of future events [Citation6]. Accurate risk stratification is therefore essential these days for quantifying an individual’s risk and tailoring management. However, existing clinical scores have a low accuracy at detecting high-risk patients [Citation7,Citation8]. To overcome this, efforts are made to understand the pathophysiology of CAD and the mechanisms associated with the formation of high-risk lesions that predispose to future cardiovascular events.
Pathological studies have demonstrated that acute coronary syndromes (ACSs) are caused either by plaque rupture, plaque erosion, or thrombosis in calcific nodules [Citation9,Citation10]. Our understanding may be limited today about the morphological characteristics of the plaques that will erode, or the calcific nodules that will cause thrombosis, but we have extensively studied the phenotypic characteristics of the lesions that will rupture – which are responsible for 73% of all ACSs [Citation11]. Majority of these lesions have specific morphological findings collectively termed thin-capped fibroatheromas (TCFA). These plaques exhibit an increased plaque burden, positive remodeling and have a large necrotic core that is covered by a thin fibrous cap (<65 µm) [Citation12,Citation13]. TCFA are also rich in macrophages, which can lead to plaque destabilization by secreting matrix metalloproteinases that readily degrade collagen and thin the fibrous cap leading to plaque rupture [Citation11,Citation12]. TCFA also contain cholesterol crystals that can penetrate and disrupt the fibrous cap and can promote the secretion of pro-inflammatory cytokines resulting in the activation of the immune system [Citation14,Citation15]. Other plaque features associated with increased vulnerability are intra-plaque hemorrhages and microcalcifications [Citation12,Citation16].
The fact that high-risk TCFA have specific morphological characteristics created hopes that their early identification would enable detection of vulnerable lesions and potentially identification of high-risk patients that would benefit from therapeutic strategies that would lead to the passivation of these plaques [Citation7]. Over the last few years, several invasive and noninvasive imaging modalities have been developed to study, with more accuracy, coronary anatomy, morphology, biology, and physiology. The aim of this review article is to describe the advantages and limitations of the invasive and noninvasive techniques, the evidence from their first applications in the study of atherosclerosis, and discuss their potential value in risk stratification and secondary prevention.
2. Invasive coronary imaging
2.1. Intravascular ultrasound
Intravascular ultrasound (IVUS) is the first imaging modality that enabled in vivo assessment of the luminal and outer vessel wall dimensions, evaluation of plaque burden, and characterization of its composition. This modality has been extensively used over the last 25 years to further our understanding about the atherosclerotic process. Preliminary IVUS studies showed that vulnerable plaques have focal manifestations and are mainly located proximally within the coronary arteries and at coronary bifurcations [Citation17–Citation19]. IVUS also enabled assessment of the effect of systemic and local factors on the progression of atherosclerosis [Citation20–Citation24], allowed morphologic comparison of symptomatic versus asymptomatic plaque rupture, serial assessment of plaque evolution [Citation25], and the effect of different treatments on this process [Citation26–Citation28].
A preliminary IVUS-based study of coronary atherosclerosis provided promise that this modality may allow accurate assessment of the phenotypic characteristics of the plaque and detection of lesions that are likely to progress and cause major adverse cardiovascular events (MACE) [Citation29]. However, three prospective studies of atherosclerotic evolution casted doubts about the accuracy of IVUS in detecting vulnerable lesions. The Prospective Natural-History Study of Coronary Atherosclerosis (PROSPECT) study was the largest study of its kind that utilized radiofrequency analysis of the backscatter IVUS signal and in particular IVUS-virtual histology (VH) to assess plaque morphology and vulnerability in 697 patients admitted with an ACS. All the studied patients had 3-vessel IVUS-VH imaging immediately after treatment of the culprit lesions and were followed up for a median of 3.4 years. On multivariate analysis, a plaque burden >70%, a minimal lumen area <4 mm2, and the presence of a TCFA phenotype were predictors of vulnerable plaques that caused MACE at follow-up. The positive predictive value of these three variables in detecting vulnerable lesions was 18.2% [Citation30].
Similar results were reported in VH-IVUS in Vulnerable Atherosclerosis study, which included 170 patients admitted with stable angina or an ACS that were referred for PCI and underwent 3-vessel IVUS-VH imaging. The presence of TCFA as identified by IVUS-VH was the only predictor of non-culprit lesion-related MACE [Citation31].
The Prediction of the Progression of Coronary Artery Disease and Clinical Outcomes Using Vascular Profiling of Shear Stress and Wall Morphology (PREDICTION) study was the only prospective study that examined the implications of the local hemodynamic forces on atherosclerotic disease progression. Five hundred and six patients with an ACS who had PCI and 3-vessel grayscale IVUS imaging at baseline and 6–10 months follow-up were included in the analysis. The IVUS data at baseline were fused with the angiographic images to reconstruct coronary artery anatomy and blood flow simulation was performed in the baseline models. Low endothelial shear stress (ESS) at baseline was a predictor of plaque progression and of lesions that required revascularization at follow-up. An increased plaque burden and low ESS enabled prediction of lesions that will require revascularization with a positive predictive value of 41% [Citation32].
Although these studies provided robust evidence that IVUS can detect vulnerable lesions, they also revealed significant limitations of intravascular imaging. First, in PROSPECT, IVUS was not able to study the entire coronary tree and thus, it assessed 53% of the lesions that caused events during the follow-up period. Second, 10.6% of the recruited patients were excluded from the PROSPECT and 33% from the PREDICTION because of incomplete data. Third, majority of the events in PROSPECT were unstable angina, rather than strong clinical end points such as cardiac death and myocardial infarction; whilst in PREDICTION, only 29% of the revascularizations were related to clinical events, as in most patients the decision to performed PCI was made based on the follow-up coronary angiography. Fourth, intravascular imaging was associated with a risk of complications – 1.6% of the patients in PROSPECT and 0.6% of patients in PREDICTION had a complication attributed to IVUS imaging [Citation30–Citation32]. Lastly, although IVUS was shown to be able to predict future events, its positive predictive value was quite low, 18.2% in PROSPECT and 41% in PREDICTION. As expected, these findings raised concerns about the role of imaging in detecting vulnerable lesions and created pessimism in the scientific community about the clinical potential of intravascular imaging to stratify cardiovascular risk [Citation33,Citation34].
2.2. Optical coherence tomography
Optical coherence tomography (OCT) with its high image resolution (10–20 vs. 150 μm for IVUS) enables more detailed assessment of vulnerable plaque morphology and visualization of plaque micro-characteristics that cannot be detected by IVUS imaging and are associated with increased vulnerability such as the presence of macrophages [Citation35], neovascularization [Citation36,Citation37], and microcalcifications [Citation38]. In addition, compared to IVUS, OCT allows more reliable characterization of plaque composition and estimation of fibrous cap thickness in fibroatheromas [Citation39,Citation40].
OCT not only allows assessment of plaque phenotype but it also enables evaluation of the effect of the local hemodynamic forces on vessel morphology. A computational fluid dynamic study that evaluated ESS in OCT-derived models showed that segments exposed to low ESS have a larger lipid burden, thinner fibrous caps, and higher prevalence of TCFA; findings that support evidence from experimental studies show that local hemodynamic forces contribute to the formation of vulnerable lesions [Citation41].
Several studies used OCT to assess the prevalence and distribution of vulnerable plaques in different populations and in patients with different clinical presentations. Reports have shown that patients with renal failure are more likely to have an increased lipid component, cholesterol crystals, calcific tissue, and vessel wall disruptions [Citation42], while these with a history of diabetes and metabolic syndrome are more likely to have plaques with an increased necrotic core component compared to normal subjects [Citation43]. Moreover, patients’ social history and gender seem to also affect plaque morphology. A 3-vessel OCT imaging study showed that smokers were more likely to have lipid-rich lesions and plaque disruptions [Citation44], while Kataoka et al. showed that female patients more often have lesions with lower cholesterol and calcium content but a higher incidence of plaque erosions [Citation45]. Females also tend to have different TCFA distribution compared to males: in males TCFA were located in the proximal segments of the coronary arteries, whereas in females they were more evenly distributed, a finding that provides mechanistic insights about the higher incidence of revascularization and adverse events noted in female patients undergoing bypass operation compared to males [Citation46].
In addition, patients admitted with a ST-elevation myocardial infarction (STEMI) are more likely to have more vulnerable plaques than those admitted with a non-ST-elevation myocardial infarction (NSTEMI) or stable angina symptoms [Citation47,Citation48].
Although OCT has been extensively used to assess plaque pathobiology, there is only one small study that investigated its efficacy in identifying lesions that are likely to progress and cause events. In this report, 53 patients were included; all the studied patients had OCT imaging at baseline and at 7 months follow-up. During this period, 13 non-flow limiting lesions exhibited disease progression. Lesions that progressed more often had vessel wall discontinuities (61.5% vs. 8.9%, P < 0.01), neo-vessels (76.9% vs. 14.3%, P < 0.01), lipid-rich plaques (100% vs. 60.7%, P = 0.02), TCFA phenotype (76.9% vs. 14.3%, P < 0.01), macrophages accumulations (61.5% vs. 14.3%, P < 0.01), and intraluminal thrombi (30.8% vs. 1.8%, P < 0.01) compared to those that remained unchanged. This analysis demonstrated for the first time the clinical implications of plaque micro-features, but it included a small number of patients that did not allow assessment of their additive value in predicting high-risk vulnerable plaques [Citation49].
OCT may has allowed evaluation of plaque characteristics that are unseen by IVUS, but it also has significant limitations in assessing plaque morphology. These include its low penetration depth of 2–3 mm that restricts its reach to the internal elastic lamina in heavily diseased vessels, its limited accuracy to detect macrophages, its limited efficacy in differentiating deeply embedded lipid cores from calcific tissue, and the fact that it doesn’t allow reliable assessment of the distribution of the plaque on vessel geometry [Citation50,Citation51].
