ABSTRACT
Introduction
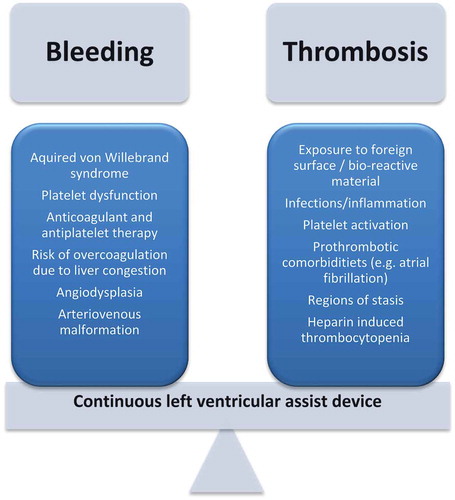
The treatment options for advanced heart failure patients drastically changed with the introduction of left ventricular assist devices (LVADs), either as bridge to transplant or as destination therapy for patients ineligible for transplant. Despite major benefits in terms of survival, functional status and quality of life, managing patients with LVADs comes with several challenges. The most significant challenge is balancing between the risks of thrombotic and bleeding complications.
Areas covered
The present review describes the pathophysiological mechanisms explaining the alterations in the hemostatic profile of LVAD patients, and summarizes current evidence to guide clinical decision making with regard to anticoagulant treatment and management of bleeding complications.
Expert opinion
LVAD patients require life-long anticoagulant therapy to reduce the risk of pump thrombosis. However, exposing LVAD patients to anticoagulant therapy, in combination with common acquired coagulopathies after LVAD implantation such as acquired von Willebrand syndrome, comes with high risks of bleeding. There is a need for randomized controlled trials in LVAD patients to determine the optimal antithrombotic regimen and find the most effective balance between thrombotic and bleeding complications. In addition, strategies to specifically target the acquired von Willebrand syndrome and its associated angiodysplasias need to be evaluated in the LVAD population.
1. Introduction
With an estimated prevalence of more than 23 million patients worldwide and a reported 1-year mortality rate as high as 20%, heart failure brings about a heavy morbidity and mortality burden [Citation1–Citation3]. For patients with advanced disease, even with optimal medical management, 1-year survival is approximately 50% [Citation4]. Although cardiac transplant greatly increases survival, with a reported median survival of 10.7 years [Citation5], limited donor supply and prevalent contraindications for transplantation makes this option available for only a minority of patients. Over the past decades, left ventricular assist devices (LVADs) have emerged as an efficacious treatment strategy for patients with advanced heart failure as a bridge to transplant. In 2016, approximately 40% of the patients undergoing transplant were bridged with LVAD therapy [Citation6]. The positive experiences of LVADs as bridge to transplant have led investigators to evaluate LVADs as treatment option as destination therapy for those patients ineligible for transplant. In 2001, the REMATCH study was the first trial randomizing patients with end-stage heart failure ineligible for transplant to LVAD implantation or conventional medical treatment. This study demonstrated a major survival benefit as well as improved quality of life for patients assigned to LVAD therapy [Citation7]. These and other observations have led to over 2500 LVADs being implanted annually in the United States to date [Citation8]. In approximately 50% of patients, LVADs are implanted as destination therapy and in 26% as bridge to transplant [Citation8]. Indications for the remaining 24% of implanted LVADs include bridge to recovery or bridge to transplant candidacy [Citation8].
LVADs support cardiac function by draining blood from the left ventricular apex via an inflow cannula and pumping it into the ascending aorta via an outflow graft. All devices require an external controller and power source that is connected to the LVAD through a tunneled percutaneous drive line. Newer LVAD devices use a continuous flow rotary-pump. Compared to previously used pulsatile devices, these newer devices have several advantages including improved hemodynamics, end-organ function and quality of life, smaller device size, and longer durability [Citation9]. Currently, the axial-flow Heartmate II device (Abbott Laboratories, Abbott Park, IL, U.S.A.) and the centrifugal-flow HeartWare LVAD device (Medtronic, Minneapolis, MN, U.S.A.) constitute the majority of devices implanted worldwide. More recently, a third-generation continuous flow LVAD, the HeartMate III (Thoratec Corp, Pleasanton, CA, U.S.A.), has been introduced in an attempt to further improve LVAD patients’ outcome.
Despite the major benefits of LVADs in terms of improved survival, functional capacity, and quality of life for patients with advanced heart failure, managing LVAD patients comes with several challenges and high rates of adverse events [Citation10]. Following the first month after LVAD implantation 31–44% of the patients are re-hospitalized at least once [Citation11,Citation12], this rate increases to 60% 6 months after LVAD implantation [Citation8]. The spectrum of complications during the course of LVAD therapy can broadly be divided into two groups. First, the continuous presence of a subcutaneous driveline exposes LVAD patients to a high risk of infections which may be difficult to eradicate [Citation13,Citation14]. Second, LVAD patients are at constant risk of pump thrombosis, whilst aggressive anticoagulant treatment to prevent this complication predisposes these patients to a high bleeding risk. Major bleeding complications represent the most common cause of hospital admission during the course of LVAD patients [Citation15].
The present review will focus on the pathophysiological basis of the difficult balance between thrombosis and hemostasis in patient with LVADs, and summarize the existing evidence to guide clinical decision making in anticoagulant therapy and bleeding complications.
2. Effect of LVAD on hemostasis
The introduction of an LVAD in the systemic circulation causes significant hemostatic alterations, in particular changes in the function of Von Willebrand Factor (VWF) and platelets ().
2.1. Acquired von Willebrand syndrome
VWF is a large multimeric glycoprotein that is released in the circulation by endothelial cells at sites of vascular injury [Citation16,Citation17]. In the circulation, VWF binds to the subendothelial matrix of the injured vessel wall and mediates platelets to adhere and aggregate at the site in order to achieve hemostasis and formation of a primary platelet plug. Furthermore, VWF functions as a binding protein and stabilizer for Factor (F) VIII [Citation18]. VWF particularly promotes platelet aggregation in high shear stress conditions, such as caused by the continuous flow-pump design of LVADs. An increase in fluid shear stress above certain levels induces structural changes in the shape of VWF molecules, resulting in proteolytic cleavage by ADAMTS‐13 of the high molecular weight multimers, which are most effective in mediating platelet function under high shear stress conditions [Citation19]. Similar to the development of acquired von Willebrand syndrome in patients with severe aortic valve stenosis [Citation20], blood flow exposed to abnormally increased shear stress forms the most plausible mechanism for the development of acquired von Willebrand syndrome in patients with LVADs [Citation21]. It is thought that this condition is present in a large proportion, if not all, LVAD patients, which may at least partly explain their bleeding tendency [Citation22,Citation23]. VWF defects are observed soon after LVAD implantation and rapidly return to normal after LVAD explantation, further supporting the hypothesis that it is induced by the LVAD [Citation24,Citation25]. In addition, VWF has been described to modulate angiogenesis [Citation26], and VWF dysfunction has been strongly associated with the development of angiodysplasia, in particular in elderly patients [Citation27,Citation28]. The combination of these two conditions could make LVAD patients particularly prone to gastrointestinal bleeding [Citation29]. However, a definite causal relation between acquired VWD and bleeding complications is yet to be established.
