ABSTRACT
Background: Volanesorsen, an investigational inhibitor of apoC-III synthesis, significantly reduced triglyceride levels in clinical trials in patients with familial chylomicronemia syndrome (FCS), a rare genetic disorder characterized by marked chylomicronemia leading to a spectrum of symptoms, including recurrent abdominal pain and episodes of potentially fatal acute pancreatitis (AP).
Objective: To determine the effect of volanesorsen on burden of disease on patients with FCS
Methods: ReFOCUS was a retrospective global web-based survey open to patients with FCS who received volanesorsen for ≥3 months in an open-label extension study. The survey included questions about patients’ experiences before and after volanesorsen treatment.
Results: Twenty-two respondents had received volanesorsen for a median of 222 days. Volanesorsen significantly reduced the number of symptoms per patient across physical, emotional, and cognitive domains. Significant reductions from baseline were reported for steatorrhea, pancreatic pain, and constant worry about an attack of pain/AP. Respondents reported that volanesorsen improved overall management of symptoms and reduced interference of FCS with work/school responsibilities. Reductions in the negative impact of FCS on personal, social, and professional life were also reported.
Conclusions: Treatment with volanesorsen has the potential to reduce disease burden in patients with FCS through modulation of multiple symptom domains.
1. Introduction
Familial chylomicronemia syndrome (FCS) is a rare metabolic disorder characterized by reduced or absent lipoprotein lipase (LPL) activity [Citation1,Citation2]. LPL catalyzes the hydrolysis of triglycerides (TG) in chylomicrons and other TG-rich lipoproteins, and its absence or functional impairment leads to marked fasting and postprandial chylomicronemia and plasma TG levels that are 10–100-fold above the normal value (150 mg/dL, 1.7 mmol/L) [Citation3]. Because of chylomicron accumulation, many patients with FCS experience a variety of symptoms and signs including frequent abdominal pain, eruptive xanthomas, lipemia retinalis, and hepatosplenomegaly. Acute pancreatitis (AP) presents the most significant risk in patients with FCS, which is associated with major morbidity and even mortality [Citation4]. Potential long-term complications as a result of AP include chronic pancreatitis, that may manifest as endocrine pancreatogenic (type 3c) diabetes and endocrine and exocrine pancreatic insufficiencies [Citation5]. It is documented that AP due to severely high TG may be more severe with worse outcomes than pancreatitis from other etiologies [Citation6]. High TG levels are independently and proportionally correlated with persistent organ failure in AP, with 48% rate of organ failure observed in AP patients with TG levels >1000 mg/dL compared with 30% and 39% in AP patients with TG levels of 150–199 mg/dL and 200–999 mg/dL, respectively; 45% of patients with HTG (TG ≥200 mg/dL) required ICU care compared with 23% of patients with normal TG (<150 mg/dL) [Citation7]. A population-based study has calculated that every 100 mg/dL increase in TG concentration increased the risk of AP by 4% [Citation8]. Although mortality associated with AP in FCS patients is not known, mortality from HTG-associated AP has been estimated at 5–6%, and as many as 30% of patients with severe complications (pancreatic necrosis in association with infected abscesses or persistent multiple organ failure) may die [Citation1,Citation2]. Pregnancy in the absence of FCS causes a 2–3-fold increase in TG levels because of the associated rise in endogenous estrogens, and thus poses a particular problem for women with FCS [Citation9–Citation11].
There are no currently available approved treatments for FCS, and long-term management relies mainly on adherence to an extremely restrictive, low-fat diet (10–20 g daily) and life-long avoidance of alcohol and medications known to increase TG levels, such as glucocorticoids, atypical antipsychotic drugs, protease inhibitors, antiretroviral agents, retinoids, thiazides, beta-blockers, and exogenous estrogen [Citation2,Citation12]. Lifetime compliance with such an extremely restrictive diet is difficult, may negatively impact quality of life, does not normalize TG levels in all patients, and therefore does not completely obviate the risk of pancreatitis or other symptoms in all patients [Citation1,Citation13]. Lipid-lowering agents such as fibrates, omega-3 fatty acids, and niacin are largely ineffective in FCS, primarily because they act by decreasing hepatic VLDL output and by enhancing LPL activity but ultimately require a functional LPL pathway, which is severely impaired or absent in FCS patients [Citation1,Citation2].