2.3. Near-infrared spectroscopy
By analyzing the reflected infrared light from the coronary wall, near-infrared spectroscopy (NIRS) can identify the chemical signature of lipid cores. Preliminary validation studies in animal models have provided encouraging results, while the first validation study of NIRS in human histological data has showed that NIRS is able to detect lipid-rich lesions with high accuracy (area under the curve, AUC: 0.86) [Citation52,Citation53]. These findings were confirmed by more recent analyses showing that NIRS is the best invasive imaging modality for the detection of fibroatheromas [Citation54–Citation56].
Several reports validated the feasibility and reproducibility of NIRS in vivo [Citation57,Citation58], and prospective studies used this modality to examine the effects of interventional and pharmacological interventions on lipid burden [Citation59,Citation60]. However, stand-alone NIRS failed to dominate in the clinical arena and in the study of atherosclerosis as it has significant inherited limitations. First, it can only detect the lipid component and it cannot give information about the other plaque components. In addition, NIRS does not enable visualization and assessment of the lumen, outer vessel wall dimensions, and plaque burden and lacks image depth resolution that enables localization of the necrotic core within the plaque and differentiation of TCFA from thick cap fibroatheromas. To overcome these limitations, efforts were made to spectroscopically assess fibrous cap thickness and develop dual-probe imaging catheters that will provide simultaneous assessment of plaque characteristics from two imaging modalities with complementary strengths, thus enabling a more accurate characterization of plaque pathobiology [Citation61,Citation62].
2.4. Multimodality imaging
Several histology-based studies have demonstrated that combined intravascular imaging provides more reliable characterization of plaque composition [Citation54,Citation55,Citation63,Citation64]. Sawada et al. were the first who used combined in vivo IVUS-VH–OCT imaging to study plaque morphology. They demonstrated significant discrepancies between the estimations of the two modalities about plaque phenotype that were attributed to the inherited limitations of each technique and concluded that combined intravascular imaging may enable more reliable evaluation of plaque characteristics () [Citation65]. Since then, several other researchers have used combined intravascular imaging to study coronary atheroma and changed our understanding about plaque evolution. Diletti et al. used serial combined IVUS-VH–OCT imaging to study plaque characteristics in bifurcation lesions and found no difference in the fibrous cap thickness and necrotic core component at 6 months follow-up concluding that plaque evolution is a slow process and contradicted the findings of a previous report that used serial stand-alone IVUS to assess changes in plaque morphology at 1-year follow-up [Citation25,Citation66]. In another report, combined IVUS–OCT imaging was used to assess plaques that ruptured and caused events, plaques that had a silent rupture and non-ruptured TCFAs. The authors showed that ruptured plaques had thinner fibrous caps – assessed by OCT – while the lesions that ruptured and caused events had a smaller lumen area and an increased plaque burden – identified by IVUS – compared to lesions that had a silent rupture. These findings indicate that combined IVUS–OCT not only detects morphological differences between these three groups but is also able to predict the natural course and the clinical implications of plaque evolution [Citation67]. In another study from the same research group, multimodality IVUS–OCT imaging was used to assess plaque morphology in angiographically significant (diameter stenosis >70%) lesions, in intermediate (diameter stenosis: 50–69%), and in non-flow limiting lesions (diameter stenosis: 30–49%) and showed that significant stenoses had a more vulnerable phenotype with a higher prevalence of TCFA, thinner fibrous caps, and a greater plaque burden compared to mild or moderate stenoses [Citation68]. These findings augmented the notion that lesions with severe stenoses are more likely to rupture and cause clinically significant events than mild or moderate stenoses [Citation69,Citation70] and questioned the results of angiographic studies conducted in 1980s suggesting that myocardial infarction is more likely to be caused by non-flow limiting lesions [Citation71].
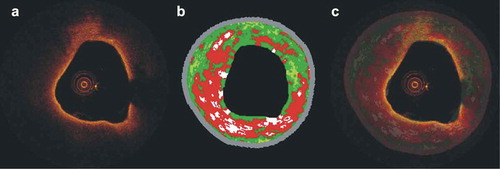
Figure 1. Efficacy of combined OCT – IVUS-VH imaging in assessing plaque morphology. (a) OCT frame showing a lipid-rich plaque with a thin fibrous cap. The corresponding IVUS-VH frame is shown in panel (b). Fusion of these images enables complete assessment of plaque characteristics: OCT allows evaluation of plaque composition and fibrous cap thickness while IVUS-VH provides quantitative information about plaque composition, quantification of plaque burden and estimation of the remodeling pattern (c).
IVUS–OCT, but also IVUS-NIRS and IVUS–angioscopic imaging have been used to assess the effect of pharmacological treatments on plaque morphology [Citation60,Citation72–Citation76]. In the Integrated Biomarkers Imaging Study (IBIS) 3 study, serial IVUS–NIRS was used to assess the implications of aggressive statin therapy (rosuvastatin 40 mg) on plaque characteristics in 164 patients undergoing coronary angiography for clinical purposes. Rosuvastatin therapy did not change the lipid component (P = 0.074) but resulted in a decrease of the plaque volume at follow-up (P = 0.006) [Citation60]. These findings contradict the results of previous smaller IVUS-angioscopic-based studies which demonstrated that treatment with statins has an effect not only on the plaque burden but also on its phenotype and vulnerability [Citation76,Citation77]. In addition, the YELLOW study implemented serial NIRS–IVUS imaging to assess plaque characteristics in obstructive lesions at baseline and after 7 weeks of treatment with either rosuvastatin 40 mg or standard lipid therapy. A significant reduction in the lipid component was noted in patients receiving high-dose statin compared to standard dose statin therapy (P = 0.01); this reduction however was not associated with changes in the atheroma burden (P = 0.86) [Citation74].
Although it is acknowledged that multimodality imaging enables more detailed assessment of plaque morphology, it is also a tedious and time-consuming process and there are concerns about its safety in the clinical arena. Taniwaki et al. were the first to explore the safety and feasibility of combined multivessel IVUS–OCT imaging. The authors presented data from the IBIS 4 which included 103 patients admitted and revascularized for a STEMI that underwent 3-vessel IVUS-VH–OCT imaging at baseline and at 13 months follow-up [Citation78]. Multimodality imaging was feasible in the majority of the patients (at baseline IVUS-VH: 85.7%, OCT: 89.9%; and at follow-up IVUS-VH: 84.8%, OCT: 86.6%). The intravascular imaging-related complications rates were low: 1.9% at baseline and 1.1% at follow-up. When the authors compared 2-year follow-up outcomes between patients who had PCI with and without multimodality intravascular imaging, they found no difference in the incidence of MACE (16.7% vs. 13.3%, P = 0.39). These findings suggest that multimodality intravascular imaging is feasible and safe, even in high-risk patients treated for STEMI.
Recently, industry has created hybrid catheters that combine two imaging probes which enable a more detailed and complete evaluation of coronary plaques. The TVC Imaging System (InfraReDx, Burlington, Massachusetts) is the first clinically available hybrid catheter and incorporates an IVUS and NIRS imaging probe enabling simultaneous data acquisition that is accurately co-registered in comprehensive images providing information about plaque composition and burden. Madder et al. used NIRS–IVUS to study 20 culprit lesions in patients presented with a STEMI and showed that an increased lipid component (lipid core burden index in a 4-mm segment, LCBI4 mm > 400) was able to differentiate the culprit from the non-ruptured plaques with a high accuracy (AUC: 0.90) [Citation79]. These findings were echoed in a larger study that demonstrated a sensitivity of 64% and a specificity of 85% for LCBI4 mm > 400 in identifying culprit lesions that caused STEMI [Citation80]. The same research group replicated the above study in patients who presented with a NSTEMI or unstable angina and showed that larger lipid cores were present in the culprit lesions of these patients as well, but in this setting, LCBI4 mm > 400 had a lower sensitivity and specificity (63.6% and 94.0% for NSTEMI and 38.5% and 89.8% for culprit lesion causing unstable angina, respectively) [Citation81]. These results created hopes that hybrid imaging may enable accurate prediction of plaques that are likely to progress and cause events and currently, two prospective imaging studies, the PROSPECT II (NCT02171065) and Lipid Rich Plaque studies (NCT02033694), are recruiting patients and aim to examine the efficacy of NIRS–IVUS in detecting vulnerable, high-risk plaques.
Apart from the NIRS–IVUS catheter, several other hybrid catheters have been designed and are currently undergoing preclinical evaluation. These include (1) the combined IVUS-OCT, (2) the OCT-NIRS, (3) the OCT-near-infrared fluorescence catheter (NIRF), (4) the IVUS-NIRF, (5) IVUS-intravascular photoacoustic (IVPA), and (6) the IVUS-time resolved fluorescence spectroscopy catheter. Moreover, efforts are made to develop other invasive imaging techniques such as intravascular magnetic resonance imaging (MRI) or Raman spectroscopy [Citation82,Citation83]. These modalities are expected to enable not only more accurate evaluation of plaque morphology but also its biology and predict atherosclerotic evolution () [Citation61,Citation62].
Figure 2. Output of the available hybrid imaging catheters: (a) combined NIRS-IVUS imaging, IVUS enables assessment of the lumen, outer vessel wall dimensions and plaque burden while NIRS allows detection of the lipid component indicated with a yellow-orange color; (b) a typical example of a combined OCT-NIRS image where spectroscopy enables reliable characterization of the composition of the plaque – the tissue extending from 4–7 o’clock could have been classified as calcific tissue according to standalone OCT, nevertheless NIRS demonstrates an increased lipid component – and classifies the plaque as fibroatheroma; (c, d) output of the combined IVUS-OCT catheter: (c) IVUS allows assessment of stent expansion and quantification of the lumen, stent, outer vessel wall dimensions and plaque burden behind the stent, while (d) OCT enables detailed assessment of stent apposition, strut endothelisation, and detection and classification of the endoluminal thrombus; (e) hybrid OCT-NIRF imaging performed after injection of the Prosense VM110 activatable marker, OCT allows visualization of plaque characteristics while NIRF identifies the presence of cathepsins B which indicate increased protease activity and inflammation; (f) combined IVUS-NIRF imaging after injection of the indocyanin green tracer which indicates macrophages accumulation; (g) hybrid IVUS-IVPA imaging, IVPA allows reliable detection of lipid component – indicated with orange color – while IVUS allows assessment of the lumen and plaque morphology; and (h) hybrid IVUS-TRFS imaging. TRFS provides assessment of the superficial plaque components and detection of collagen (orange color), macrophages and lipid component (green color) while IVUS allows visualization of the lumen and quantification of the plaque burden. Images modified and obtained with permission from Bourantas et al [Citation62] and Abran et al [Citation84]. Full color available online.