2.2. Platelet dysfunction
Platelets play an essential role in primary hemostasis. Upon vascular injury, platelets promptly adhere to the extracellular matrix. At low shear rates platelet adhesion primarily involves binding to collagen, fibronectin, and laminin. However, at higher shear rates the interaction between the platelet surface receptor glycoprotein Ibα (GPIbα) and VWF (either in the extracellular matrix or immobilized on exposed collagen) becomes of critical importance important in order to slow down fast-flowing platelets [Citation30]. Increased shear stress can lead to shedding of the GPIbα receptor, which has been found to be associated with bleeding in patients with continuous flow LVAD devices [Citation31]. Furthermore, in an observational study of 12 LVAD patients and matched controls, platelet function was markedly compromised under high shear rates [Citation32]. Impairment of ristocetin-induced aggregation was observed, which was only partly attributable to a low VWF activity. In another study, increased rates of platelet apoptosis were reported in LVAD patients with bleeding complications [Citation33].
3. Thromboembolic complications
LVAD pump thrombosis is a major complication after LVAD implantation, causing significant morbidity and mortality. Pump thrombosis is defined by the formation of a blood clot within any or all of the components of the LVAD system, including the titanium inflow cannula, the pump rotator, and the outflow graft [Citation34]. Thrombus can either originate in the pump itself or travel to the pump from the left atrium or ventricle, or from right-sided cardiac chambers through a septal defect. Pump thrombosis results in pump failure leading to acute heart failure and is associated with embolic complications such as ischemic stroke. In the previously mentioned REMATCH trial, 16% of the LVAD patients were diagnosed with stroke, accounting for a yearly incidence of 0.19. The majority of events occurred in the direct postoperative period [Citation35].
Although pump thrombosis was reported relatively infrequent in the initial LVAD trials, an abrupt increase was observed in patients with HeartMate II devices implanted in 837 patients in three institutions, showing a drastic rise from 2.2% to 8.4% at 3 months after implantation before and after 2011 [Citation36]. For the HeartWare HVAD devices, the overall rate of pump thrombosis has reported to be 8% 1 year after implantation [Citation37]. Although the reasons for the increase in incidence of pump thrombosis are not completely understood and probably multi-factorial, it has been hypothesized that suboptimal anticoagulant regimens to prevent bleeding complications may have largely contributed [Citation15].
In an attempt to reduce the risk of pump thrombosis and subsequent thromboembolic complications, the Heartmate III has been designed with full magnetic levitation and thereby avoiding the need mechanical bearing to enhance biocompatibility by minimizing shear force effects [Citation38]. In the Momentum 3 trial, 1028 patients with advanced heart failure were randomly assigned to receive a Heartmate III or the Heartmate II LVAD [Citation39]. During 2 years of follow-up, suspected or confirmed pump thrombosis occurred in 1.4% of patients with a Heartmate III device versus 13.9% of patients assigned to a Heartmate II device. The respective rates of stroke were 9.9 versus 19.9%.
3.1. Diagnosis of pump thrombosis
The consequences that come with a missed diagnosis of pump thrombosis make promptly recognizing this condition of vital importance. It typically presents with increased LVAD pump power, flow abnormalities, signs of hemolysis caused by nonlaminar blood flow, or signs and symptoms of heart failure. Increased levels of lactate dehydrogenase (LDH) have been shown to be a sensitive marker for even very early stages of pump thrombosis [Citation40,Citation41]. LDH levels greater than 3 times the upper limit and/or free plasma hemoglobin levels greater than 40 mg/dL are suggestive of pump thrombosis [Citation34]. Echocardiography may reveal indirect evidence of thrombosis such as an increase in aortic valve opening, severe mitral regurgitation, and/or elevated right ventricular systolic pressure. Lack of contrast in the outflow graft on thoracic computed tomography is also suggestive of pump thrombosis [Citation41].
Ramp studies, in which left ventricular end-diastolic diameter are assessed using echocardiography at increasing device speed (rotations per minute), may also be useful to evaluate potential LVAD obstruction [Citation41]. In a prospective study of 17 patients with Heartmate II devices and clinically suspected pump thrombosis, failure to reduce left ventricular end-diastolic diameter with increased LVAD speed, in conjunction with elevated LDH levels, was found to be highly specific in the detection of obstruction to flow [Citation41].
Finally, right-heart catheterization could reveal elevated right-sided pressures suggestive of pump thrombosis and left-sided heart catheterization may be used to identify or rule out the presence of contrast flow across the pump and into outflow graft [Citation34].
4. Anticoagulant treatment
The high incidences of LVAD pump thrombosis and thromboembolic complications, both in the early post-operative phase and during long-term follow-up, necessitates anticoagulant treatment.
4.1. Early postoperative period
In the early post-operative phase, the International Society for Heart and Lung Transplantation (ISHLT) guidelines recommend to initiate intravenous unfractionated heparin (UFH) within 48 hours after LVAD implantation. The aimed activated partial thromboplastin time (APTT) is 40 to 60 seconds [Citation42]. From the third postoperative day, upward titration of UFH is recommended to achieve an APTT in the regular therapeutic range of 60 to 80 seconds. This should be continued till international normalized ratio (INR) levels are within target range after initiation of vitamin k antagonist therapy. These recommendations account for HeartMate II devices as well as other centrifugal devices. Despite these recommendations, management in clinical practice is highly variable. For instance, in a retrospective cohort of 418 patients with HeartMate II devices implanted as bridge to transplant, 122 (29%) patients were not bridged with UFH in transition to warfarin [Citation43]. Compared to patients who did receive UFH, the risk of thromboembolic complications seemed to be similar on the third post-operative day. In patients in whom UFH was withheld, there were significantly less bleeding complications during the first post-operative month. Given the observational nature of this study and the known procoagulant effect early after vitamin K antagonists initiation due to reduction of protein C and S levels, such a strategy cannot be recommended. In fact, the observed increased incidence in pump thrombosis mentioned previously has led to the initiation of the PREVENT trial. This trial was specifically designed to evaluate strategies to reduce this risk, including strict adherence to anticoagulant regimens [Citation15]. In this multi-center prospective trial, 95% of patients were bridged with UFH. Combined with recommendations regarding implant technique and pump-speed, this resulted in a low 3-month rate of pump thrombosis of 2.9%.