A recently discovered method to lower TG levels in patients with FCS is to target plasma apoC-III, a small, 79-amino-acid glycoprotein that raises plasma TG levels through potent inhibition of LPL activity and also through a non-LPL-dependent mechanism [Citation14–Citation16]. Volanesorsen sodium, an investigational drug being developed for the reduction of TGs in patients with FCS, is a second-generation antisense inhibitor of apoC-III synthesis, which binds to hepatic APOC3 mRNA and elicits the degradation of mRNA by the endogenous ribonuclease RNase H1 [Citation16]. The APPROACH trial was a randomized, double-blind, placebo-controlled phase III study (ISIS 304801-CS6; APPROACH: A Randomized, Double-Blind, Placebo-Controlled, Phase 3 Study of ISIS 304801 Administered Subcutaneously to 66 Patients With Familial Chylomicronemia Syndrome (FCS); NCT02211209) conducted to evaluate the efficacy and safety of volanesorsen (300 mg subcutaneously once a week) compared with placebo in patients over the age of 18 years with FCS. In this study, currently the largest in this population, the primary end point at 3 months was met with a 94% decrease in TG in the volanesorsen-treated group compared to the placebo group (P < 0.001). Treatment with volanesorsen reduced TG levels by 77% when compared to baseline at 3 months, as opposed to an 18% increase with placebo (P < 0.001). The incidence of AP events was also reduced during treatment, with 1 AP event in a volanesorsen-treated patient compared with 4 events in 3 placebo-treated patients. However, the low frequency of recorded AP events over the 52-week duration of the study limited statistical comparison. The most common adverse events with volanesorsen were injection site reactions (17% of all injections). Declines in platelet counts led to 5 early terminations in APPROACH, 2 of which had platelets <25,000/µl; platelet counts recovered to normal following treatment with corticosteroids and cessation of volanesorsen [Citation17].
Patient-reported outcomes, including health-related quality of life (QoL), are key considerations for understanding the burden and impairment associated with a chronic disease, and for assessing the effectiveness of treatment. Although the physical symptoms and other clinical features of FCS are well defined in the existing literature, the effects of FCS on patients’ QoL, including emotional, cognitive, and psychosocial well-being were, until recently, poorly understood. To address this gap in the literature on QoL of patients with FCS, a multinational web-based survey, Investigation of Findings and Observations Captured in Burden of Illness Survey in FCS Patients (IN-FOCUS), was conducted to assess the QoL and multidimensional burden in patients with FCS; the survey design and interim analysis have been published [Citation4]. An interim analysis of 60 patients in this study highlighted the multiple and heterogeneous symptoms experienced by these patients [Citation4]. Between 22% and 35% of patients experienced severe physical symptoms such as bloating, abdominal pain, asthenia, fatigue, indigestion, and pancreatic pain; 42% reported an history of AP. The constant threat of debilitating or potentially fatal AP severely impacted the well-being of one third of patients. Patients with FCS generally reported themselves to be underemployed and more than 90% of respondents in the IN-FOCUS study indicated that FCS had negatively influenced their employment opportunities and career choices. FCS had directly caused 68% of full- or part-time employed patients to take time off from work, reporting missing a mean of 30 days of work in a year because of their FCS symptoms. Additionally, 81% and 70% of patients in the global IN-FOCUS study reported that applying the dietary restriction was extremely time consuming and energy draining, respectively, and the constant planning and management of their diet was a source of anxiety and social withdrawal. Further, 53% of patients continued to experience symptoms despite compliance to their extremely restricted low-fat diet and lifestyle prescriptions [Citation18].
Upon completion of the APPROACH study, eligible patients could elect to receive volanesorsen in an open-label extension (OLE) study. Since the IN-FOCUS study revealed the extensive burden of disease and the poor QoL experienced by patients with FCS, it is important to determine whether the reduction of TGs with volanesorsen in APPROACH translates into improved QoL for these patients or a mitigation of the myriad burdens identified in IN-FOCUS. The Retrospective Findings and Observations Captured in Burden of Illness Survey in FCS Patients (ReFOCUS) study was conducted to evaluate burden of illness and QoL of patients with FCS before and during treatment with volanesorsen. A questionnaire, adapted from the IN-FOCUS questionnaire, was applied to an eligible population from the APPROACH OLE study to determine whether treatment with volanesorsen also reduced the clinical and psychosocial burdens that impact patients’ QoL and interfered with their personal, social, and professional well-being.
2. Methods
2.1. Study design
ReFOCUS was a global online, quantitative research study that consisted of a web-based survey designed to retrospectively record the burden of living with FCS in patients before and after treatment with volanesorsen in the APPROACH OLE study (NCT02658175). The survey was open to patients in the USA, Canada, the UK, South Africa, Spain, France, Netherlands, Germany, Italy, Brazil, and Israel and was available in multiple languages in addition to English. Respondents had the option to complete the survey over multiple sessions.
All current or former participants in the APPROACH OLE study of volanesorsen for the treatment of FCS were invited to participate in the survey. To be considered for participation in the ReFOCUS study, patients must have been enrolled in the APPROACH OLE and have received one or more injections of volanesorsen. All eligible patients at the time of study recruitment (N = 58) were invited to participate in the survey by the OLE Principal Investigators, who directed potential respondents to the study website. All patients were requested to complete the ‘pre-volanesorsen’ portion of the questionnaire; no response was received from patients who chose not to participate (n = 23) despite multiple follow ups. Only patients who received treatment with volanesorsen for ≥3 months in APPROACH OLE completed sections pertaining to their experience during the most recent 3-month period while on the treatment. ‘Before and after’ data were available for 22 respondents. Patients continued all other management strategies per APPROACH OLE protocol (i.e. maintained a fat-restrictive diet, take TG-lowering medication, and alcohol avoidance). The research and study team were blinded to the identity of survey respondents. Respondents who completed the full survey were offered a nominal honorarium to compensate for time spent completing the survey.