![Figure 2. Output of the available hybrid imaging catheters: (a) combined NIRS-IVUS imaging, IVUS enables assessment of the lumen, outer vessel wall dimensions and plaque burden while NIRS allows detection of the lipid component indicated with a yellow-orange color; (b) a typical example of a combined OCT-NIRS image where spectroscopy enables reliable characterization of the composition of the plaque – the tissue extending from 4–7 o’clock could have been classified as calcific tissue according to standalone OCT, nevertheless NIRS demonstrates an increased lipid component – and classifies the plaque as fibroatheroma; (c, d) output of the combined IVUS-OCT catheter: (c) IVUS allows assessment of stent expansion and quantification of the lumen, stent, outer vessel wall dimensions and plaque burden behind the stent, while (d) OCT enables detailed assessment of stent apposition, strut endothelisation, and detection and classification of the endoluminal thrombus; (e) hybrid OCT-NIRF imaging performed after injection of the Prosense VM110 activatable marker, OCT allows visualization of plaque characteristics while NIRF identifies the presence of cathepsins B which indicate increased protease activity and inflammation; (f) combined IVUS-NIRF imaging after injection of the indocyanin green tracer which indicates macrophages accumulation; (g) hybrid IVUS-IVPA imaging, IVPA allows reliable detection of lipid component – indicated with orange color – while IVUS allows assessment of the lumen and plaque morphology; and (h) hybrid IVUS-TRFS imaging. TRFS provides assessment of the superficial plaque components and detection of collagen (orange color), macrophages and lipid component (green color) while IVUS allows visualization of the lumen and quantification of the plaque burden. Images modified and obtained with permission from Bourantas et al [Citation62] and Abran et al [Citation84]. Full color available online.](/cms/asset/ccfa8179-4436-4ff7-86f1-5fae8dbbbf56/ierk_a_1297231_f0002_oc.jpg)
3. Noninvasive imaging
3.1. Computed tomographic coronary angiography
Computed tomographic coronary angiography (CTCA) has been recently introduced as an attractive alternative for the study of coronary atherosclerosis as it enables noninvasive assessment of atheroma characteristics. Several histology and intravascular-based imaging studies have shown that CTCA allows accurate evaluation of the luminal and outer vessel wall dimensions, assessment of plaque burden and remodeling pattern, and characterization of its composition [Citation85–Citation92]. Reports have demonstrated that CTCA enables detection of calcific tissue but it has a limited accuracy in differentiating lipid from fibrotic tissue component [Citation86,Citation87,Citation89,Citation91,Citation92]; while recent histology-based studies have shown that CTCA – despite its limited imaging resolution – allows characterization of the phenotype of the plaque and detection of high-risk vulnerable lesions – which on CTCA exhibit a napkin-ring sign morphology – with high specificity but low sensitivity [Citation93,Citation94].
Despite the limited accuracy of CTCA in evaluating plaque morphology and composition, there is consistent evidence that this modality is able to identify lesions that are likely to progress and cause cardiovascular events [Citation95–Citation98]. Two retrospective studies conducted by Motoyama et al. that included 1059 and 3158 patients showed that the presence of attenuated plaques and positive remodeling indicated plaque vulnerability [Citation95,Citation98]. These findings were confirmed by Otsuka et al. in a retrospective analysis that included 895 patients who underwent CTCA for suspected CAD and were followed up for 2.3 years [Citation96]. During this period, 24 ACSs occurred. Positive remodeling, low attenuated plaques, and a napkin-ring sign were predictors of vulnerable lesions. The sensitivity and specificity of the napkin-ring sign in detecting lesions that caused events was 41% and 97%, respectively, while the positive predictive value was 22%, which is slightly higher than the positive predictive value of IVUS-derived plaque characteristics reported in the PROSPECT study [Citation30]. This is likely to be due to the different study design (e.g. retrospective vs. prospective design), the smaller follow-up period in the study of Otsuka et al. (2.3 vs. 3.4 years), and to the different clinical presentations and baseline characteristics of the patients recruited into these studies. The value of CTCA in detecting vulnerable lesions is also supported by a recent retrospective analysis which included 1650 patients with suspected CAD which showed that lesions that caused cardiovascular events had specific morphological characteristics on CTCA and in particular increased plaque burden, lower attenuation, and a smaller lumen area compared to those that remained silent [Citation97]. Nevertheless, all these studies were performed in patients with suspected CAD and not in those with established CAD who are likely to have extensive and advanced atherosclerotic lesions; therefore, the efficacy of CTCA to detect vulnerable lesions in this vulnerable population, who may be studied by invasive imaging techniques, remains unclear.
In contrast to intravascular imaging, CTCA enables complete assessment of coronary artery tree pathology, reconstruction of the coronary arteries, and generation of three dimensional (3D) geometries that can be processed with computational fluid dynamic techniques to estimate vessel wall biomechanics (). Several reports used CTCA to examine the association between plaque morphology and local hemodynamic forces and studies examined the value of CTCA modeling in predicting atherosclerotic disease progression [Citation99–Citation101]. In the study of Bourantas et al. that included 32 patients admitted with an ACS who had CTCA imaging following complete revascularization and at 3 years follow-up, low baseline ESS was an independent predictor of lumen reduction (β = −0.47 95% confidence interval: −0.78 to −0.16; P < 0.001) and plaque burden increase (β = 0.11, 95% confidence interval: 0.02–0.21; P = 0.018) at follow-up [Citation102]. These findings were confirmed by the analysis of Sakellarios et al. who simulated the LDL transport process into the vessel wall and showed that increased LDL accumulation was independently associated with a reduction in lumen area (β = −0.53, 95% confidence interval: −0.86 to −0.20; P = 0.002) and an increase in plaque burden (β = 0.19, 95% confidence interval: 0.08–0.29; P < 0.001) [Citation103]. The results of these two small scale studies are promising and support the use of CTCA-based modeling to assess vessel physiology; however, it is still unclear whether the ESS estimated by CTCA can improve prediction of high-risk plaques that will progress and cause cardiovascular events.
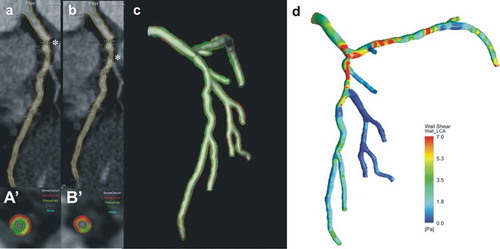
Figure 3. A case example that highlights the role of CTCA in assessing plaque morphology and physiology.The raw CTCA data demonstrates two tight lipid-rich lesions in the ostium (a) and the proximal segment (b) of the left anterior descending artery. Panels (a‘) and (b’) portray the cross sections with the minimal lumen area, their locations in panels (a) and (b) are indicated with asterisks. The annotated borders in the CTCA were used to reconstruct coronary artery anatomy, (c) assess the distribution of the plaque in the model (red indicates lipid tissue, green fibrotic, light-green fibrofatty and white calcific tissue) and perform blood flow simulation, estimate the local hemodynamic forces and their association with plaque morphology (d). Full color available online.
3.2. Magnetic resonance imaging
Comparing to CTCA MRI has significant advantages in the study of atherosclerosis as it enables better evaluation of soft tissue characteristics, lacks of the blooming artifacts seen in the calcified plaques, and does not require radiation exposure. Although there is today convincing evidence about the efficacy of MRI in assessing plaque morphology in the carotids, its role in the study of coronary atherosclerosis is limited [Citation104,Citation105]. This should be attributed to the fact that coronary imaging requires increased imaging time to enhance spatial resolution and the need to reduce motion arterfacts created during the cardiac circle. The first studies investigating the efficacy of MRI in detecting obstructive CAD demonstrated a moderate accuracy [Citation106] which however improved in recent reports [Citation107,Citation108] that implemented advanced imaging but remained inferior to CTCA [Citation109]. Two studies compared the plaque burden estimations of black-blood MRI and IVUS and the first showed a good correlation between MRI and IVUS estimations while in the other report, there was a weak association between MRI and IVUS [Citation110,Citation111].
Several reports have examined the efficacy of MRI in detecting plaque characteristics associated with increased vulnerability. The Multi-Ethnic Study of Atherosclerosis and the Atherosclerotic Disease, Vascular Function, and Genetic Epidemiology study have shown that black-blood MRI imaging can detect positive remodeling [Citation112,Citation113]; while T1-weitghted MRI imaging studies have shown that this technique can identify the presence of thrombus and high-risk plaques [Citation114,Citation115]. T1-weighted imaging can also provide useful prognostic information: in a study that included 568 patients with suspected CAD, an increased coronary plaque intensity in T1-weigthed imaging was independent predictor of future adverse cardiovascular events at 55 months follow-up (hazard ratio: 3.96; 95% confidence interval: 1.92–8.17; P < 0.001); nevertheless, there is no data today about the efficacy of MRI in detecting lesions that are likely to progress and cause cardiovascular events [Citation116].
3.3. Positron emission tomography
Molecular imaging using positron emission tomography (PET) can be applied to detect inflammation and metabolic processes occurring within atherosclerotic plaques, including microcalcification, hypoxia, and neo-angiogenesis. PET is a highly sensitive noninvasive nuclear imaging technique that involves intravenous injection of radio-labeled tracers with a range of molecular targets. PET scanners detect annihilation events that occur as a result of beta-decay of the positron-emitting radio-isotope and use this data to generate 2D or 3D tomographic maps displaying the distribution of the radio-ligand within the body at specific time points. As the spatial resolution of PET is limited (roughly 5 mm), PET images are typically fused with CT or MRI for accurate anatomical signal localization.
