ABSTRACT
Rare cases of myocarditis and pericarditis after COVID-19 mRNA vaccination have been recently reported in male adolescents and young adults. Acute myocarditis and pericarditis following COVID-19 mRNA vaccination in male adolescents and young adults may be connected with age-related lower levels of T-bet and PD-1 in predisposed individulas with T-bet polymorphisms by the release od autoreactive CD8+CTL. Upregulatiomn of T-Bet and PD-1 by estrogen might explai the higher incidence of men developing myocarditis or pericarditis in comparison to women.
Rare cases of myocarditis and pericarditis after Coronavirus disease 2019 (COVID-19) messenger RNA (mRNA) vaccination have been recently reported in male adolescents and young adults [Citation1–4]. Most myocarditis and pericarditis have been described in mRNA vaccine recipients, predominately after the second dose, within the first week [Citation1,Citation2]. The short time interval between vaccination and the onset of symptoms have raised concern about a possible causative role for mRNA COVID-19 vaccines [Citation1–8]. The Advisory Committee on Immunization Practices (ACIP) have examined reported cases of myocarditis and pericarditis highlighting the importance of alerting vaccination providers and the public about the benefits and the risks, including the risk for myocarditis, following the administration of mRNA COVID-19 vaccine, mainly for males aged 12–29 years, using myocarditis to refer to myocarditis, pericarditis, or myopericarditis [Citation5]. The analysis of adverse event reports from the Vaccine Adverse Event Reporting System (VAERS) has found that post-vaccine myocarditis and pericarditis are associated most frequently with mRNA COVID-19 vaccines followed by live or live-attenuated non–COVID-19 vaccines including smallpox and anthrax vaccines [Citation2,Citation9]. Fortunately, both myocarditis and pericarditis have been observed to be mild and self-limited in almost all patients [Citation1–9]. At present, there is no definite causal association between these cases and vaccine administration [Citation10]. Until today, there is no conclusive evidence about the machinery underlying myocarditis and pericarditis following mRNA COVID-19 vaccination in younger patients [Citation10]. Uncertainties also exist concerning risk factors, reasons for sex differences and the development of myocarditis and pericarditis particularly during the secondary immune response, which remain to be clarified [Citation1–6]. It has been proposed that the association of myocarditis with male sex and younger age may be attributed to sex hormones which may account for a more intense inflammatory response [Citation1,Citation3–8]. A number of possible mechanisms linking COVID-19 mRNA vaccination to myocarditis onset have been suggested including the hypothesis that the immune system may identify the mRNA in the vaccine as an antigen eliciting a pro-inflammatory cascade and immunologic signaling pathways resulting in myocarditis as a part of a systemic reaction in certain subjects [Citation1,Citation3,Citation5,Citation8]. This conjecture appears to be corroborated by the fact that endomyocardial biopsy specimens from patients with myocarditis after COVID-19 mRNA vaccination show similar inflammatory infiltrate predominantly composed of T-cells and a substantial CD-68-positive macrophages, CD3-positive T-lymphocytes admixed with eosinophils, B-cells and plasma cells [Citation11,Citation12]. COVID-19 mRNA vaccines consist of nucleoside-modified mRNA encoding the viral spike glycoprotein of SARS-CoV2, without live virus or DNA [Citation1,Citation3]. mRNA vaccines stimulate the cells to shape the spike protein which then produce an adaptive immune response to recognize and destroy SARS-CoV2 expressing spike-protein [Citation1,Citation3]. Following infection, inflammatory cues appear to induce core transcriptional programs to attain pathogen-specific protection [Citation13]. In viral infection or vaccination, it has been shown that T follicular helper cells elicit the prototypical T helper 1 (TH1) transcription factor T-bet that represents the lineage–defining transcription factor for TH1 cells [Citation13]. T-bet is also greatly shaped in CD8+ cytotoxic T lymphocytes (CTLs) playing the role of a molecular switch between effector and memory differentiation [Citation11]. T-bet has been described to play a significant context-dependent role between extrinsic inflammatory and intrinsic cellular differentiation programs in triggering protective, neutralizing antibodies following viral infection or vaccination in the determination of cell fate [Citation13]. CD8+ CTLs represent the best characterized subpopulation of CD8+ T cells that play an important role in adaptive immunity contributing to clearance of intracellular pathogens and providing long-term protection [Citation14,Citation15]. There is increasing evidence for alternative CD8+ T cell fates controlling CD4+ T-cell-mediated responses in the context of a number of processes including autoimmunity [Citation14]. CD8 + T lymphocyte damage to tissue cells have been demonstrated to play a key role in several disease processes such as organ-specific autoimmunity [Citation16]. Inappropriate Th cell activation and polarization appear to lead to autoimmunity [Citation17]. T-bet has been found to play a significant role in the development of autoimmune diseases [Citation18]. Recently, T-bet has been reported to cause inflammation in cardiovascular disease (CVD) [Citation19]. It has been written that T-bet functions as a double-edged sword playing the dual role of triggering and