All research materials were approved by the Institutional Review Board of the University of Mississippi in the USA and the National Health Service South West – Frenchay Research Ethics Committee in the UK. Ethics Committee approval was sought and received for centers based outside the USA and UK, as required.
2.2. Questionnaire
The questionnaire used in ReFOCUS was adapted from the IN-FOCUS questionnaire, a prior burden of illness study conducted in patients with FCS [Citation4]. The questionnaire consisted of sections on screening criteria and demographics; diagnosis; symptoms; FCS management; impact of FCS on personal, social, and professional life, mental and physical health, and diet; and comorbidities and lifetime events. ReFOCUS was designed as a classic, pre- vs. post-study, to retrospectively capture the daily burden of living with FCS across two different time-periods, the 3 months prior to receiving volanesorsen and the most recent 3 months since on volanesorsen. The draft questionnaire was tested through a direct interview with two eligible respondents from the USA to ensure comprehensiveness and relevance to respondents’ condition and situation, before the survey was launched. The survey website (www.FCSReFOCUS.com) was active between 12 July 2017 and 3 November 2017. Toluna USA Inc., a third-party programming and data hosting company, hosted the website and housed data generated by the study.
2.3. Statistical analysis
Data are shown for 22 respondents who completed the questionnaire for both ‘before’ and ‘after’ time periods. Continuous variables were presented as mean with standard deviation, or median with ranges. Categorical variables were presented as frequencies and percentage of occurrence of each category. Rating scales additionally were presented as medians with interquartile ranges (Q1–Q3) and percent changes in mean rank from baseline were calculated using geometric means. Wilcoxon Signed Rank tests were conducted to test for differences in mean ranks before and after taking volanesorsen. This test was used over a t-test to account for the ordinal nature of these data. Data analysis was performed using SPSS Statistics 22 (Armonk, NY). P < 0.05 was considered to be statistically significant.
3. Results
3.1. Respondent characteristics
A total of 58 eligible patients from the APPROACH OLE (41 of whom had been on volanesorsen for at least 3 months) were invited to participate in the study. Exclusions are explained in the flowchart in ; these include 23 patients who did not visit the website, 7 patients who did not complete screening and 3 additional patients who did not complete the survey. Of a final study sample of 25 respondents, 22 had been treated with volanesorsen for at least 3 months; 3 were excluded as they were treated with volanesorsen for <3 months (). Baseline characteristics are shown in . A majority of respondents were female (73%) and 55% were from North America (the USA and Canada). The median age at the time of the survey was 51 years (range, 26–65 years), with a majority of responders (68%) between 41 and 60 years. The median age at diagnosis was 24 years (range, 0–63 years); 9 respondents (41%) were diagnosed with FCS before the age of 10 years. Of 11 patients who could recall their TG levels at diagnosis, 10 had TG levels ≥1500 mg/dL. The median duration on volanesorsen therapy was 222 days (~7.5 months); 68% had been treated for >6 months when they completed the survey. Following observation for platelet decline, the protocol was amended to include alternative dosing schedule including biweekly dosing and 64% of respondents were taking volanesorsen on a biweekly basis.
Table 1. Baseline demographics and characteristics.
Figure 1. Approaches used to manage symptoms of FCS.
N = 20 unless otherwise stated. *P < 0.05 vs. baseline. aN for PRE = 21; bN = 16; cN for PRE = 16; dImprovement defined as decrease in mean rank rating in past 3 months versus 3 months prior to starting treatment with volanesorsen; exception: ‘My approach to managing…,’ where improvement is defined as an increase in mean rank rating. Medians for the ratings scales pre- and post-treatment are shown with the interquartile ranges (Q1–Q3).
Survey respondents (19 who could recall their path to diagnosis) had been seen by an average of 3 physicians (range, 1–15) before being diagnosed with FCS; however, 5 patients (23%) had visited 4–6 physicians and 1 had visited 13–15 physicians before diagnosis. Most commonly, the diagnosis of FCS was made by a metabolic specialist (N = 5; 23%) or lipidologist (N = 4; 18%). Misdiagnoses were common; 12 (55%) respondents reported a misdiagnosis, most commonly hypertriglyceridemia (N = 6; 50%), other (N = 5; 42%), or AP of unknown cause (N = 4; 33%) before being diagnosed correctly with FCS. Misdiagnoses were most commonly made by a primary care physician (N = 5, 42%), ER doctor/intensivist (N = 4, 33%), pediatrician (N = 4, 33%), or gastroenterologist (N = 2, 25%).