Several PET ligands with established roles in clinical cancer imaging have been repurposed for use in atherosclerosis research. Of these PET tracers, 18F-fluorodeoxyglucose (FDG) is the most well studied in atherosclerosis. 18F-FDG signals within atherosclerotic plaques reflect the metabolic activity of macrophages and therefore plaque inflammation. Indeed, in vivo 18F-FDG uptake is strongly correlated with macrophages density within excised carotid plaques, as well as gene expression associated with vascular inflammation [Citation117,Citation118]. Vascular 18F-FDG signals have also been shown to be significantly correlated with presence of traditional cardiovascular risk factors (e.g. older age, smoking, hypertension, diabetes mellitus, and hyperlipidemia) and are elevated in patients with systemic inflammatory conditions conferring increased cardiovascular risk, such as rheumatoid arthritis and psoriasis [Citation119–Citation121]. Data from the prospective Dublin Carotid Atherosclerosis Stroke Study showed that increased carotid artery 18F-FDG uptake can identify patients with increased risk of early stroke recurrence, independent of age and stenosis severity (a,b) [Citation122]. Moreover, a retrospective study of imaging from 513 cancer-free patients examined over a 4-year period found that aortic 18F-FDG uptake strongly predicted risk of cardiovascular events independent of traditional risk factors (hazard ratio: 4.71, P < 0.001), with nearly 30% net reclassification improvement over Framingham risk score in the highest risk group [Citation123].
Figure 4. Representative images of vascular PET-CT imaging of disease activity in patients with atherosclerosis. Top: CT (a) and 18F-fluordeoxyglucose (FDG) PET-CT (b) images from a patient with recent transient ischemic attack resulting from a symptomatic right internal carotid artery stenosis (circle) demonstrating an intense focal inflammatory signal (arrow) on PET imaging. Bottom: CT (c) and 18F-sodium fluoride (NaF) PET-CT (d) images from a patient with stable angina showing high tracer uptake representing micro-calcification in relation to left anterior descending artery atherosclerosis (arrows), as well as the ascending aorta (arrowhead). Figure adapted from Tarkin et al [Citation124].
![Figure 4. Representative images of vascular PET-CT imaging of disease activity in patients with atherosclerosis. Top: CT (a) and 18F-fluordeoxyglucose (FDG) PET-CT (b) images from a patient with recent transient ischemic attack resulting from a symptomatic right internal carotid artery stenosis (circle) demonstrating an intense focal inflammatory signal (arrow) on PET imaging. Bottom: CT (c) and 18F-sodium fluoride (NaF) PET-CT (d) images from a patient with stable angina showing high tracer uptake representing micro-calcification in relation to left anterior descending artery atherosclerosis (arrows), as well as the ascending aorta (arrowhead). Figure adapted from Tarkin et al [Citation124].](/cms/asset/8b19b471-6a55-4c99-9afa-fbd642f6fecd/ierk_a_1297231_f0004_oc.jpg)
Although 18F-FDG PET has well-established, evidence-based roles for imaging vascular inflammation, there are several limitations to this technique in atherosclerosis. First, as most metabolically active cells take up glucose, it is unclear how much of the observed signal is influenced by cells other than macrophages within plaques, including neutrophils, lymphocytes, endothelial cells, and vascular smooth muscle cells. Vascular 18F-FDG signal intensity is also significantly influenced by plaque hypoxia and therefore might not be purely representative of inflammation per se [Citation125]. Perhaps most importantly, using 18F-FDG to image the coronary vasculature is particularly difficult because of high background myocardial uptake of 18F-FDG, even with strict dietary manipulation or prolonged fasting [Citation126]. Nonetheless, in a feasibility study of coronary 18F-FDG imaging, increased tracer uptake was observed in proximal culprit coronary lesions in patients with ACS compared to non-culprit lesions in patients with stable angina [Citation127]. Several other PET tracers have been tested for use in atherosclerosis imaging, which might offer more specific markers of inflammation than 18F-FDG or provide better methods for coronary artery imaging owing to inherently low myocardial tracer activity. These include 18F-fluorodeoxymannose, the somatostatin receptor subtype-2 PET ligand 68Ga-DOTATATE, 11F-fluorocholine, and transient receptor protein receptor tracers, including 11C-PK11195 [Citation128–Citation131].
Aside from inflammation, several other pathogenic mechanisms of atherosclerosis can be imaged using PET, including microcalcification, hypoxia, neo-angiogenesis, hematopoiesis, and HDL accumulation [Citation126,Citation132–Citation135]. For example, early vascular calcification occurring in response to intense plaque inflammation, and below the resolution of CT, can be detected using 18F-sodium fluoride (NaF) PET. In carotid plaques, 18F-NaF binding takes place in areas of pathological mineralization and is related to the surface area of exposed hydroxyapatite (c,d) [Citation136]. Increased vascular 18F-NaF accumulation has also been shown to occur during early stages of neointima thickening, while a prospective clinical study showed that plaque microcalcification detected by 18F-NaF PET enabled accurate identification of culprit coronary lesions in patients with a myocardial infarction [Citation126,Citation137]. The ongoing multicenter Prediction of Recurrent Events With 18F-Fluoride (PREFFIR, NCT02278211) study aims to evaluate the prognostic value of coronary 18F-NaF PET-CT imaging in 700 patients with myocardial infarction and proven multivessel CAD followed up over 2 years.
4. Expert commentary
Imaging of coronary atherosclerosis has provided unique insights about atherosclerotic evolution and has enabled in vivo identification of vulnerable, high-risk plaques (). Prospective invasive imaging-based studies have shown that IVUS has a low accuracy in detecting these lesions, but this increases considerably when the local hemodynamic patterns are included in the prediction model () [Citation138]. These findings highlight the need for a complete and detailed evaluation of plaque morphology, physiology, and biology to predict its evolution. Invasive imaging – with its high resolution – and in particular hybrid intravascular imaging appears as the ideal approach to study atherosclerotic disease progression and detect vulnerable plaques. Emerging hybrid dual-probe catheters are anticipated to allow precise assessment of plaque characteristics and evaluation of the interplay between plaque micro-features that are unseen by stand-alone IVUS – such as neovessels (given by IVPA), inflammation (provided by NIRF), macrophages (detected by IVPA), or cholesterol crystals detected by OCT – and established markers of plaque vulnerability, such as plaque burden, lipid component, and ESS and their synergistic effect on the formation of high-risk lesions. Hybrid intravascular imaging is also anticipated to shed light into the pathophysiological mechanisms that are involved in plaque erosion and allow us to appreciate the role of microcalcification and cholesterol crystals on plaque destabilization. Finally, hybrid intravascular imaging may also enable prediction of the clinical implications of plaque rupture and differentiation of the lesions that will rupture and cause cardiovascular events from those that will sustain a clinically silent rupture. Considering the limitations of intravascular imaging that restricts its broad use – i.e., the increased time required for the processing of the acquired data, the fact that it can be used only in symptomatic patients undergoing coronary angiography for clinical purposes and that it does not enable complete assessment of coronary artery tree – future intravascular imaging studies assessing vulnerable lesions are anticipated to include small number of patients and focus on the mechanisms regulating atherosclerotic disease progression.
Table 1. Prospective and retrospective studies investigating the efficacy of invasive and noninvasive imaging in detecting vulnerable lesions.
Figure 5. Efficacy of plaque characteristics and vessel physiology in detecting lesions prone to progress. (a) In the PROSPECT study an increased plaque burden, a TCFA phenotype and a minimum lumen area <4 mm2 were able to detect lesions that will progress and cause events with a positive predictive value of 18.2%. (b) In the PREDICTION study the positive predictive value of the model built from the plaque burden and ESS was 41% and increased considerably to 53% after including information about the composition of the plaque. Panels a and b were obtained with permission from Stone et al [Citation30] and Papafaklis et al [Citation138].
![Figure 5. Efficacy of plaque characteristics and vessel physiology in detecting lesions prone to progress. (a) In the PROSPECT study an increased plaque burden, a TCFA phenotype and a minimum lumen area <4 mm2 were able to detect lesions that will progress and cause events with a positive predictive value of 18.2%. (b) In the PREDICTION study the positive predictive value of the model built from the plaque burden and ESS was 41% and increased considerably to 53% after including information about the composition of the plaque. Panels a and b were obtained with permission from Stone et al [Citation30] and Papafaklis et al [Citation138].](/cms/asset/70208b40-5907-4645-af84-adcae0194c1e/ierk_a_1297231_f0005_oc.jpg)
On the other hand, CTCA overcomes these limitations and carries a unique potential for the conduction of large scale outcome studies. Several software have been developed for the fast processing of CTCA imaging data, coronary reconstruction, and blood flow simulation [Citation85,Citation87,Citation139] and evidence supports the use of CTCA in detecting vulnerable plaques in low risk patients [Citation95–Citation98]; nevertheless, there is lack of prospective data, and the efficacy of CTCA in identifying vulnerable lesions in patients with established CAD and extensive atherosclerotic burden remains unclear. Future studies are anticipated to explore the efficacy of CTCA in detecting vulnerable lesions in high-risk patients and the additive value of PET imaging in this challenging setting. Positive results are likely to change clinical practice and justify the focal treatment of vulnerable lesions not only with novel endovascular devices with a better safety profile, but also with nanotechnology-based therapies that target high-risk plaques and modify vulnerable plaque physiology [Citation7,Citation140,Citation141].
Irrespective of the potential value of invasive and noninvasive imaging in detecting lesions that will progress and cause events, cumulative evidence has demonstrated that imaging of atherosclerosis may also be useful in stratifying cardiovascular risk and detecting high-risk patients that are likely to suffer a cardiovascular event. Angioscopic, IVUS, and NIRS-based studies have shown that plaque imaging can provide useful prognostic information [Citation142–Citation145]. However, the above studies included a small number of patients and therefore, the number of events reported was too small to allow us to examine the additive predictive value of intravascular imaging over clinical or angiographic variables.