One small observational study, including 78 LVAD patients, assessed the feasibility of low-molecular-weight heparin (LMWH) as transition to warfarin [Citation44]. The outcomes of this study were favorable with rapid and constant biologic activity monitored by determination of anti-factor Xa levels, and low risks of adverse events. Although attractive, in particular after the initial post-operative phase, UFH may still be preferred in the immediate post-operative phase given its easier reversibility by protamine and better feasibility in patients with renal failure.
Of note, high levels of discordance between APTT and anti-Xa levels during monitoring of UFH treatment in LVAD patients have been reported, in particular, once INR levels are >1.8 or in the setting of diffuse intravascular coagulation [Citation45]. Whether anti-Xa levels better reflect the heparin-induced anticoagulant effect than APTT in patients after LVAD implantation is currently being evaluated by a randomized controlled trial (NCT03143569).
During UFH or LMWH administration, physicians should be aware of the occurrence of heparin-induced thrombocytopenia (HIT). Most frequently this occurs 5–10 days post initiation of heparin therapy [Citation46]. An incidence of 4% was reported in a single-center cohort of 358 LVAD patients [Citation47]. HIT is an immune-mediated adverse effect characterized by antibody-induced activation of platelets, resulting in thrombin generation and a paradoxical hypercoagulable state despite low platelet counts. Once confirmed or strongly suspected, immediate implementation of an alternative non-heparin anticoagulation regimen such as argatroban, danaparoid, or is indicated [Citation48].
4.2. Long-term management
VKA represent the mainstay of anticoagulant treatment during the long-term course of LVAD patients in order to prevent pump thrombosis. For patients with continues-flow devices, achieving target INR levels of 2.0–3.0 is recommended by current guidelines [Citation42]. Maintaining therapeutic INR in LVAD patients is however challenging. For instance, it has been demonstrated that more than half of the patients on long-term warfarin treatment required adjustment in warfarin dosing after LVAD placement [Citation49]. In addition, time spent within therapeutic range for LVAD patients is reported to be lower than for patients receiving VKA in the general population [Citation50]. Right heart failure, which is found to be present in 20–50% of LVAD patients [Citation51], may be one of the contributors to this problem, given that liver congestion due to right heart failure may cause increased coumarin responsiveness, thereby potentially increasing the risk of bleeding [Citation52].
Not surprisingly, the risk of thrombotic events is inversely related to INR levels with the highest event rate reported for an INR range <1.5 [Citation53]. In addition, it has been demonstrated that LVAD patients developing pump thrombosis spent significantly less time within therapeutic INR range in the 2 months prior to the event compared to those without pump thrombosis [Citation54]. Bridging LVAD patients with subtherapeutic INR levels with heparin treatment may therefore, be considered. Traditionally, UFH is used for this purpose. Over the years, LMWH have however been regarded more attractive given its ability for outpatient management. Still, this strategy may come at the expense of bleeding complications during the potential overlap with therapeutic INR levels [Citation55]. The most practical strategy to reduce the need for bridging is to lower the threshold for subtherapeutic INR levels. Such a strategy has been investigated in a recent small observational study where LVAD patients were not bridged unless INR levels were below 1.8 [Citation56]. In addition, reduced doses of enoxaparin (0.5 mg/kg twice daily) or fondaparinux (2.5 mg once daily) were used. The anticoagulant effect, as measured with native thromboelastography (n-TEG), as well as the clinical outcomes was found to be comparable for patients receiving UFH, enoxaparin, or fondaparinux.
Point-of-care INR testing may further improve anticoagulant care by providing a more convenient option, and possibly improving compliance. However, experience with point-of-care INR testing among LVAD patients to date is limited. A recent multicenter study evaluated the possibility of point-of-care INR testing in a cohort of 279 LVAD patients [Citation57]. No significant differences were observed between point-of-care and plasma INR values, in particular if measured within less than 4 hours of each other. It should be stated that these analyzes were performed retrospectively and the effect of point-of-care INR testing on the outcome of LVAD patients was not assessed.
4.3. Direct oral anticoagulants
In the general population, VKA have now largely been replaced by direct oral anticoagulants (DOACs) for venous thromboembolism and non-valvular atrial fibrillation. These novel anticoagulants directly and specifically inhibit thrombin (dabigatran) or factor Xa (apixaban, rivaroxaban and edoxaban). Compared to VKA, DOACs display several advantages including a predictable pharmacological profile, a broad therapeutic window, and a low potential for drug and food interactions [Citation58]. Moreover, randomized controlled trials have established a lower tendency for bleeding complications associated with DOACs, including significantly less intracranial hemorrhage (ICH) [Citation59–Citation62]. These advantages appear to be particularly attractive for the LVAD population displaying a significant bleeding risk. Furthermore, the rapid onset and offset of action of DOACs avoid the need for bridging. However, experience with DOAC in LVAD patients is limited. To date, only one randomized controlled trial compared DOACs to VKA in patients with a HeartWare HVAD [Citation63]. In this trial, patients were randomized to either dabigatran or warfarin 1-month post-LVAD implantation. After inclusion of 16 patients, the trial was halted prematurely because of an excess in thromboembolic complications (50%) in the interventional arm. A similar excess of thromboembolic complications has previously been demonstrated in patients with mechanical heart valves receiving dabigatran versus warfarin [Citation64]. A possible explanation for these observations may be that the pathways of coagulation activation differ between patients with atrial fibrillation versus those with LVADs or mechanical valves. In the former group, stasis and endothelial dysfunction in the left atrial appendage are the main factors to drive thrombus formation, representing a low-flow and low shear stress area. Inhibiting one key factor in the coagulation cascade in these patients appears to be sufficient. In patients with mechanical heart valves or LVADs, coagulation is primarily triggered by blood contact with artificial surfaces activating the contact pathway [Citation65]. Under these circumstances, VKA may be more effective by inhibiting not only thrombin but also the tissue factor induced pathway (by inhibiting Factor VII synthesis), the contact pathway (by inhibiting factor IX synthesis), as well as factor X [Citation64].
Although positive experiences have been reported with the anti-Xa inhibitor apixaban in one LVAD patient [Citation66], the use of any of the DOACs in the LVAD population is at this time not recommend, leaving VKA as the anticoagulant agents of choice.