3.2. Management of FCS
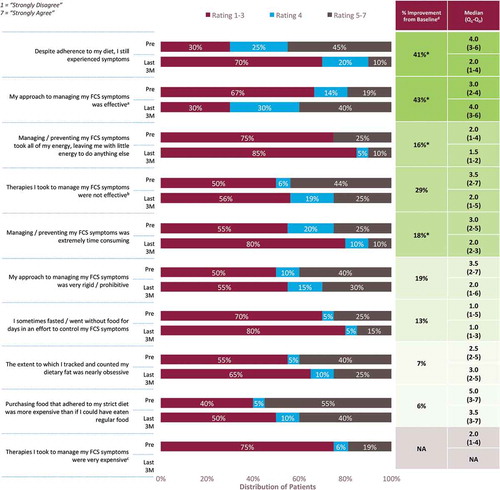
A 7-point Likert scale (1 = ‘strongly disagree;’ 7 = ‘strongly agree’) was used to assess patients’ perceptions of and experience with managing the symptoms of FCS before starting volanesorsen and after at least 3 months of treatment. Respondents indicated that they believed their FCS was being more effectively managed when treated with volanesorsen compared with their previous management regimen (). Notably, after volanesorsen treatment, more respondents reported that their strategies for managing the symptoms of FCS were effective (40% vs. 19%) and that their symptoms were controlled with adherence to diet (90% vs. 55%). Managing symptoms during the 3-month treatment period was also reported to be significantly less time consuming and energy draining, compared with the pretreatment period. Despite noting the greater efficacy and ease of dietary management while on volanesorsen treatment, patients continued to be highly focused and obsessive/rigid about adhering to their diet during treatment.
3.3. Symptomology
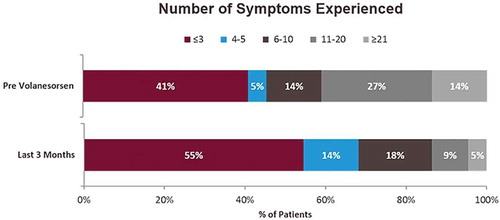
Respondents were asked to indicate symptoms they experienced during pre- and post-volanesorsen treatment periods from a list of 41 symptoms categorized into three domains: physical, emotional, and cognitive. Overall, the median number of symptoms experienced after initiating volanesorsen treatment decreased from 6.5 (Q1–Q3 = 2.0–13.0) prior to volanesorsen treatment to 3.5 (Q1–Q3 = 1.5–6.5) after at least 3 months of treatment (34% improvement; P < 0.05). Likewise, the number of respondents who experienced greater than 10 symptoms decreased from 41% prior to volanesorsen treatment to 14% after treatment (improvement from baseline, 66%) (). Treatment significantly reduced the number of symptoms experienced per patient in all three domains, with decreases from baseline of 47% (2.4 vs. 4.5; P = 0.009), 47% (1.9 vs. 3.5; P = 0.007), and 46% (0.3 vs. 0.6; P = 0.030) in physical, emotional, and cognitive domains, respectively.
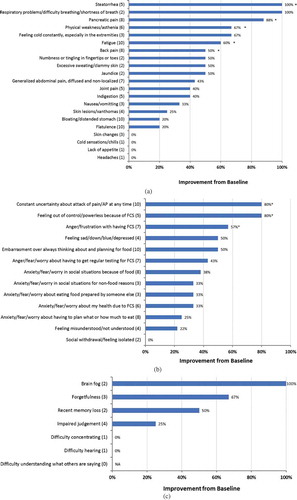
Among physical symptoms, a significant reduction in incidence was reported for pancreatic pain (from 8 respondents to 1, P < 0.05), steatorrhea (from 5 respondents to 0, P < 0.05), physical weakness/asthenia (from 6 respondents to 2, P < 0.05), fatigue (from 10 respondents to 4, P < 0.05) and back pain (from 8 respondents to 4, P < 0.05) (). All but four physical symptoms were reported by fewer respondents following ≥3 months of volanesorsen treatment; skin rash, chills, lack of appetite, and headaches remained unchanged. Among emotional symptoms, the constant worry about having an attack of pain or AP at any time was reduced from 10 respondents to 2 (P < 0.05), and similar significant reduction in incidence was reported for feeling out of control because of FCS (from 5 respondents to 1, P < 0.05) and anger/frustration with having FCS (from 7 respondents to 3, P < 0.05) (). All emotional symptoms, other than social withdrawal/feeling isolated also showed reduced incidence. In general, <20% of patients experienced cognitive symptoms at baseline (); the numbers of patients experiencing these declined or remained steady after 3 months of treatment. Notably, no respondent reported an increase in the number of symptoms experienced while taking volanesorsen. For many of the symptoms, the sample size was too small to analyze. However, the reported average severity was lower for indigestion (27%), generalized, diffuse, non-localized abdominal pain (18%), bloating (17%), skin changes (20%), and xanthoma (18%). Emotional symptoms whose average severity was lowered following volanesorsen treatment were feeling misunderstood/not understood (42%), embarrassment over constantly thinking about/planning for food (30%), anger/fear/worry in social situations because of food (22%).