In parallel, several retrospective analyses have demonstrated that CTCA-based imaging provides useful prognostic information and enables detection of patients that are likely to suffer a cardiovascular event amongst individuals with suspected CAD [Citation95–Citation98,Citation146,Citation147]. In patients with established CAD, a small scale study showed that CTCA-derived variables were predictors of MACE at 5-year follow-up and improved considerably the prognostic accuracy of the model developed from the clinical variables (from 0.68 to 0.76) [Citation148]. However, the small number of the reported events and the fact that this analysis did not take into account the angiographic variables associated with clinical outcomes (i.e. the Syntax score, or the residual Syntax score) did not allow us to draw safe conclusions about the additive prognostic value of CTCA in this population [Citation149–Citation152].
The accurate risk stratification and identification of high-risk individuals has recently attracted attention as several new therapies have been introduced that appear capable of modifying atherosclerotic disease progression [Citation6,Citation153]. Nevertheless, all these new therapies have significant limitations either from administration route (intravenous or subcutaneous), from side effects (bleeding, infection, or bone marrow suppression), or cost. Invasive or noninvasive imaging may have a role in this setting and facilitate accurate risk stratification and detection of high-risk patients that would benefit from an individualized, aggressive treatment for coronary atherosclerosis.
5. Five-year view
Future hybrid intravascular imaging-based studies are expected to shed light into the mechanisms regulating atherosclerotic evolution and predict more accurately lesions that will progress and cause cardiovascular events. Noninvasive imaging and in particular CTCA or combined PET-CTCA imaging are anticipated to have increased applications in the study of atherosclerosis and used to identify noninvasively vulnerable plaques and stratify more accurately cardiovascular risk. Future studies sought also investigate the clinical feasibility and cost-effectiveness of noninvasive imaging in identifying vulnerable patients and guiding treatment in patients with established CAD.
6. Key issues
Intravascular imaging modalities enable assessment of plaque characteristics and can identify with low accuracy lesions that are likely to progress and cause cardiovascular events
Combined intravascular imaging appears able to overcome limitations of standalone imaging modalities and provide detailed and complete assessment of plaque pathobiology
CTCA can assess atherosclerotic disease burden in the entire coronary tree, identify plaque features related with increased vulnerability and provide useful prognostic information in low risk-individuals
PET imaging provides complementary information to CTCA as it enables non-invasive assessment of plaque inflammation and biology
Future studies sought to evaluate the value of CTCA, MRI or PET-CTCA imaging in stratifying cardiovascular risk in patients with established CAD
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Additional information
Funding
References
- Organisation WH. The top ten causes of death. 2011.
- Fox KA, Poole-Wilson PA, Henderson RA, et al. Interventional versus conservative treatment for patients with unstable angina or non-ST-elevation myocardial infarction: the British heart foundation RITA 3 randomised trial. Randomized intervention trial of unstable angina. Lancet. 2002;360:743–751.
- [Anonymous]. Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Lancet. 1988;2:349–360.
- Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. DOI:10.1056/NEJMoa0904327
- Foundation BH. cardiovascular disease statistics. 2015:
- Ridker PM, Luscher TF. Anti-inflammatory therapies for cardiovascular disease. Eur Heart J. 2014;35:1782–1791.
- Bourantas CV, Garcia-Garcia HM, Diletti R, et al. Early detection and invasive passivation of future culprit lesions: a future potential or an unrealistic pursuit of chimeras? Am Heart J. 2013;165:869–881 e864.
- Bourantas CV, Garcia-Garcia HM, Torii R, et al. Vulnerable plaque detection: an unrealistic quest or a feasible objective with a clinical value? Heart. 2016;102:581–589.
- Finn AV, Nakano M, Narula J, et al. Concept of vulnerable/unstable plaque. Arterioscler Thromb Vasc Biol. 2010;30:1282–1292. DOI:10.1161/ATVBAHA.108.179739
- Kragel AH, Reddy SG, Wittes JT, et al. Morphometric analysis of the composition of atherosclerotic plaques in the four major epicardial coronary arteries in acute myocardial infarction and in sudden coronary death. Circulation. 1989;80:1747–1756.
- Falk E, Nakano M, Bentzon JF, et al. Update on acute coronary syndromes: the pathologists’ view. Eur Heart J. 2013;34:719–728. DOI:10.1093/eurheartj/ehs411
- Burke AP, Farb A, Malcom GT, et al. Coronary risk factors and plaque morphology in men with coronary disease who died suddenly. N Engl J Med. 1997;336:1276–1282. DOI:10.1056/NEJM199705013361802
- Falk E. Plaque rupture with severe pre-existing stenosis precipitating coronary thrombosis. Characteristics of coronary atherosclerotic plaques underlying fatal occlusive thrombi. Br Heart J. 1983;50:127–134.
- Grebe A, Latz E. Cholesterol crystals and inflammation. Curr Rheumatol Rep. 2013;15:313. DOI:10.1007/s11926-012-0313-z
- Janoudi A, Shamoun FE, Kalavakunta JK, et al. Cholesterol crystal induced arterial inflammation and destabilization of atherosclerotic plaque. Eur Heart J. 2016;37:1959–1967.
- Virmani R, Burke AP, Farb A, et al. Pathology of the vulnerable plaque. J Am Coll Cardiol. 2006;47:C13–18. DOI:10.1016/j.jacc.2005.10.065
- Rodriguez-Granillo GA, Garcia-Garcia HM, Mc Fadden EP, et al. In vivo intravascular ultrasound-derived thin-cap fibroatheroma detection using ultrasound radiofrequency data analysis. J Am Coll Cardiol. 2005;46:2038–2042.
- Hong MK, Mintz GS, Lee CW, et al. A three-vessel virtual histology intravascular ultrasound analysis of frequency and distribution of thin-cap fibroatheromas in patients with acute coronary syndrome or stable angina pectoris. Am J Cardiol. 2008;101:568–572.
- Garcia-Garcia HM, Gomez-Lara J, Gonzalo N, et al. A comparison of the distribution of necrotic core in bifurcation and non-bifurcation coronary lesions: an in vivo assessment using intravascular ultrasound radiofrequency data analysis. EuroIntervention, 2010;6:321–327. DOI:10.4244/EIJV6I3A54
- Philipp S, Bose D, Wijns W, et al. Do systemic risk factors impact invasive findings from virtual histology? Insights from the international virtual histology registry. Eur Heart J. 2010;31:196–202.
- Stone PH, Coskun AU, Kinlay S, et al. Regions of low endothelial shear stress are the sites where coronary plaque progresses and vascular remodelling occurs in humans: an in vivo serial study. Eur Heart J. 2007;28:705–710.
- Wentzel JJ, Krams R, Schuurbiers JC, et al. Relationship between neointimal thickness and shear stress after Wallstent implantation in human coronary arteries. Circulation. 2001;103:1740–1745.
- Marso SP, Frutkin AD, Mehta SK, et al. Intravascular ultrasound measures of coronary atherosclerosis are associated with the Framingham risk score: an analysis from a global IVUS registry. EuroIntervention. 2009;5:212–218.
- Samady H, Eshtehardi P, McDaniel MC, et al. Coronary artery wall shear stress is associated with progression and transformation of atherosclerotic plaque and arterial remodeling in patients with coronary artery disease. Circulation. 2011;124:779–788.
- Kubo T, Maehara A, Mintz GS, et al. The dynamic nature of coronary artery lesion morphology assessed by serial virtual histology intravascular ultrasound tissue characterization. J Am Coll Cardiol. 2010;55:1590–1597.
- Nicholls SJ, Ballantyne CM, Barter PJ, et al. Effect of two intensive statin regimens on progression of coronary disease. N Engl J Med. 2011;365:2078–2087. DOI:10.1056/NEJMoa1110874
- Nicholls SJ, Puri R, Anderson T, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV randomized clinical trial. Jama, 2016. DOI:10.1001/jama.2016.16951.
- Choi SY, Mintz GS. What have we learned about plaque rupture in acute coronary syndromes?. Curr Cardiol Rep. 2010;12:338–343. DOI:10.1007/s11886-010-0113-x
- Sano K, Kawasaki M, Ishihara Y, et al. Assessment of vulnerable plaques causing acute coronary syndrome using integrated backscatter intravascular ultrasound. J Am Coll Cardiol. 2006;47:734–741. DOI:10.1016/j.jacc.2005.09.061
- Stone GW, Maehara A, Lansky AJ, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364:226–235. DOI:10.1056/NEJMoa1002358
- Calvert PA, Obaid DR, O’Sullivan M, et al. Association between IVUS findings and adverse outcomes in patients with coronary artery disease: the VIVA (VH-IVUS in Vulnerable Atherosclerosis) Study. JACC Cardiovasc Imaging. 2011;4:894–901. DOI:10.1016/j.jcmg.2011.05.005
- Stone PH, Saito S, Takahashi S, et al. Prediction of progression of coronary artery disease and clinical outcomes using vascular profiling of endothelial shear stress and arterial plaque characteristics: the PREDICTION Study. Circulation. 2012;126:172–181. DOI:10.1161/CIRCULATIONAHA.112.096438
- Arbab-Zadeh A, Fuster V. The myth of the “vulnerable plaque”: transitioning from a focus on individual lesions to atherosclerotic disease burden for coronary artery disease risk assessment. J Am Coll Cardiol. 2015;65:846–855. DOI:10.1016/j.jacc.2014.11.041
- Libby P, Pasterkamp G. Requiem for the ‘vulnerable plaque’. Eur Heart J. 2015;36:2984–2987.
- Tearney GJ, Yabushita H, Houser SL, et al. Quantification of macrophage content in atherosclerotic plaques by optical coherence tomography. Circulation. 2003;107:113–119.
- Kitabata H, Tanaka A, Kubo T, et al. Relation of microchannel structure identified by optical coherence tomography to plaque vulnerability in patients with coronary artery disease. Am J Cardiol. 2010;105:1673–1678. DOI:10.1016/j.amjcard.2010.01.346
- Tian J, Hou J, Xing L, et al. Significance of intraplaque neovascularisation for vulnerability: optical coherence tomography study. Heart. 2012;98:1504–1509. DOI:10.1136/heartjnl-2012-302445
- Tearney GJ, Regar E, Akasaka T et al. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the international working group for intravascular optical coherence tomography standardization and validation. J Am Coll Cardiol 2012; 59: 1058–1072 DOI: 10.1016/j.jacc.2011.09.079
- Kume T, Akasaka T, Kawamoto T, et al. Measurement of the thickness of the fibrous cap by optical coherence tomography. Am Heart J. 2006;152:755 e751–754. DOI:10.1016/j.ahj.2006.06.030
- Jang IK, Bouma BE, Kang DH, et al. Visualization of coronary atherosclerotic plaques in patients using optical coherence tomography: comparison with intravascular ultrasound. J Am Coll Cardiol. 2002;39:604–609.