inhibiting inflammatory reactions as well as angiogenesis stimulation as result of inflammatory cytokines in CVD [Citation19]. T-bet has been detected to control the differentiation of CD8 + T cells into pathogenic CTLs that can infiltrate and cause lethal myocardial injury by a separate impact on migration and effector activity implying that targeting T-bet-regulated gene expression may have potential therapeutic benefit in immune-mediate heart disease [Citation20]. It is well-known that CD8+ CTLs mediate much of the damage in myocarditis [Citation20,Citation21]. It has been revealed that T-bet controls the migratory phenotype of autoreactive effector CD8 + T cells, and notably, that this T-bet peculiarity controls the ability of tissue Ag-specific CD8 + T cells to cause disease in the heart [Citation20]. It has also been written that loss of T-bet in the CD8+ compartment exacerbates experimental autoimmune myocarditis [Citation21]. Expression of T-bet in heart-infiltrating CD8 + T cells is considered important for their capability to arbitrate antigen-nonspecific bystander suppression of local inflammation in the experimental autoimmune myocarditis [Citation22]. It has been observed that genetic polymorphisms may predispose individuals to inflammatory bowel diseases through alterations in T-bet binding and gene expression implying that other disease-associated variants may similarly act by controlling the binding of lineage-specifying transcription factors such as T-bet in a tissue selective and disease-specific manner governing cell fate [Citation17]. T-bet levels appear to be increased in CD8 T cells from aged individuals [Citation23]. T-bet mRNA expression has been verified to be low in naïve CD 8 T cells in young individuals, but to be increased in non-naïve CD8 T cells from aged individuals [Citation23]. However, CD8 T cells from aged subjects have been detected to reveal decreased functionally resulting in ineffective CD8 T cells responses [Citation23]. Age-associated exhausted CD8 T cells have been linked to increased expression of inhibitor receptor programmed death (PD)-1 [Citation24]. T-bet has been detected to interfere with programmed death (PD)-1 and its ligand PD-L1 pathway [Citation25]. The PD-1/PD-L1 coinhibitory pathway have been shown to play critical roles in the immune response and autoimmunity via the regulation of T cell activity [Citation26]. The PD-1/PD-L1 pathway has been stated to control the expansion and function of cytotoxic CD8 + T cells [Citation27]. The interplay between T-bet and PD-1 has been described to vary according to the context [Citation26]. Interfering with PD-1 has been demonstrated to cause a number of immune-related adverse conditions including autoimmune myocarditis and pericarditis [Citation28]. PD-1 has been recognized to protect against inflammation and myocyte damage in T cell mediated myocarditis [Citation29]. Interestingly, it has been demonstrated that estrogen-mediated immunomodulation relates to enhanced expression of the PD-1 [Citation30]. Remarkably, it has been verified that in vivo estrogen exposure primes lymphocytes toward Th1 type development by promoting up-regulation of T-bet expression [Citation31]. Interestingly, distinct immunotypes showing different patterns of lymphocyte responses including hyperactived or exhausted CD8 T cells and T-bet have been shown in hospitalized COVID-19 patients resulting in different clinical features, disease severity, and temporal changes in response and pathogenesis [Citation32]. Intriguingly, SARSCoV2 infection has been revealed to be linked to CD8 T Cell activation only in a subset of patients [Citation32]. With respect to the above, I hypothesize that myocarditis and pericarditis following COVID-19 mRNA vaccination may be triggered by genetic variation at the T-bet locus in certain individuals by the release of autoreactive CD8+ CTL cells (). I assume that myocarditis and pericarditis following COVID-19 mRNA vaccines may be more frequent in younger patients compared with elderly subjects as result of age-related lower levels of T-bet and PD-1. However, I presume that only patients with specific T-bet polymorphisms may experience myocarditis or pericarditis after COVID-19 mRNA vaccination. On this regard, I suppose that T-bet polymorphisms may worsen the condition of age-related lower levels of T-bet and PD-1 levels in younger subjects when compared to older individuals that have both increased levels of T-bet and PD-1 and exhaustion of CD8 T cells. The fact that the majority of cases have largely been described within few days after the second dose of mRNA vaccination led me to conjecture that the first vaccination might initially function as an antigen-driven autoreactive effector CD8+ CTLs cell by genetic variants of T-Bet producing an overly aggressive immune system response with the second dose as a booster shot for strong autoimmune reactions against the heart resulting in rapidly evolving form of acute myocarditis or pericarditis in predisposed individuals. Finally, I suppose that up-regulation of T-bet and PD-1 by estrogen might explain the higher incidence of men developing myocarditis or pericarditis compared to women. I conclude that prospective observational studies and genome-wide association studies are warranted to better define pathophysiology of acute myocarditis and pericarditis following mRNA COVID-19 vaccination and identify single nucleotide polymorphisms that may be responsible for myocarditis and pericarditis susceptibility.
Figure 1. Myocarditis and pericarditis after COVID-19 mRNA vaccination in the young: implications of T-Bet?