Figure 3. (A)(B)(C). Effect of volanesorsen treatment on (A) physical symptoms, (B) emotional symptoms, and (C) cognitive symptoms.
Numbers in parentheses indicate the number of patients in the pretreatment period who experienced each symptom. *P < 0.05
In the 3 months before starting volanesorsen, 10 respondents (45%) experienced general abdominal pain or pancreatic pain. Abdominal pain was defined as ‘generalized abdominal pain, diffuse and non-localized’ while pancreatic pain was defined as ‘deep-seated pain that is most often felt in the back, makes it hard to get comfortable.’ Of those, 2 patients (20%) required hospitalization. After at least 3 months of treatment with volanesorsen, only 4 respondents (18%) reported these symptoms, reflecting a 60% decrease. Of these 4 respondents, none required hospitalization during treatment. Following treatment with volanesorsen, the incidence of AP decreased by 80% from 5 patients (4 had experienced 1–2 episodes and 1 with >5 episodes) pretreatment to 1 patient (experiencing between 1 and 2 episodes while on treatment). Because of the small sample size, improvement in the average severity of pancreatic pain could not be determined.
3.4. Impact of disease on personal, social, and professional life
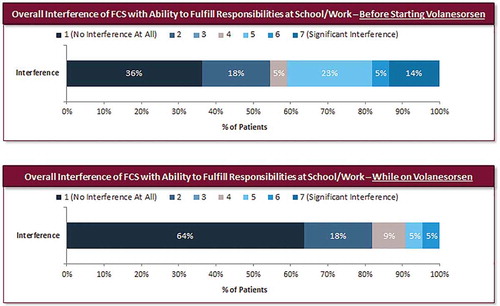
The impact of FCS on the respondents’ personal, social, and professional life was assessed on a 7-point Likert scale (1 = ‘no interference at all;’ 7 = ‘significant interference’). When considering the overall impact of FCS on their lives, the proportion of respondents who reported ‘no interference’ increased from 5% prior to volanesorsen to 23% while on therapy, whereas those reporting a high level of interference (rating of 5–7) decreased from 59% prior to treatment to 37% while on volanesorsen (). Symptoms of FCS had a significantly lower impact on respondents’ lives during volanesorsen treatment, with a 31% reduction in mean score from baseline (median 5.0 (Q1–Q3 = 2.0–6.0) versus 2.5 (Q1–Q3 = 2.0–5.0); P < 0.05). The ability to fulfill their responsibilities at work or school while being treated with volanesorsen improved; the score for overall FCS-related interference with work/school decreased 39% compared with before starting volanesorsen (median: 2.0 (Q1–Q3 = 1.0–5.0) versus 1.0 (Q1–Q3 = 1.0–2.0); P < 0.05). The proportion of respondents who reported no interference of FCS with work or school increased from 36% before starting volanesorsen to 64% during treatment ().
Figure 4. Overall impact of FCS on patients’ lives before and after volanesorsen treatment (N = 22).
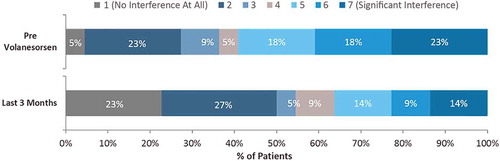
Figure 5. Interference of FCS with work/school attendance and responsibilities before and after treatment with volanesorsen (N = 22).
Participation in household and personal activities improved during volanesorsen treatment, with a lower proportion of respondents reporting a high level of interference (rating 5–7) of FCS with eating out (23% vs. 59%), taking vacations (14% vs. 38%), pursuing hobbies (5% vs. 23%) and taking care of others (0% vs. 10%) (). Treatment with volanesorsen improved their reported ability to participate in social activities, such as entertaining and traveling, and the ability to socialize and engage with others (). Respondents reported reduced stress over managing diet and difficulty over food planning while on volanesorsen treatment. Several aspects of emotional and mental well-being, including stress and anxiety, feelings of self-worth, and sleep quality also improved significantly after treatment with volanesorsen (). Importantly, there was a shift in attitude about the future; at baseline, 67% of respondents were worried about ever being able to lead a normal life, compared with 36% who expressed this concern after volanesorsen treatment ().
When time to feeling better was examined, 14% and 43% of respondents felt markedly better within a month and between 1 and 2 months, respectively, and 25% and 56% felt somewhat better within a month and between 1 and 2 months, respectively (Supplementary Figure 6). Overall, the median time to feeling ‘somewhat better’ was 42 days and for ‘markedly better,’ 60 days.