- Vergallo R, Papafaklis MI, Yonetsu T, et al. Endothelial shear stress and coronary plaque characteristics in humans: combined frequency-domain optical coherence tomography and computational fluid dynamics study. Circ Cardiovasc Imaging. 2014;7:905–911. DOI:10.1161/CIRCIMAGING.114.001932
- Kato K, Yonetsu T, Jia H, et al. Nonculprit coronary plaque characteristics of chronic kidney disease. Circ Cardiovasc Imaging. 2013;6:448–456. DOI:10.1161/CIRCIMAGING.112.000165
- Yonetsu T, Kato K, Uemura S, et al. Features of coronary plaque in patients with metabolic syndrome and diabetes mellitus assessed by 3-vessel optical coherence tomography. Circ Cardiovasc Imaging. 2013;6:665–673. DOI:10.1161/CIRCIMAGING.113.000345
- Abtahian F, Yonetsu T, Kato K, et al. Comparison by optical coherence tomography of the frequency of lipid coronary plaques in current smokers, former smokers, and nonsmokers. Am J Cardiol. 2014;114:674–680. DOI:10.1016/j.amjcard.2014.05.056
- Kataoka Y, Puri R, Hammadah M, et al. Sex differences in nonculprit coronary plaque microstructures on frequency-domain optical coherence tomography in acute coronary syndromes and stable coronary artery disease. Circ Cardiovasc Imaging. 2016;9:e004506. DOI:10.1161/CIRCIMAGING.116.004506
- Hassan A, Chiasson M, Buth K, et al. Women have worse long-term outcomes after coronary artery bypass grafting than men. Can J Cardiol. 2005;21:757–762.
- Ino Y, Kubo T, Tanaka A, et al. Difference of culprit lesion morphologies between ST-segment elevation myocardial infarction and non-ST-segment elevation acute coronary syndrome: an optical coherence tomography study. JACC Cardiovasc Interv. 2011;4:76–82. DOI:10.1016/j.jcin.2010.09.022
- Kato K, Yonetsu T, Kim SJ, et al. Nonculprit plaques in patients with acute coronary syndromes have more vulnerable features compared with those with non-acute coronary syndromes: a 3-vessel optical coherence tomography study. Circ Cardiovasc Imaging. 2012;5:433–440. DOI:10.1161/CIRCIMAGING.112.973701
- Uemura S, Ishigami K, Soeda T, et al. Thin-cap fibroatheroma and microchannel findings in optical coherence tomography correlate with subsequent progression of coronary atheromatous plaques. Eur Heart J. 2012;33:78–85.
- Manfrini O, Mont E, Leone O, et al. Sources of error and interpretation of plaque morphology by optical coherence tomography. Am J Cardiol. 2006;98:156–159.
- Phipps JE, Vela D, Hoyt T, et al. Macrophages and intravascular OCT bright spots: a quantitative study. JACC Cardiovasc Imaging. 2015;8:63–72.
- Cassis LA, Lodder RA. Near-IR imaging of atheromas in living arterial tissue. Anal Chem. 1993;65:1247–1256.
- Gardner CM, Tan H, Hull EL, et al. Detection of lipid core coronary plaques in autopsy specimens with a novel catheter-based near-infrared spectroscopy system. JACC Cardiovasc Imaging. 2008;1:638–648. DOI:10.1016/j.jcmg.2008.06.001
- Kang SJ, Mintz GS, Pu J, et al. Combined IVUS and NIRS detection of fibroatheromas: histopathological validation in human coronary arteries. JACC Cardiovasc Imaging. 2015;8:184–194. DOI:10.1016/j.jcmg.2014.09.021
- Puri R, Madder RD, Madden SP, et al. Near-infrared spectroscopy enhances intravascular ultrasound assessment of vulnerable coronary plaque: a combined pathological and in vivo study. Arterioscler Thromb Vasc Biol. 2015;35:2423–2431. DOI:10.1161/ATVBAHA.115.306118
- Inaba S, Mintz GS, Burke AP, et al. Intravascular ultrasound and near-infrared spectroscopic characterization of thin-cap fibroatheroma. Am J Cardiol, 2016. DOI:10.1016/j.amjcard.2016.10.031.
- Garcia BA, Wood F, Cipher D, et al. Reproducibility of near-infrared spectroscopy for the detection of lipid core coronary plaques and observed changes after coronary stent implantation. Catheter Cardiovasc Interv. 2010;76: 359-365. DOI:10.1002/ccd.22500
- Waxman S, Dixon SR, L’Allier P, et al. In vivo validation of a catheter-based near-infrared spectroscopy system for detection of lipid core coronary plaques: initial results of the SPECTACL study. JACC Cardiovasc Imaging. 2009;2:858–868.
- Goldstein JA, Maini B, Dixon SR, et al. Detection of lipid-core plaques by intracoronary near-infrared spectroscopy identifies high risk of periprocedural myocardial infarction. Circ Cardiovasc Interv. 2011;4:429–437.
- Oemrawsingh RM, Garcia-Garcia HM, van Geuns RJ, et al. Integrated biomarker and imaging study 3 (IBIS-3) to assess the ability of rosuvastatin to decrease necrotic core in coronary arteries. EuroIntervention. 2016;12:734–739.
- Bourantas CV, Garcia-Garcia HM, Naka KK, et al. Hybrid intravascular imaging: current applications and prospective potential in the study of coronary atherosclerosis. J Am Coll Cardiol. 2013;61:1369–1378.
- Bourantas CV, Jaffer FA, Gijsen FJ, et al. Hybrid intravascular imaging: recent advances, technical considerations, and current applications in the study of plaque pathophysiology. Eur Heart J, 2016. DOI:10.1093/eurheartj/ehw097.
- Brown AJ, Obaid DR, Costopoulos C, et al. Direct comparison of virtual-histology intravascular ultrasound and optical coherence tomography imaging for identification of thin-cap fibroatheroma. Circ Cardiovasc Imaging. 2015;8:e003487.
- Fujii K, Hao H, Shibuya M, et al. Accuracy of OCT, grayscale IVUS, and their combination for the diagnosis of coronary TCFA: an ex vivo validation study. JACC Cardiovasc Imaging. 2015;8:451–460.
- Sawada T, Shite J, Garcia-Garcia HM, et al. Feasibility of combined use of intravascular ultrasound radiofrequency data analysis and optical coherence tomography for detecting thin-cap fibroatheroma. Eur Heart J. 2008;29:1136–1146.
- Diletti R, Garcia-Garcia HM, Gomez-Lara J, et al. Assessment of coronary atherosclerosis progression and regression at bifurcations using combined IVUS and OCT. JACC Cardiovasc Imaging. 2011;4:774–780. DOI:10.1016/j.jcmg.2011.04.007
- Tian J, Ren X, Vergallo R, et al. Distinct morphological features of ruptured culprit plaque for acute coronary events compared to those with silent rupture and thin-cap fibroatheroma: a combined optical coherence tomography and intravascular ultrasound study. J Am Coll Cardiol. 2014;63:2209–2216. DOI:10.1016/j.jacc.2014.01.061
- Tian J, Dauerman H, Toma C, et al. Prevalence and characteristics of TCFA and degree of coronary artery stenosis: an OCT, IVUS, and angiographic study. J Am Coll Cardiol. 2014;64:672–680. DOI:10.1016/j.jacc.2014.05.052
- Zaman T, Agarwal S, Anabtawi AG, et al. Angiographic lesion severity and subsequent myocardial infarction. Am J Cardiol. 2012;110:167–172. DOI:10.1016/j.amjcard.2012.03.008
- Ahmadi A, Leipsic J, Blankstein R, et al. Do plaques rapidly progress prior to myocardial infarction? The interplay between plaque vulnerability and progression. Circ Res. 2015;117:99–104.
- Ambrose JA, Tannenbaum MA, Alexopoulos D, et al. Angiographic progression of coronary artery disease and the development of myocardial infarction. J Am Coll Cardiol. 1988;12:56–62.
- Hou J, Xing L, Jia H, et al. Comparison of intensive versus moderate lipid-lowering therapy on fibrous cap and atheroma volume of coronary lipid-rich plaque using serial optical coherence tomography and intravascular ultrasound imaging. Am J Cardiol. 2016;117:800–806.
- Gin AL, Vergallo R, Minami Y, et al. Changes in coronary plaque morphology in patients with acute coronary syndrome versus stable angina pectoris after initiation of statin therapy. Coron Artery Dis. 2016;27:629–635. DOI:10.1097/MCA.0000000000000415
- Kini AS, Baber U, Kovacic JC, et al. Changes in plaque lipid content after short-term intensive versus standard statin therapy: the YELLOW trial (reduction in yellow plaque by aggressive lipid-lowering therapy). J Am Coll Cardiol. 2013;62:21–29. DOI:10.1016/j.jacc.2013.03.058
- Kodama K, Komatsu S, Ueda Y, et al. Stabilization and regression of coronary plaques treated with pitavastatin proven by angioscopy and intravascular ultrasound–the TOGETHAR trial. Circ J. 2010;74:1922–1928.
- Takayama T, Komatsu S, Ueda Y, et al. Comparison of the effect of rosuvastatin 2.5 mg vs 20 mg on coronary plaque determined by angioscopy and intravascular ultrasound in japanese with stable angina pectoris (from the aggressive lipid-lowering treatment approach using intensive rosuvastatin for vulnerable coronary artery plaque [ALTAIR] randomized trial). Am J Cardiol. 2016;117:1206–1212.
- Hirayama A, Saito S, Ueda Y, et al. Plaque-stabilizing effect of atorvastatin is stronger for plaques evaluated as more unstable by angioscopy and intravenous ultrasound. Circ J. 2011;75:1448–1454.