4.4. Antiplatelet therapy
Consensus guidelines recommend the use of aspirin (at a dose of 81–325 mg daily) starting 24 to 72 hours after LVAD implantation [Citation42]. Still, the need for antiplatelet therapy in addition to VKA is a topic of ongoing debate. This is illustrated by great variety in antiplatelet therapy regimens among different centers and devices, as demonstrated by a systematic review including 24 mainly observational studies [Citation67]. Most likely, this variety is driven by a fear for bleeding events when antiplatelet therapy is combined with VKA in patients already prone to acquired von Willebrand syndrome and disturbed platelet function. In some studies, antiplatelet therapy is completely avoided whereas in other studies dual antiplatelet therapy is prescribed combining aspirin with dipyridamole or clopidogrel. In patients with axial devices who were treated with aspirin and dipyridamole, thromboembolic events were significantly lower compared to those patients treated with aspirin alone (10% vs 19%, RR 0.50; 95% CI 0.36–0.68) [Citation67]. The respective rates of ischemic stroke were 6% and 10%. For those patients treated without aspirin, the rate of thromboembolic events was 14% which was not higher than in those treated with aspirin (RR 1.43, 95% CI 0.81–2.5). All these results should however be interpreted with caution as INR intensity as well as patient and device characteristics were not equally balanced among studies. In the European TRACE study, a total of 101 patients with HeartMate II devices were managed with VKA monotherapy [Citation68]. After a follow-up of 2 years, freedom from bleeding, ischemic stroke, hemorrhagic stroke, and pump thrombosis after initiation was 81%, 96%, 94%, and 94%, respectively. The authors concluded from this observational study that avoiding antiplatelet therapy might have lowered the risk of bleeding, whilst maintaining stroke and pump thrombosis rates similar to previous trials.
A randomized controlled trial comparing aspirin versus placebo in patients with HeartMate II devices is currently ongoing (NCT02836652); the results of this trial should be awaited before firm conclusions can be drawn upon the need for antiplatelet therapy in LVAD patients. Whilst awaiting these results, it is recommended to initiate antiplatelet therapy with at least low-dose aspirin in LVAD patients in the absence of significant bleeding complications.
5. Management of pump thrombosis
The optimal management approach to LVAD pump thrombosis has not been completely established. Uptitration of anticoagulant treatment, such as increasing the INR range, initiating high dose aspiring or a second antiplatelet agent, or initiating intravenous heparin has been suggested as a first-line management strategy in hemodynamically stable patients with highly suspected pump thrombosis. Pump exchange has been regarded the treatment of choice once conservative treatment of pump thrombosis has failed [Citation34,Citation69]. Alternatively, for patients with an acceptable risk of bleeding, several small case series and case reports have reported successful management of pump thrombosis with administration of thrombolytic agents [Citation70–Citation73]. No studies have compared the efficacy and adverse events associated with systemic or catheter-directed thrombolysis versus pump exchange in patients with pump thrombosis. Therefore, no definite recommendation toward the use of thrombolytic agents in LVAD patients can be made and its use should always be discussed on a case-by-case basis by a multidisciplinary team of experts.
6. Bleeding complications
Bleeding events represent the most common cause of readmission after LVAD implantation [Citation15]. Early post-operative bleeding, frequently requiring re-operation, has been described to occur in 6% −69% of LVAD patients, whereas 30% of the patients experience major bleeding complications beyond the post-operative phase [Citation67]. Of these, gastro-intestinal bleedings are the most common with a reported pooled prevalence of 23%, particularly among older LVAD recipients and those with a history of gastro-intestinal bleedings [Citation74,Citation75].
Factors that contribute to these high bleeding risks are multifactorial and include the routine administration of VKA and antiplatelet therapy, combined with the above described risk of acquired coagulopathies and angiodysplasias. The precise mechanisms for the development of angiodysplasias in LVAD patients are not completely understood. Recent evidence has suggested that angiopoietin 2, a potent angiogenic factor that is stored in endothelial cells within Weibel-Palade bodies, is dysregulated in patients with continuous flow LVADs [Citation76]. Compared to patients with heart failure or orthotopic heart transplantation, serum levels and endothelial expression of angiopoietin 2 were higher in LVAD patients. Elevated levels of angiopoietin 2 increased angiogenesis in vitro, which was normalized with angiopoietin −2 blockade. Whether this represents a pharmaceutical target to prevent bleeding complications in LVAD patients remains to be investigated.
6.1. Management of bleeding complications
Depending on the severity of the bleeding, in addition to universal measures, direct anticoagulation reversal should be discussed on a case-by-case basis with the cardiology and/or hemostasis consultant, weighing the competing risk of thrombosis. Four-factor prothrombin complex concentrate (4 F-PCC) has been shown to be superior in acquiring rapid INR reversal and effective hemostasis compared to fresh frozen plasma in non-LVAD patients using VKA [Citation77,Citation78]. Although only small observation studies have reported the use of 4 F-PCC among LVAD patients requiring rapid anticoagulant reversal in case of bleeding or urgent surgery, these studies do not point toward an excess in thrombotic complications and suggest more rapid and predictable reversal when 4 F-PCC is used compared to fresh frozen plasma [Citation79,Citation80]. Four F-PCC displays the additional advantage that it requires lower volume administrations, thereby decreasing the risk of volume overload.
ISHLT guidelines also recommend to withhold antiplatelet therapy in the setting of clinically significant bleeding [Citation42]. No data exist on the use of platelet transfusion to reverse antiplatelet therapy in critical bleeding or urgent surgery settings in LVAD patients.
6.2. Gastro-intestinal bleeding
Following conservative management steps including withholding or reversing anticoagulant agents, treatment with proton-pump inhibitors and treatment with blood product if indicated, esophagogastroduodenoscopy is the preferred initial endoscopic strategy to identify and treat the culprit lesion in the setting of gastro-intestinal bleeding, given the high probability of upper gastro-intestinal tract angiodysplasias. Still, this procedure may be non-diagnostic in over two-thirds of patients [Citation81]. The routine use of push endoscopy may increase the diagnostic yield of the initial diagnostic assessment and thereby reduce the risk of re-bleed and need for re-investigation [Citation82].
Agents specifically treating the acquired von Willebrand syndrome such as VWF/FVIII or VWF concentrates may also be of use to manage gastro-intestinal bleeding, although experience with these agents in the LVAD population is limited [Citation83]. Prophylactic VWF concentrate has been described to prevent angiodysplastic gastrointestinal bleeding in von Willebrand disease [Citation27]. To date, one case has been reported of an LVAD patient with recurrent severe gastrointestinal bleeding which was finally controlled using repeated transfusions of VWF concentrate [Citation84]. It should be noted that VWF(/FVIII) concentrates are not yet registered for use in LVAD patients with acquired von Willebrand syndrome and require confirmation in prospective trials ().