4. Discussion
Patients with FCS describe frequent chronic, debilitating abdominal pain and less frequent but significant and potentially life-threatening bouts of AP, despite following a strict, extremely restrictive low-fat diet. In addition to the burden of physical symptoms, patients with FCS describe significant cognitive, psychosocial, emotional, and economic burden. Specifically, their testimonies reference the impact of FCS symptoms on QoL (i.e. fatigue, mental fog, anxiety/fear about next attack) and job performance (missed days due to sickness or hospitalization). The phase III APPROACH study demonstrated that treatment with volanesorsen significantly reduced TG levels by 94% in patients with FCS when compared to placebo at the primary end point [Citation17]. While receiving treatment with volanesorsen, respondents in the ReFOCUS study described an overall improvement in their health and QoL, reporting a significant decrease in the total number of FCS-related symptoms, across all domains assessed.
Respondents reported more effective overall FCS management during treatment with volanesorsen. Patients with FCS generally adhere to a strict very low-fat diet. The IN-FOCUS study has chronicled the stress and anxiety and the interference with all spheres of life attendant to following such a restricted diet to control symptoms [Citation4]. Before starting volanesorsen treatment, many respondents noted that, despite adhering to their diet, they still experienced symptoms and that their approach to managing symptoms was time consuming, energy draining, and not completely effective. The addition of volanesorsen to dietary management resulted in better symptom control and, overall, respondents felt that their FCS management strategies became more effective, and dietary management easier. Regardless, most patients reported continued adherence to their diet when on volanesorsen treatment.
The symptoms experienced by the respondents in ReFOCUS before initiating treatment with volanesorsen were broadly similar to those captured in the IN-FOCUS study and were poorly controlled despite adherence to a highly restrictive very low-fat diet. Notably, patients treated with volanesorsen reported a significant decrease in the total number of FCS-related symptoms experienced. The proportion of patients experiencing steatorrhea and pancreatic pain declined significantly. The incidence of AP and hospitalization due to generalized abdominal pain/pancreatic pain also decreased following volanesorsen treatment. The reduction in physical symptoms, especially attacks of pain or AP, was accompanied by an improvement in respondents’ emotional outlook; in particular, the constant uncertainty of having an attack of pain or AP was significantly reduced (P < 0.05). Although only a few respondents reported cognitive decrements at baseline, volanesorsen treatment reduced brain fog and symptoms such as forgetfulness and recent memory loss. It is notable that these improvements occurred even though two-thirds of the patients on the alternative dosing schedule took volanesorsen every 2 weeks.
Beyond the direct improvement on FCS symptomology, volanesorsen treatment had a broader impact on patients’ ability to fulfill their responsibility at work or school, and to perform and participate in household and personal activities, all of which were improved with volanesorsen. The baseline data from this study and data from IN-FOCUS [Citation4,Citation18] both highlighted the negative influence of FCS on careers, employment opportunities, and work productivity. Treatment with volanesorsen decreased the reported overall interference of FCS with work or school (the proportion of respondents reporting no interference of FCS with work/school increased by 77%), suggesting that improved disease management with volanesorsen treatment may increase productivity and reduce absenteeism. Likewise, reduced symptom prevalence may translate into broadly reduced healthcare resource utilization [Citation19].
The reduction in the assessed variables and burdens also translated to functional and social outcomes. For example, improvement was noted in patients’ reported ability to go out to restaurants, take family vacations, actively engage in various hobbies, and entertain and socialize. Perhaps the most impactful finding from the ReFOCUS survey was the improvement in respondents’ mental wellbeing and future outlook on their life and health compared to before they started on volanesorsen treatment.
While the available literature discusses the physical, emotional, and cognitive manifestations associated with FCS, there is little, if any evidence on the impact of pharmacotherapy on the QoL in patients with FCS. The phase III APPROACH study showed that volanesorsen significantly reduced TG levels in patients with FCS. Following 3 months of treatment with volanesorsen, mean TG decreased 77% from baseline, compared with an increase of 18% with placebo; 77% and 50% of volanesorsen-treated patients had TG reductions to <750 mg/dL and 500 mg/dL, respectively [Citation17]. These are levels generally below the threshold for AP [Citation20]. TG levels were not measured in the ReFOCUS study, but together with the data from APPROACH, the ReFOCUS findings suggest that the lower TG levels following volanesorsen treatment may be associated with the reduction in AP and abdominal pain and a generally improved symptom profile reported by the respondents. The present survey demonstrated that treatment with volanesorsen improved the physical, emotional and cognitive domains associated with FCS. These improvements led to a significantly improved overall ability to perform professional, academic, and social obligations. The research highlights considerable improvement across multiple domains achieved in this patient population with volanesorsen treatment and provides encouraging data that underscores the need for a therapeutic option such as volanesorsen.