- Taniwaki M, Radu MD, Garcia-Garcia HM, et al. Long-term safety and feasibility of three-vessel multimodality intravascular imaging in patients with ST-elevation myocardial infarction: the IBIS-4 (integrated biomarker and imaging study) substudy. Int J Cardiovasc Imaging. 2015;31:915–926. DOI:10.1007/s10554-015-0631-0
- Madder RD, Goldstein JA, Madden SP, et al. Detection by near-infrared spectroscopy of large lipid core plaques at culprit sites in patients with acute ST-segment elevation myocardial infarction. JACC Cardiovasc Interv. 2013;6:838–846. DOI:10.1016/j.jcin.2013.04.012
- Madder RD, Puri R, Muller JE, et al. Confirmation of the intracoronary near-infrared spectroscopy threshold of lipid-rich plaques that underlie ST-Segment-elevation myocardial infarction. Arterioscler Thromb Vasc Biol. 2016;36:1010–1015. DOI:10.1161/ATVBAHA.115.306849
- Madder RD, Husaini M, Davis AT, et al. Detection by near-infrared spectroscopy of large lipid cores at culprit sites in patients with non-ST-segment elevation myocardial infarction and unstable angina. Catheter Cardiovasc Interv, 2015;86: 1014-1021. DOI:10.1002/ccd.25754.
- Sathyanarayana S, Schar M, Kraitchman DL, et al. Towards real-time intravascular endoscopic magnetic resonance imaging. JACC Cardiovasc Imaging. 2010;3:1158–1165.
- Brennan JF 3rd, Nazemi J, Motz J, et al. The vPredict optical catheter system: intravascular raman spectroscopy. EuroIntervention. 2008;3:635–638.
- Abran M, Stahli BE, Merlet N, et al. Validating a bimodal intravascular ultrasound (IVUS) and near-infrared fluorescence (NIRF) catheter for atherosclerotic plaque detection in rabbits. Biomed Opt Express. 2015;6:3989–3999. doi:10.1364/BOE.6.003989.
- Boogers MJ, Broersen A, van Velzen JE, et al. Automated quantification of coronary plaque with computed tomography: comparison with intravascular ultrasound using a dedicated registration algorithm for fusion-based quantification. Eur Heart J. 2012;33:1007–1016.
- Broersen A, De Graaf MA, Eggermont J, et al. Enhanced characterization of calcified areas in intravascular ultrasound virtual histology images by quantification of the acoustic shadow: validation against computed tomography coronary angiography. Int J Cardiovasc Imaging. 2016;32:543–552. DOI:10.1007/s10554-015-0820-x
- De Graaf MA, Broersen A, Kitslaar PH, et al. Automatic quantification and characterization of coronary atherosclerosis with computed tomography coronary angiography: cross-correlation with intravascular ultrasound virtual histology. Int J Cardiovasc Imaging. 2013;29:1177–1190. DOI:10.1007/s10554-013-0194-x
- Kruk M, Wardziak L, Mintz GS, et al. Accuracy of coronary computed tomography angiography vs intravascular ultrasound for evaluation of vessel area. J Cardiovasc Comput Tomogr. 2014;8:141–148. DOI:10.1016/j.jcct.2013.12.014
- Obaid DR, Calvert PA, Gopalan D, et al. Atherosclerotic plaque composition and classification identified by coronary computed tomography: assessment of computed tomography-generated plaque maps compared with virtual histology intravascular ultrasound and histology. Circ Cardiovasc Imaging. 2013;6:655–664. DOI:10.1161/CIRCIMAGING.112.000250
- Papadopoulou SL, Neefjes LA, Schaap M, et al. Detection and quantification of coronary atherosclerotic plaque by 64-slice multidetector CT: a systematic head-to-head comparison with intravascular ultrasound. Atherosclerosis. 2011;219:163–170. DOI:10.1016/j.atherosclerosis.2011.07.005
- Takahashi S, Kawasaki M, Miyata S, et al. Feasibility of tissue characterization of coronary plaques using 320-detector row computed tomography: comparison with integrated backscatter intravascular ultrasound. Heart Vessels. 2016;31:29–37.
- Voros S, Rinehart S, Qian Z, et al. Prospective validation of standardized, 3-dimensional, quantitative coronary computed tomographic plaque measurements using radiofrequency backscatter intravascular ultrasound as reference standard in intermediate coronary arterial lesions: results from the ATLANTA (assessment of tissue characteristics, lesion morphology, and hemodynamics by angiography with fractional flow reserve, intravascular ultrasound and virtual histology, and noninvasive computed tomography in atherosclerotic plaques) I study. JACC Cardiovasc Interv. 2011;4:198–208.
- Maurovich-Horvat P, Schlett CL, Alkadhi H, et al. The napkin-ring sign indicates advanced atherosclerotic lesions in coronary CT angiography. JACC Cardiovasc Imaging. 2012;5:1243–1252.
- Maurovich-Horvat P, Hoffmann U, Vorpahl M, et al. The napkin-ring sign: CT signature of high-risk coronary plaques? JACC Cardiovasc Imaging. 2010;3:440–444.
- Motoyama S, Sarai M, Harigaya H, et al. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol. 2009;54:49–57.
- Otsuka K, Fukuda S, Tanaka A, et al. Napkin-ring sign on coronary CT angiography for the prediction of acute coronary syndrome. JACC Cardiovasc Imaging. 2013;6:448–457.
- Versteylen MO, Kietselaer BL, Dagnelie PC, et al. Additive value of semiautomated quantification of coronary artery disease using cardiac computed tomographic angiography to predict future acute coronary syndrome. J Am Coll Cardiol. 2013;61:2296–2305.
- Motoyama S, Ito H, Sarai M, et al. Plaque characterization by coronary computed tomography angiography and the likelihood of acute coronary events in mid-term follow-up. J Am Coll Cardiol. 2015;66:337–346.
- Gitsioudis G, Chatzizisis YS, Wolf P, et al. Combined non-invasive assessment of endothelial shear stress and molecular imaging of inflammation for the prediction of inflamed plaque in hyperlipidaemic rabbit aortas. Eur Heart J Cardiovasc Imaging, 2016. DOI:10.1093/ehjci/jew048.
- Park HB, Heo R, O Hartaigh B, et al. Atherosclerotic plaque characteristics by CT angiography identify coronary lesions that cause ischemia: a direct comparison to fractional flow reserve. JACC Cardiovasc Imaging. 2015;8:1–10.
- Park JB, Choi G, Chun EJ, et al. Computational fluid dynamic measures of wall shear stress are related to coronary lesion characteristics. Heart. 2016;102:1655–1661.
- Bourantas CV, Papadopoulou SL, Serruys PW, et al. Noninvasive prediction of atherosclerotic progression: the PROSPECT-MSCT study. JACC Cardiovasc Imaging. 2016;9:1009–1011.
- Sakellarios A, Bourantas CV, Papadopoulou SL, et al. Prediction of atherosclerotic disease progression using LDL transport modelling: a serial computed tomographic coronary angiographic study. Eur Heart J Cardiovasc Imaging, 2016. DOI:10.1093/ehjci/jew035.
- Keegan J. Coronary artery wall imaging. J Magn Reson Imaging. 2015;41:1190–1202. DOI:10.1002/jmri.24766
- Dweck MR, Puntman V, Vesey AT, et al. Imaging of coronary arteries and plaques. JACC Cardiovasc Imaging. 2016;9:306–316.
- Kim WY, Danias PG, Stuber M, et al. Coronary magnetic resonance angiography for the detection of coronary stenoses. N Engl J Med. 2001;345:1863–1869.
- Kato S, Kitagawa K, Ishida N, et al. Assessment of coronary artery disease using magnetic resonance coronary angiography: a national multicenter trial. J Am Coll Cardiol. 2010;56:983–991. DOI:10.1016/j.jacc.2010.01.071
- Yonezawa M, Nagata M, Kitagawa K, et al. Quantitative analysis of 1.5-T whole-heart coronary MR angiograms obtained with 32-channel cardiac coils: a comparison with conventional quantitative coronary angiography. Radiology. 2014;271:356–364. DOI:10.1148/radiol.13122491
- Schuetz GM, Zacharopoulou NM, Schlattmann P, et al. Meta-analysis: noninvasive coronary angiography using computed tomography versus magnetic resonance imaging. Ann Intern Med. 2010;152:167–177.
- He Y, Zhang Z, Dai Q, et al. Accuracy of MRI to identify the coronary artery plaque: a comparative study with intravascular ultrasound. J Magn Reson Imaging. 2012;35:72–78. DOI:10.1002/jmri.22652
- Gerretsen S, Kessels AG, Nelemans PJ, et al. Detection of coronary plaques using MR coronary vessel wall imaging: validation of findings with intravascular ultrasound. Eur Radiol. 2013;23:115–124. DOI:10.1007/s00330-012-2576-1
- Miao C, Chen S, Macedo R, et al. Positive remodeling of the coronary arteries detected by magnetic resonance imaging in an asymptomatic population: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2009;53:1708–1715.
- Terashima M, Nguyen PK, Rubin GD, et al. Right coronary wall CMR in the older asymptomatic advance cohort: positive remodeling and associations with type 2 diabetes and coronary calcium. J Cardiovasc Magn Reson. 2010;12:75.
- Jansen CH, Perera D, Makowski MR, et al. Detection of intracoronary thrombus by magnetic resonance imaging in patients with acute myocardial infarction. Circulation. 2011;124:416–424.
- Kawasaki T, Koga S, Koga N, et al. Characterization of hyperintense plaque with noncontrast T(1)-weighted cardiac magnetic resonance coronary plaque imaging: comparison with multislice computed tomography and intravascular ultrasound. JACC Cardiovasc Imaging. 2009;2:720–728.
- Noguchi T, Kawasaki T, Tanaka A, et al. High-intensity signals in coronary plaques on noncontrast T1-weighted magnetic resonance imaging as a novel determinant of coronary events. J Am Coll Cardiol. 2014;63:989–999.
- Tawakol A, Migrino RQ, Bashian GG, et al. In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am Coll Cardiol. 2006;48:1818–1824.