Table 1. Directions for future research with regard to bleeding complications in LVAD patients.
Some evidence suggests that octreotide may reduce the risk of transfusion and re-admission in LVAD patients with refractory gastro-intestinal bleeding [Citation85]. Thalidomide is another agent that has gained attention for managing gastrointestinal bleeding in VWD patients due to its anti-angiogenic properties. Again, evidence for the use of this agent in LVAD patients is anecdotal.
A final strategy to reduce the risk of bleeding may be to reduce the pump speed in order to improve LVAD pulsatility and thereby decrease VWF degradation. In one study, reduced pulsatility was associated with an increased risk of nonsurgical bleeding during the first 3 months after device implantation [Citation86]. In this respect, the Heartmate III LVAD which functions at a lower RPM, combined with its fully magnetically levitation to enhance hemocompatibility, may reduce circulatory shear stress and improve the LVAD patients’ bleeding profile [Citation87]. Indeed, an improved preservation of VWF high-molecular-weight multimers has been observed in patients with Heartmate III compared to Heartmate II LVADs [Citation87]. In the previously mentioned MOMENTUM 3 trial, the incidence of gastro-intestinal bleeding was 24.5% in patients with Heartmate III LVADs versus 30.9% for those with Heartmate LVADs [Citation39].
6.3. Intracerebral hemorrhage
Although the risk of ICH among LVAD patients is low with a reported rate of 0.05–0.31 events per patient-year [Citation88], its occurrence poses a particular challenge in the management of LVAD patients. For patients with intraparenchymal bleeding, the 30-day mortality rate may be as high as 59% [Citation89]. Guidance regarding reversal and duration of discontinuation of anticoagulant management in the setting of ICH only comes from small retrospective case series. In a cohort of 31 LVAD patients with ICH who were all on aspirin and warfarin therapy, aspirin was withheld in 47% of patients for a median duration of 6 days, whereas warfarin was withheld in 61% for a mean duration of 10.5 days [Citation89]. Re-hemorrhage was not observed after resuming aspirin and warfarin. In addition, no thromboembolic events or pump failures occurred during follow-up. In a recently published series of 41 LVAD patients with ICH, warfarin was resumed after a median of 6.5 days and aspirin after 5.5 days [Citation90]. In 39% of the patients, bridging with UFH was initiated at a median of 2.5 days post-ICH. No thromboembolic events occurred during discontinuation of anticoagulant treatment. Secondary ICH was observed in 27% of the patients during one year follow-up, after a median time of 229 days post-anticoagulant resumption. A stepwise approach for managing ICH in LVAD patients has previously been proposed, only performing active anticoagulant reversal in case of large ICH (defined as intraparenchymal hemorrhage >3 cm or subarachnoid hemorrhage >1-2 mm thickness), with no specific guidance for duration of anticoagulation [Citation91]. Such a strategy has however not been validated by an outcome study.
7. Conclusion
Despite more than 30 years of experience and evolving technical improvements of LVADs, thromboembolic complications and pump thrombosis remain a substantial risk requiring continuous and intensive anticoagulant treatment combining VKA with antiplatelet therapy. Although strict adherence to such an anticoagulant strategy reduces the risk of pump thrombosis, this comes at the expense of bleeding complications of which gastro-intestinal bleeds are most incident. There is a need for randomized controlled trials in LVAD patients to determine the optimal antithrombotic regimen and find the most effective balance between thrombotic and bleeding complications.
8. Expert opinion
Considering the high rates of thromboembolic as well as bleeding complications in LVAD patients, current anticoagulant regimes in these patients are still far from optimal. This underlines the need for more effective and safer anticoagulant strategies. Although attractive because of their predictable course of action, ease of use with fixed dosing and lower risk of bleeding, DOACs appear to be inferior to VKA in preventing thrombosis in LVAD patients. As discussed in this review, this may be explained by the fact that thrombus formation in LVAD patients is primarily induced by activation of the contact pathway, a process contributing to the host defense to foreign substances. Recently, a new class of anticoagulant agents has entered clinical research that specifically target factor XI and XII, which display an important role in the contact pathway. These agents may, therefore, be particularly effective for thrombosis prevention in patients with medical devices such as LVADs. Importantly, no bleeding tendency has been reported in patients with severe deficiencies of FXII. Patients with FXI deficiency usually display a relatively mild bleeding tendency and rarely develop spontaneous bleeding. Therefore, agents targeting FXI or FXII may not only be more effective in preventing thrombosis in LVAD patients, they may also display an improved safety profile, reducing the risk of hemorrhagic complications. The clinical potential anticoagulant strategies directed at FXI or FXII thus appear an important future direction for research in LVAD patients.
Once a diagnosis of LVAD pump thrombosis is established, current guidelines recommend LVAD pump exchange as preferred management approach. This however is an invasive surgical procedure associated with significant morbidity and mortality. Identifying alternative management strategies which could avoid reoperation thus forms another key area for improvement in the management of LVAD patients. One such strategy could be the use of systemic or catheter-directed thrombolysis. Several case reports and case series have reported positive outcomes with this approach, however to date these results have not been confirmed in prospective trials. In our view, based on our experience and reported literature, catheter-directed or systemic thrombolysis forms an approach that should be considered particularly for those patients with an acceptable bleeding risk, in a final attempt to avoid pump exchange.
Lastly, strategies to specifically target the acquired von Willebrand syndrome and its associated angiodysplasias need to be evaluated in the LVAD population, in order to provide physicians with an improved therapeutic arsenal in case bleeding complications occur.
Article highlights
Blood flow exposed to abnormally increased shear stress induces an acquired von Willebrand syndrome in a large proportion, if not all, LVAD patients, contributing to their increased bleeding tendency.
Intensive anticoagulant treatment combining VKA with antiplatelet therapy is indicated to reduce the risk of LVAD pump thrombosis and other thromboembolic complications.
Direct anticoagulant agents cannot be recommended in LVAD patients because of a decreased efficacy compared to vitamin K antagonists.
Once conservative management has failed, thrombolysis may be regarded an possible approach to avoid LVAD pump exchange in patients with established pump thrombosis and an acceptable bleeding risk.
In case of bleeding complications, direct anticoagulation reversal should be considered on a case-by-case bases, balancing the severity of the bleeding to the risk of thromboembolic complications.