The study has several limitations, which should be considered when assessing the significance of the study findings. In particular, when enrolling patients with a rare disease in clinical studies, there are inherent restrictions on potential sample size, study design, and respondent recruitment options. In this case, the sample size was limited to 22 self-selected respondents enrolled in the APPROACH OLE study, representing only 6 of the 12 countries that participated in the APPROACH study. Second, as the survey findings were self-reported, it was not possible to independently verify the experiences reported by patients. Since all the patients were recruited from the OLE, there was no comparator group to distinguish the differences seen in patients receiving volanesorsen treatment versus patients without treatment. Finally, with a retrospective pre-/posttreatment study design the potential influence of recall bias in patient reported outcomes, particularly the risk of overstating the efficacy in the context of a new treatment option being offered for an underserved population and/or misremembering their symptoms, must also be considered. To mitigate this effect in the ReFOCUS study, survey responses were gathered several months following volanesorsen initiation (median time on therapy of 222 days) and from respondents from both arms of the randomized, placebo-controlled APPROACH study who entered the OLE study. However, given that FCS is a chronic disease that patients in the APPROACH study had lived with for many years, study respondents were likely to accurately recall their symptoms and life experiences from the period before initiating volanesorsen. The study design for ReFOCUS did not include collecting clinical information in the form of TG values, which in turn does not allow for direct correlation of improvements in QoL to the corresponding physiologic change. While the data from the ReFOCUS study complement the clinical findings in the APPROACH phase III clinical trial, further studies are planned to show a direct relationship between the effect of volanesorsen on TG lowering and improvements in patients’ QoL.
5. Conclusions
The ReFOCUS study shows that, following treatment with volanesorsen, several measures of the QoL of respondents with FCS improved, including physical, emotional, and cognitive symptoms, and FCS had a reduced impact on personal, social and professional life. The patient-oriented outcomes data of this study complement the clinical outcomes reported from the APPROACH study and suggest that treatment with volanesorsen has the potential to reduce disease burden and improve QoL for patients with FCS, whose disease confers a heavy lifelong clinical and psychosocial burden.
Key issues
Familial chylomicronemia syndrome (FCS) is a rare metabolic disease caused by the build-up of chylomicrons (chylomicronemia), lipoprotein particles that transport predominantly dietary fat as well as cholesterol
The chylomicronemia in FCS brings risks of significant morbidity and mortality, including the threat of acute pancreatitis, which can be lethal
The IN-FOCUS study demonstrated that patients with FCS experience significant clinical and psychosocial burdens that reduce their quality of life and limit employment and social interactions
Since FCS is a disorder of impaired chylomicron clearance, treatment has primarily focused on reducing chylomicron production via restriction of fat intake, that does not guarantee sustained reduction of chylomicrons or absence of risk of acute pancreatitis episodes
Treatment of FCS patients with the investigational drug volanesorsen has significantly reduced TG levels in FCS patients enrolled in clinical trials
The ReFOCUS study demonstrated that treatment with volanesorsen had a positive impact on patients’ QoL and improvement in patients’ activities of daily living across all domains assessed
Respondents noted that dietary management of their disease was easier, and their symptoms more effectively controlled during volanesorsen treatment.
The survey demonstrated that treatment with volanesorsen improved the physical, emotional, and neurocognitive domains associated with FCS
Improvements lead to a significantly improved overall ability to perform professional, academic, and social obligations and improved future outlook on life.
The research highlights in this patient population a considerable improvement across multiple domains, encouraging data that underscores the need for a therapeutic option such as volanesorsen.
Declaration of interest
M Arca has received grant/honoraria from Amgen, Sanofi, Pfizer, Aegerion, MSD, Mylan, Akcea Therapeutics, Inc., and Ionis Pharmaceuticals. H Soran has received research grants from Alexion, Amgen, Akcea Therapeutics, Inc., Pfizer, MSD, Genzyme-Sanofi and has also received personal fees/honorarium and education grants from Aegerion, Amgen, Janssen Cilag Ltd, Lilly, MSD, Pfizer, Sanofi, NAPP, Link-Medical, Akcea Therapeutics, Inc and Alexion. A Hsieh, Louis O’Dea and Michael Stevenson are employees of Akcea Therapeutics, Inc. P Rosenblit has served on the speaker’s bureau for Abbvie, Akcea Therapeutics, Inc., Boehringer Ingelheim-Lilly, Merck, Janssen and Novo Nordisk and has received grant support from Amgen, Akcea Therapeutics, Inc., Ionis Pharmaceuticals, AstraZeneca-Bristol Myers Squibb, Boehringer Ingelheim, Merck, GlaxoSmithKline, Novo Nordisk, Lexicon, Roche and Sanofi-Regeneron. P Rosenblit has also served as a consultant for Amgen, Akcea Therapeutics Inc, Ionis Pharmaceuticals, Amarin, Novo Nordisk, and Sanofi-Regeneron.