- Pedersen SF, Graebe M, Fisker Hag AM, et al. Gene expression and 18FDG uptake in atherosclerotic carotid plaques. Nucl Med Commun. 2010;31:423–429. DOI:10.1097/MNM.0b013e32833767e0
- Bucerius J, Mani V, Moncrieff C, et al. Impact of noninsulin-dependent type 2 diabetes on carotid wall 18F-fluorodeoxyglucose positron emission tomography uptake. J Am Coll Cardiol. 2012;59:2080–2088.
- Naik HB, Natarajan B, Stansky E, et al. Severity of psoriasis associates with aortic vascular inflammation detected by FDG PET/CT and neutrophil activation in a prospective observational study. Arterioscler Thromb Vasc Biol. 2015;35:2667–2676.
- Emami H, Vijayakumar J, Subramanian S, et al. Arterial 18F-FDG uptake in rheumatoid arthritis correlates with synovial activity. JACC Cardiovasc Imaging. 2014;7:959–960.
- Marnane M, Merwick A, Sheehan OC, et al. Carotid plaque inflammation on 18F-fluorodeoxyglucose positron emission tomography predicts early stroke recurrence. Ann Neurol. 2012;71:709–718. DOI:10.1002/ana.23553
- Figueroa AL, Abdelbaky A, Truong QA, et al. Measurement of arterial activity on routine FDG PET/CT images improves prediction of risk of future CV events. JACC Cardiovasc Imaging. 2013;6:1250–1259.
- Tarkin JM, Joshi FR, Rudd JH. Pet imaging of inflammation in atherosclerosis. Nat Rev Cardiol. 2014;11:443–457. doi:10.1038/nrcardio.2014.80.
- Folco EJ, Sheikine Y, Rocha VZ, et al. Hypoxia but not inflammation augments glucose uptake in human macrophages: implications for imaging atherosclerosis with 18fluorine-labeled 2-deoxy-D-glucose positron emission tomography. J Am Coll Cardiol. 2011;58:603–614. DOI:10.1016/j.jacc.2011.03.044
- Joshi NV, Vesey AT, Williams MC, et al. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: a prospective clinical trial. Lancet. 2014;383:705–713. DOI:10.1016/S0140-6736(13)61754-7
- Rogers IS, Nasir K, Figueroa AL, et al. Feasibility of FDG imaging of the coronary arteries: comparison between acute coronary syndrome and stable angina. JACC Cardiovasc Imaging. 2010;3:388–397. DOI:10.1016/j.jcmg.2010.01.004
- Tahara N, Mukherjee J, De Haas HJ, et al. 2-deoxy-2-[18F]fluoro-D-mannose positron emission tomography imaging in atherosclerosis. Nat Med. 2014;20:215–219. DOI:10.1038/nm.3437
- Rominger A, Saam T, Wolpers S, et al. 18F-FDG PET/CT identifies patients at risk for future vascular events in an otherwise asymptomatic cohort with neoplastic disease. J Nucl Med. 2009;50:1611–1620. DOI:10.2967/jnumed.109.065151
- Voo S, Kwee RM, Sluimer JC, et al. Imaging intraplaque inflammation in carotid atherosclerosis with 18F-fluorocholine positron emission tomography-computed tomography: prospective study on vulnerable atheroma with immunohistochemical validation. Circ Cardiovasc Imaging, 2016;9. DOI:10.1161/CIRCIMAGING.115.004467.
- Gaemperli O, Shalhoub J, Owen DR, et al. Imaging intraplaque inflammation in carotid atherosclerosis with 11C-PK11195 positron emission tomography/computed tomography. Eur Heart J. 2012;33:1902–1910. DOI:10.1093/eurheartj/ehr367
- van der Valk FM, Sluimer JC, Voo SA, et al. In vivo imaging of hypoxia in atherosclerotic plaques in humans. JACC Cardiovasc Imaging. 2015;8:1340–1341. DOI:10.1016/j.jcmg.2014.12.015
- Beer AJ, Pelisek J, Heider P, et al. PET/CT imaging of integrin alphavbeta3 expression in human carotid atherosclerosis. JACC Cardiovasc Imaging. 2014;7:178–187. DOI:10.1016/j.jcmg.2013.12.003
- Ye YX, Calcagno C, Binderup T, et al. Imaging macrophage and hematopoietic progenitor proliferation in atherosclerosis. Circ Res. 2015;117:835–845. DOI:10.1161/CIRCRESAHA.115.307024
- Perez-Medina C, Binderup T, Lobatto ME, et al. In vivo PET Imaging of HDL in multiple atherosclerosis models. JACC Cardiovasc Imaging. 2016;9:950–961. DOI:10.1016/j.jcmg.2016.01.020
- Irkle A, Vesey AT, Lewis DY, et al. Identifying active vascular microcalcification by (18)F-sodium fluoride positron emission tomography. Nat Commun. 2015;6:7495. DOI:10.1038/ncomms8495
- McKenney-Drake ML, Territo PR, Salavati A, et al. (18)F-NaF PET imaging of early coronary artery calcification. JACC Cardiovasc Imaging. 2016;9:627–628. DOI:10.1016/j.jcmg.2015.02.026
- Papafaklis MI, Mizuno S, Takahashi S, et al. Incremental predictive value of combined endothelial shear stress, plaque necrotic core, and plaque burden for future cardiac events: a post-hoc analysis of the PREDICTION study. Int J Cardiol. 2016;202:64–66. DOI:10.1016/j.ijcard.2015.08.208
- Baumann S, Wang R, Schoepf UJ, et al. Coronary CT angiography-derived fractional flow reserve correlated with invasive fractional flow reserve measurements–initial experience with a novel physician-driven algorithm. Eur Radiol. 2015;25:1201–1207. DOI:10.1007/s00330-014-3482-5
- Bourantas CV, Serruys PW, Nakatani S, et al. Bioresorbable vascular scaffold treatment induces the formation of neointimal cap that seals the underlying plaque without compromising the luminal dimensions: a concept based on serial optical coherence tomography data. EuroIntervention. 2015;11:746–756. DOI:10.4244/EIJY14M10_06
- Duivenvoorden R, Tang J, Cormode DP, et al. A statin-loaded reconstituted high-density lipoprotein nanoparticle inhibits atherosclerotic plaque inflammation. Nat Commun. 2014;5:3065. DOI:10.1038/ncomms4065
- Ohtani T, Ueda Y, Mizote I, et al. Number of yellow plaques detected in a coronary artery is associated with future risk of acute coronary syndrome: detection of vulnerable patients by angioscopy. J Am Coll Cardiol. 2006;47:2194–2200. DOI:10.1016/j.jacc.2006.01.064
- Bourantas CV, Garcia-Garcia HM, Farooq V, et al. Clinical and angiographic characteristics of patients likely to have vulnerable plaques: analysis from the PROSPECT study. JACC Cardiovasc Imaging. 2013;6:1263–1272. DOI:10.1016/j.jcmg.2013.04.015
- Oemrawsingh RM, Cheng JM, Garcia-Garcia HM, et al. Near-infrared spectroscopy predicts cardiovascular outcome in patients with coronary artery disease. J Am Coll Cardiol. 2014;64:2510–2518. DOI:10.1016/j.jacc.2014.07.998
- Cheng JM, Garcia-Garcia HM, De Boer SP, et al. In vivo detection of high-risk coronary plaques by radiofrequency intravascular ultrasound and cardiovascular outcome: results of the ATHEROREMO-IVUS study. Eur Heart J. 2014;35:639–647. DOI:10.1093/eurheartj/eht484
- Feuchtner G, Kerber J, Burghard P, et al. The high-risk criteria low-attenuation plaque <60 HU and the napkin-ring sign are the most powerful predictors of MACE: a long-term follow-up study. Eur Heart J Cardiovasc Imaging, 2016. DOI:10.1093/ehjci/jew167.
- Hadamitzky M, Taubert S, Deseive S, et al. Prognostic value of coronary computed tomography angiography during 5 years of follow-up in patients with suspected coronary artery disease. Eur Heart J. 2013;34:3277–3285. DOI:10.1093/eurheartj/eht293
- Dedic A, Kurata A, Lubbers M, et al. Prognostic implications of non-culprit plaques in acute coronary syndrome: non-invasive assessment with coronary CT angiography. Eur Heart J Cardiovasc Imaging. 2014;15:1231–1237. DOI:10.1093/ehjci/jeu111
- Farooq V, Serruys PW, Bourantas CV, et al. Quantification of incomplete revascularization and its association with five-year mortality in the synergy between percutaneous coronary intervention with taxus and cardiac surgery (SYNTAX) trial validation of the residual SYNTAX score. Circulation. 2013;128:141–151. DOI:10.1161/CIRCULATIONAHA.113.001803
- Genereux P, Palmerini T, Caixeta A, et al. Quantification and impact of untreated coronary artery disease after percutaneous coronary intervention: the residual SYNTAX (Synergy Between PCI with Taxus and Cardiac Surgery) score. J Am Coll Cardiol. 2012;59:2165–2174. DOI:10.1016/j.jacc.2012.03.010
- Garg S, Sarno G, Serruys PW, et al. Prediction of 1-year clinical outcomes using the SYNTAX score in patients with acute ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention: a substudy of the STRATEGY (Single high-dose bolus tirofiban and sirolimus-eluting stent versus abciximab and bare-metal stent in acute myocardial infarction) and MULTISTRATEGY (Multicenter evaluation of single high-dose bolus tirofiban versus abciximab with sirolimus-eluting stent or bare-metal stent in acute myocardial infarction study) trials. JACC Cardiovasc Interv. 2011;4:66–75. DOI:10.1016/j.jcin.2010.09.017
- Garg S, Serruys PW, Silber S, et al. The prognostic utility of the SYNTAX score on 1-year outcomes after revascularization with zotarolimus- and everolimus-eluting stents: a substudy of the RESOLUTE All comers trial. JACC Cardiovasc Interv. 2011;4:432–441. DOI:10.1016/j.jcin.2011.01.008
- Bonaca MP, Braunwald E, Sabatine MS. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;373:1274–1275. DOI:10.1056/NEJMc1508692