Declaration of interest
J Eikenboom received Research funding from CSL Behring, outside the submitted work. E Klok reports research grants from Bayer, Bristol-Myers Squibb, Boehringer-Ingelheim, Daiichi-Sankyo, MSD and Actelion, the Dutch Heart foundation and the Netherlands Thrombosis Foundation, outside the submitted work. M Huisman reports grants from ZonMW Dutch Healthcare Fund, grants and personal fees from Boehringer Ingelheim, grants and personal fees from Pfizer-BMS, grants and personal fees from Bayer Health Care, grants from Aspen, grants and personal fees from Daiichi-Sankyo, outside the submitted work. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Lloyd-Jones D, Lloyd-Jones D, Adams RJ, et al. Heart disease and stroke statistics–2010 update: a report from the American heart association. Circulation. 2010;121(7):e46–e215.
- Heron M. Deaths: leading causes for 2010. Natl Vital Stat Rep. 2013;62(6):1–96.
- Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol. 2011;8(1):30–41.
- Califf RM, Adams KF, McKenna WJ, et al. A randomized controlled trial of epoprostenol therapy for severe congestive heart failure: the flolan international randomized survival trial (FIRST). Am Heart J. 1997;134(1):44–54.
- Stehlik J, Edwards LB, Kucheryavaya AY, et al. The registry of the international society for heart and lung transplantation: twenty-eighth adult heart transplant report–2011. J Heart Lung Transplant. 2011;30(10):1078–1094.
- Lund LH, Khush KK, Cherikh WS, et al. The registry of the international society for heart and lung transplantation: thirty-fourth adult heart transplantation report-2017; Focus theme: allograft ischemic time. J Heart Lung Transplant. 2017;36(10):1037–1046.
- Rose EA, Gelijns AC, Moskowitz AJ, et al. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001;345(20):1435–1443.
- Kirklin JK, Pagani FD, Kormos RL, et al. Eighth annual INTERMACS report: special focus on framing the impact of adverse events. J Heart Lung Transplant. 2017;36(10):1080–1086.
- Slaughter MS, Rogers JG, Milano CA, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361(23):2241–2251.
- Eckman PM, John R. Bleeding and thrombosis in patients with continuous-flow ventricular assist devices. Circulation. 2012;125(24):3038–3047.
- Akhter SA, Badami A, Murray M, et al. Hospital readmissions after continuous-flow left ventricular assist device implantation: incidence, causes, and cost analysis. Ann Thorac Surg. 2015;100(3):884–889.
- Agrawal S, Garg L, Shah M, et al. Thirty-day readmissions after left ventricular assist device implantation in the United States: insights from the nationwide readmissions database. Circ Heart Fail. 2018;11(3):e004628.
- Koval CE, Thuita L, Moazami N, et al. Evolution and impact of drive-line infection in a large cohort of continuous-flow ventricular assist device recipients. J Heart Lung Transplant. 2014;33(11):1164–1172.
- Goldstein DJ, Naftel D, Holman W, et al. Continuous-flow devices and percutaneous site infections: clinical outcomes. J Heart Lung Transplant. 2012;31(11):1151–1157.
- Maltais S, Kilic A, Nathan S, et al. PREVENtion of Heartmate II pump thrombosis through clinical management: the PREVENT multi-center study. J Heart Lung Transplant. 2017;36(1):1–12.
- De Jong A, Eikenboom J. Developments in the diagnostic procedures for von Willebrand disease. J Thromb Haemost. 2016;14(3):449–460.
- Leebeek FW, Eikenboom JC. Von Willebrand’s disease. N Engl J Med. 2016;375(21):2067–2080.
- Weiss HJ, Sussman II, Hoyer LW. Stabilization of factor VIII in plasma by the von Willebrand factor. Studies on posttransfusion and dissociated factor VIII and in patients with von Willebrand’s disease. J Clin Invest. 1977;60(2):390–404.
- Tsai HM, Sussman II, Nagel RL. Shear stress enhances the proteolysis of von Willebrand factor in normal plasma. Blood. 1994;83(8):2171–2179.
- Hollestelle MJ, LOOTS CM, SQUIZZATO A, et al. Decreased active von Willebrand factor level owing to shear stress in aortic stenosis patients. J Thromb Haemost. 2011;9(5):953–958.
- Klovaite J, Gustafsson F, Mortensen SA, et al. Severely impaired von Willebrand factor-dependent platelet aggregation in patients with a continuous-flow left ventricular assist device (HeartMate II). J Am Coll Cardiol. 2009;53(23):2162–2167.
- Sakamoto K, Yamamoto Y, Okamatsu H, et al. D-dimer is helpful for differentiating acute aortic dissection and acute pulmonary embolism from acute myocardial infarction. Hellenic J Cardiol. 2011;52(2):123–127.
- Meyer AL, Malehsa D, Budde U, et al. Acquired von Willebrand syndrome in patients with a centrifugal or axial continuous flow left ventricular assist device. JACC Heart Fail. 2014;2(2):141–145.
- Goda M, Jacobs S, Rega F, et al. Time course of acquired von Willebrand disease associated with two types of continuous-flow left ventricular assist devices: Heartmate II and circulate synergy pocket micro-pump. J Heart Lung Transplant. 2013;32(5):539–545.
- Meyer AL, Malehsa D, Bara C, et al. Acquired von Willebrand syndrome in patients with an axial flow left ventricular assist device. Circ Heart Fail. 2010;3(6):675–681.
- Starke RD, Ferraro F, Paschalaki KE, et al. Endothelial von Willebrand factor regulates angiogenesis. Blood. 2011;117(3):1071–1080.
- Makris M, Federici AB, Mannucci PM, et al. The natural history of occult or angiodysplastic gastrointestinal bleeding in von Willebrand disease. Haemophilia. 2015;21(3):338–342.
- Randi AM, Laffan MA. Von Willebrand factor and angiogenesis: basic and applied issues. J Thromb Haemost. 2017;15(1):13–20.
- Demirozu ZT, Radovancevic R, Hochman LF, et al. Arteriovenous malformation and gastrointestinal bleeding in patients with the HeartMate II left ventricular assist device. J Heart Lung Transplant. 2011;30(8):849–853.
- Broos K, Feys HB, De Meyer SF, et al. Platelets at work in primary hemostasis. Blood Rev. 2011;25(4):155–167.
- Hu J, Mondal NK, Sorensen EN, et al. Platelet glycoprotein Ibalpha ectodomain shedding and non-surgical bleeding in heart failure patients supported by continuous-flow left ventricular assist devices. J Heart Lung Transplant. 2014;33(1):71–79.