Supplemental figures
Download Zip (3.7 MB)Acknowledgments
The authors would like to first and foremost thank the patients with FCS and their families for participating in this study. Additionally, the authors would like to acknowledge Trinity Partners, Waltham, MA for their collaboration on survey design and execution. The authors would also like to thank ApotheCom and S Thorat, PhD for assistance with medical writing.
Supplemental meterial
Supplementary data can be accessed here.
Additional information
Funding
Notes on contributors
Michael Stevenson
M Stevenson, A Hsieh and L O’Dea were responsible for the conception and design of the study. A Hsieh, M Stevenson and L O’Dea acquired data for the study. M Arca, H Soran, P Rosenblit, M Stevenson, A Hsieh and L O’Dea were responsible for analysis and interpretation of the data, drafting the manuscript or revising it critically for important intellectual content. All authors approved the final manuscript for submission.
References
- Stroes E, Moulin P, Parhofer KG, et al. Diagnostic algorithm for familial chylomicronemia syndrome. Atheroscler Suppl. 2017;23:1–7.
- Brahm AJ, Hegele RA. Chylomicronaemia—current diagnosis and future therapies. Nat Rev Endocrinol. 2015;11:352–362
- Chait A, Brunzell JD. Chylomicronemia syndrome. Adv Intern Med. 1992;37:249–273.
- Davidson M, Stevenson M, Hsieh A, et al. The burden of familial chylomicronemia syndrome: interim results from the IN-FOCUS study. Expert Rev Cardiovasc Ther. 2017;15(5):415–423.
- Das SLM, Kennedy JIC, Murphy R, et al. Relationship between the exocrine and endocrine pancreas after acute pancreatitis. World Journal of Gastroenterology: WJG. 2014;20(45):17196–17205.
- Valdivielso P, Ramirez-Bueno A, Ewald N,J. Current knowledge of hypertriglyceridemic pancreatitis. Eur Med. 2014;25:689–694.
- Nawaz H, Koutroumpakis E, Easler J, et al. Elevated serum triglycerides are independently associated with persistent organ failure in acute pancreatitis. Am J Gastroenterol. 2015;110(10):1497–1503.
- Murphy MJ, Sheng X, MacDonald TM, et al. Hypertriglyceridemia and acute pancreatitis. JAMA Intern Med. 2013;173(2):162–164.
- Gupta N, Ahmed S, Shaffer L, et al. Severe hypertriglyceridemia induced pancreatitis in pregnancy. Case Rep Obstet Gynecol. 2014;2014:485493. doi: 10.1155/2014/485493.
- Kayataş SE, Eser M, Cam C, et al. Acute pancreatitis associated with hypertriglyceridemia: a life-threatening complication. Arch Gynecol Obstet. 2010;281(3):427–429.
- Watts G, Morton K, Jackson P, et al. Management of patients with severe hypertriglyceridaemia during pregnancy: report of two cases with familial lipoprotein lipase deficiency. Br J Obstet Gynaecol. 1992;99(2):163–166.
- Williams L, Wilson DP. Editorial commentary: dietary management of familial chylomicronemia syndrome. J Clin Lipidol. 2016;10(3):462–465.
- Gaudet D, De Wal J, Tremblay K, et al. Review of the clinical development of alipogene tiparvovec gene therapy for lipoprotein lipase deficiency. Atherosclerosis Supplements. 2010;11(1):55–60.
- Gangabadage CS, Zdunek J, Tessari M, et al. Structure and dynamics of human apolipoprotein CIII. J Biol Chem. 2008;283(25):17416–17427.
- Gaudet D, Brisson D, Tremblay K, et al. Targeting APOC3 in the familial chylomicronemia syndrome. N Engl J Med. 2014;371(23):2200–2206.
- Graham MJ, Lee RG, Bell TA, et al. Antisense oligonucleotide inhibition of apolipoprotein C-III reduces plasma triglycerides in rodents, nonhuman primates, and humans: novelty and significance. Circ Res. 2013;112(11):1479.
- Gaudet D, Digenio A, Alexander V, et al. The APPROACH study: a randomized, double-blind, placebo-controlled, phase 3 study of volanesorsen administered subcutaneously to patients with familial chylomicronemia syndrome (FCS). Atherosclerosis. 2017;263:e10.
- Davidson M, Stevenson M, Hsieh A, et al. Examining the high disease burden and impact on quality of life in familial chylomicronemia syndrome - results from the IN-FOCUS study. Clin Cardiol. 2017;40:4–15.
- Gaudet D, Signorovitch J, Swallow E, et al. Medical resource use and costs associated with chylomicronemia. J Med Econ. 2013;16:657-666.
- Ž R. Developed with the special contribution of: European Association for Cardiovascular P, rehabilitation et al. ESC/EAS guidelines for the management of dyslipidaemias. The task force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J. 2011;32(14):1769–1818.