- Steinlechner B, Dworschak M, Birkenberg B, et al. Platelet dysfunction in outpatients with left ventricular assist devices. Ann Thorac Surg. 2009;87(1):131–137.
- Mondal NK, Li T, Chen Z, et al. Mechanistic insight of platelet apoptosis leading to non-surgical bleeding among heart failure patients supported by continuous-flow left ventricular assist devices. Mol Cell Biochem. 2017;433(1–2):125–137.
- Goldstein DJ, John R, Salerno C, et al. Algorithm for the diagnosis and management of suspected pump thrombus. J Heart Lung Transplant. 2013;32(7):667–670.
- Lazar RM, Shapiro PA, Jaski BE, et al. Neurological events during long-term mechanical circulatory support for heart failure: the randomized evaluation of mechanical assistance for the treatment of congestive heart failure (REMATCH) experience. Circulation. 2004;109(20):2423–2427.
- Starling RC, Moazami N, Silvestry SC, et al. Unexpected abrupt increase in left ventricular assist device thrombosis. N Engl J Med. 2014;370(1):33–40.
- Najjar SS, Slaughter MS, Pagani FD, et al. An analysis of pump thrombus events in patients in the HeartWare ADVANCE bridge to transplant and continued access protocol trial. J Heart Lung Transplant. 2014;33(1):23–34.
- Schmitto JD, Hanke JS, Rojas SV, et al. First implantation in man of a new magnetically levitated left ventricular assist device (HeartMate III). J Heart Lung Transplant. 2015;34(6):858–860.
- Mehra MR, Uriel N, Naka Y, et al. A fully magnetically levitated left ventricular assist device — final report. N Engl J Med. 2019;380(17):1618–1627.
- Shah P, Mehta VM, Cowger JA, et al. Diagnosis of hemolysis and device thrombosis with lactate dehydrogenase during left ventricular assist device support. J Heart Lung Transplant. 2014;33(1):102–104.
- Uriel N, Morrison KA, Garan AR, et al. Development of a novel echocardiography ramp test for speed optimization and diagnosis of device thrombosis in continuous-flow left ventricular assist devices: the Columbia ramp study. J Am Coll Cardiol. 2012;60(18):1764–1775.
- Feldman D, Pamboukian SV, Teuteberg JJ, et al. The 2013 international society for heart and lung transplantation guidelines for mechanical circulatory support: executive summary. J Heart Lung Transplant. 2013;32(2):157–187.
- Slaughter MS, Naka Y, John R, et al. Post-operative heparin may not be required for transitioning patients with a HeartMate II left ventricular assist system to long-term warfarin therapy. J Heart Lung Transplant. 2010;29(6):616–624.
- Sandner SE, Riebandt J, Haberl T, et al. Low-molecular-weight heparin for anti-coagulation after left ventricular assist device implantation. J Heart Lung Transplant. 2014;33(1):88–93.
- Adatya S, Sunny R, Fitzpatrick MJ, et al. Coagulation factor abnormalities related to discordance between anti-factor Xa and activated partial thromboplastin time in patients supported with continuous-flow left ventricular assist devices. J Heart Lung Transplant. 2016;35(11):1311–1320.
- Warkentin TE, Greinacher A. Heparin-induced thrombocytopenia and cardiac surgery. Ann Thorac Surg. 2003;76(6):2121–2131.
- Koster A, Huebler S, Potapov E, et al. Impact of heparin-induced thrombocytopenia on outcome in patients with ventricular assist device support: single-institution experience in 358 consecutive patients. Ann Thorac Surg. 2007;83(1):72–76.
- Warkentin TE, Greinacher A, Koster A, et al. Treatment and prevention of heparin-induced thrombocytopenia: american college of chest physicians evidence-based clinical practice guidelines (8th edition). Chest. 2008;133(6 Suppl):340S–380S.
- Jennings DL, Brewer R, Williams C. Impact of continuous flow left ventricular assist device on the pharmacodynamic response to warfarin early after implantation. Ann Pharmacother. 2012;46(9):1266–1267.
- Jennings D, McDonnell J, Schillig J. Assessment of long-term anticoagulation in patients with a continuous-flow left-ventricular assist device: a pilot study. J Thorac Cardiovasc Surg. 2011;142(1):e1–2.
- Argiriou M, Kolokotron, SM, Sakellaridis, T, et al. Right heart failure post left ventricular assist device implantation. J Thorac Dis. 2014;6(Suppl 1):S52–9.
- Visser LE, Bleumink GS, Trienekens PH, et al. The risk of over anticoagulation in patients with heart failure on coumarin anticoagulants. Br J Haematol. 2004;127(1):85–89.
- Nassif ME, LaRue, SJ, Raymer, DS, et al. Relationship between anticoagulation intensity and thrombotic or bleeding outcomes among outpatients with continuous-flow left ventricular assist devices. Circ Heart Fail. 2016;9(5):e002680.
- Grabska J, Schlöglhofer T, Gross C, et al. Early detection of pump thrombosis in patients with left ventricular assist device. ASAIO J. 2020;66(4):348–354.
- Bhatia A, Juricek C, Sarswat N, et al. Increased risk of bleeding in left ventricular assist device patients treated with enoxaparin as bridge to therapeutic international normalized ratio. Asaio J. 2018;64(2):140–146.
- Cosgrove RH, Basken RL, Smith RG, et al. Anticoagulant bridge comparison in mechanical circulatory support patients. Asaio J. 2019;65(1):54–58.
- Schettle S, Schlöglhofer T, Zimpfer D, et al. International analysis of LVAD point-of-care versus plasma INR: A multicenter study. Asaio J. 2018;64(6):e161–e165. DOI:10.1097/MAT.0000000000000845.
- Es van J, EERENBERG ES, KAMPHUISEN PW, et al. How to prevent, treat, and overcome current clinical challenges of VTE. J Thromb Haemost. 2011;9(Suppl 1):265–274.
- van Es N, Coppens M, Schulman S, et al. Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: evidence from phase 3 trials. Blood. 2014;124(12):1968–1975.
- Lopez-Lopez JA, Sterne JAC, Thom HHZ, et al. Oral anticoagulants for prevention of stroke in atrial fibrillation: systematic review, network meta-analysis, and cost effectiveness analysis. Bmj. 2017;359:j5058.
- Miller CS, Grandi SM, Shimony A, et al. Meta-analysis of efficacy and safety of new oral anticoagulants (dabigatran, rivaroxaban, apixaban) versus warfarin in patients with atrial fibrillation. Am J Cardiol. 2012;110(3):453–460.
- van der Hulle T, Kooiman J, den Exter PL, et al. Effectiveness and safety of novel oral anticoagulants as compared with vitamin K antagonists in the treatment of acute symptomatic venous thromboembolism: a systematic review and meta-analysis. J Thromb Haemost. 2014;12(3):320–328.
- Andreas M, Moayedifar, R, Wieselthaler, G, et al. Increased thromboembolic events with dabigatran compared with vitamin K antagonism in left ventricular assist device patients: A randomized controlled pilot trial. Circ Heart Fail. 2017;10(5):e003709.
- Eikelboom JW, Connolly SJ, Brueckmann M, et al. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. 2013;369(13):1206–1214.
- Dewanjee MK, Gross DR, Zhai P, et al. Thrombogenicity of polyethylene oxide-bonded Dacron sewing ring in a mechanical heart valve. J Heart Valve Dis. 1999;8(3):324–330.
- Pollari F, Fischlein T, Fittkau M, et al. Anticoagulation with apixaban in a patient with a left ventricular assist device and gastrointestinal bleeding: A viable alternative to warfarin? J Thorac Cardiovasc Surg. 2016;151(4):e79–81.
- Baumann Kreuziger LM, Kim B, Wieselthaler GM. Antithrombotic therapy for left ventricular assist devices in adults: a systematic review. J Thromb Haemost. 2015;13(6):946–955.
- Netuka I, Litzler P-Y, Berchtold-Herz M, et al. Outcomes in HeartMate II patients with no antiplatelet therapy: 2-year results from the European TRACE study. Ann Thorac Surg. 2017;103(4):1262–1268.
- Stulak JM, Cowger J, Haft JW, et al. Device exchange after primary left ventricular assist device implantation: indications and outcomes. Ann Thorac Surg. 2013;95(4):1262–1267; discussion 1267–8.
- Muthiah K, Robson D, Macdonald PS, et al. Thrombolysis for suspected intrapump thrombosis in patients with continuous flow centrifugal left ventricular assist device. Artif Organs. 2013;37(3):313–318.
- Kamouh A, John R, Eckman P. Successful treatment of early thrombosis of HeartWare left ventricular assist device with intraventricular thrombolytics. Ann Thorac Surg. 2012;94(1):281–283.
- Delgado R 3rd, Frazier OH, Myers TJ, et al. Direct thrombolytic therapy for intraventricular thrombosis in patients with the Jarvik 2000 left ventricular assist device. J Heart Lung Transplant. 2005;24(2):231–233.
- Hayes H, Dembo L, Larbalestier R, et al. Successful treatment of ventricular assist device associated ventricular thrombus with systemic tenecteplase. Heart Lung Circ. 2008;17(3):253–255.
- Joy PS, Kumar G, Guddati AK, et al. Risk factors and outcomes of gastrointestinal bleeding in left ventricular assist device recipients. Am J Cardiol. 2016;117(2):240–244.
- Draper KV, Huang RJ, Gerson LB. GI bleeding in patients with continuous-flow left ventricular assist devices: a systematic review and meta-analysis. Gastrointest Endosc. 2014;80(3):435–446.e1.
- Tabit CE, Chen P, Kim GH, et al. Elevated angiopoietin-2 level in patients with continuous-flow left ventricular assist devices leads to altered angiogenesis and is associated with higher nonsurgical bleeding. Circulation. 2016;134(2):141–152.
- Sarode R, Milling TJ, Refaai MA, et al. Efficacy and safety of a 4-factor prothrombin complex concentrate in patients on vitamin K antagonists presenting with major bleeding: a randomized, plasma-controlled, phase IIIb study. Circulation. 2013;128(11):1234–1243.
- Goldstein JN, Refaai MA, Milling TJ, et al. Four-factor prothrombin complex concentrate versus plasma for rapid vitamin K antagonist reversal in patients needing urgent surgical or invasive interventions: a phase 3b, open-label, non-inferiority, randomised trial. Lancet. 2015;385(9982):2077–2087.
- Rimsans J, Levesque A, Lyons E, et al. Four factor prothrombin complex concentrate for warfarin reversal in patients with left ventricular assist devices. J Thromb Thrombolysis. 2018;46(2):180–185.
- Jennings DL, Rimsans J, Connors JM. Prothrombin Complex Concentrate for Warfarin Reversal in Patients with Continuous-Flow Left Ventricular Assist Devices: A Narrative Review. Asaio J. 2019.
- Gurvits GE, Fradkov E. Bleeding with the artificial heart: gastrointestinal hemorrhage in CF-LVAD patients. World J Gastroenterol. 2017;23(22):3945–3953.
- Marsano J, Desai J, Chang S, et al. Characteristics of gastrointestinal bleeding after placement of continuous-flow left ventricular assist device: a case series. Dig Dis Sci. 2015;60(6):1859–1867.
- Muslem R, Caliskan K, Leebeek FWG. Acquired coagulopathy in patients with left ventricular assist devices. J Thromb Haemost. 2018;16(3):429–440.
- Fischer Q, Huisse M-G, Voiriot G, et al. Von Willebrand factor, a versatile player in gastrointestinal bleeding in left ventricular assist device recipients? Transfusion. 2015;55(1):51–54.
- Loyaga-Rendon RY, Hashim T, Tallaj JA, et al. Octreotide in the management of recurrent gastrointestinal bleed in patients supported by continuous flow left ventricular assist devices. Asaio J. 2015;61(1):107–109.
- Wever-Pinzon O, Selzman CH, Drakos SG, et al. Pulsatility and the risk of nonsurgical bleeding in patients supported with the continuous-flow left ventricular assist device HeartMate II. Circ Heart Fail. 2013;6(3):517–526.
- Netuka I, Kvasnička T, Kvasnička J, et al. Evaluation of von Willebrand factor with a fully magnetically levitated centrifugal continuous-flow left ventricular assist device in advanced heart failure. J Heart Lung Transplant. 2016;35(7):860–867.
- Backes D, van den Bergh WM, van Duijn AL, et al. Cerebrovascular complications of left ventricular assist devices. ASAIO J. 2020;66(5):482–488.
- Wilson TJ, Stetler WR, Al-Holou WN, et al. Management of intracranial hemorrhage in patients with left ventricular assist devices. J Neurosurg. 2013;118(5):1063–1068.
- Santos CD, Matos NL, Asleh R, et al. The dilemma of resuming antithrombotic therapy after intracranial hemorrhage in patients with left ventricular assist devices. Neurocrit Care. 2019. DOI:10.1007/s12028-019-00836-y.
- Ramey WL, Basken RL, Walter CM, et al. Intracranial hemorrhage in patients with durable mechanical circulatory support devices: institutional review and proposed treatment algorithm. World Neurosurg. 2017;108:826–835.