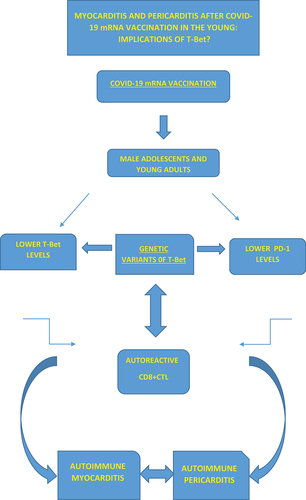
Expert Opinion – Acute myocarditis and pericarditis following COVID-19 mRNA vaccination in male adolescents and young adults may be linked to age-related lower levels of T-Bet and PD-1 in predisposed individuals suffering from T-bet polymorphisms by the release of autoreactive CD8+ CTL. Up-regulation of T-bet and PD-1 by estrogen might explain the higher incidence of men developing myocarditis or pericarditis compared to women.
Declaration of Interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Luk A, Clarke B, and Dahdah N, et al. Myocarditis and pericarditis after covid-19 mrna vaccination: practical considerations for care providers. Can J Cardiol. 2021;8: S0828-282X(21)00624-3. doi:https://doi.org/10.1016/j.cjca.2021.
- Hajjo R, Sabbah DA, Bardaweel SK, et al. Shedding the Light on post-vaccine myocarditis and pericarditis in covid-19 and non-covid-19 vaccine recipients. Vaccines (Basel). 2021;9(10):1186. 15.
- Bozkurt B, Kamat I, Hotez PJ. Myocarditis with COVID-19 mRNA Vaccines. Circulation. 2021;144(6):471–484. 10.
- Dickey JB, Albert E, Badr M, et al. A series of patients with myocarditis following sars-cov-2 vaccination with mrna-1279 and bnt162b2. Jacc Cardiovasc Imaging. 2021;14(9):1862–1863.
- Gargano JW, Wallace M, Hadler SC, et al. Use of mrna covid-19 vaccine after reports of myocarditis among vaccine recipients: update from the advisory committee on immunization practices - United States. MMWR Morb Mortal Wkly Rep. 2021;70(27):977–982. 9.
- Caso F, Costa L, Ruscitti P, et al. Could Sars-Coronavirus-2 trigger autoimmune and/or autoinflammatory mechanisms in genetically predisposed subjects? Autoimmun Rev. 2020;19(5):102524. 102524.
- Diaz GA, Parsons GT, Gering SK, et al. Pericarditis After Vaccination for COVID-19. JAMA. 2021 Sep 28;326(12):1210–1212.
- Lazaros G, Klein AL, Hatziantoniou S, et al. The novel platform of mrna covid-19 vaccines and myocarditis: clues into the potential underlying mechanism. Vaccine. 2021 Aug 16;39(35):4925–4927.
- Su JR, McNeil MM, Welsh KJ, et al. Myopericarditis after vaccination, vaccine adverse event reporting system (VAERS). Vaccine 1990-2018. 29 2021;39(5):839–845.
- Marshall M, Ferguson ID, Lewis P, et al. Symptomatic acute myocarditis in 7 adolescents after pfizer-biontech covid-19 vaccination. Pediatrics. 2021;148(3):e2021052478.
- Nguyen TD, Mall G, and Westphal JG, et al. Acute myocarditis after COVID-19 vaccination with mRNA-1273 in a patient with former SARS-CoV-2 infection. In: ESC Heart Fail. Sep 18; 2020.
- Verma AK, Lavine KJ, Lin CY. Myocarditis after Covid-19 mRNA Vaccination. N Engl J Med. 2021;385(14):1332–1334. 30.
- Sheikh AA, Cooper L, Feng M, et al. Context-dependent role for t-bet in t follicular helper differentiation and germinal center function following viral infection. Cell Rep. 2019;28(7):1758–72.e4. 13.
- Mittrücker HW, Visekruna A, Huber M. Heterogeneity in the differentiation and function of cd8+ t cells. Arch. Immunol. Ther. Exp 2014;62(6):449–458.
- Joshi NS, Cui W, Chandele A, et al. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27(2):281–295.
- Grabie N, Hsieh DT, Buono C, et al. Neutrophils sustain pathogenic CD8+ T cell responses in the heart. Am J Pathol. 2003;163(6):2413–2420.
- Soderquest K, Hertweck A, Giambartolomei C, et al. Genetic variants alter T-bet binding and gene expression in mucosal inflammatory disease. PLoS Genet. 2017;13(2):e1006587. 10.
- Ji N, Sosa RA, Forsthuber TG. More than just a T-box: the role of T-bet as a possible biomarker and therapeutic target in autoimmune diseases. Immunotherapy. 2011;3(3):435–441.
- Haybar H, Rezaeeyan H, Shahjahani M, et al. T-bet transcription factor in cardiovascular disease: attenuation or inflammation factor? J Cell Physiol. 2019;234(6):7915–7922.
- Taqueti VR, Grabie N, Colvin R, et al. T-bet controls pathogenicity of CTLs in the heart by separable effects on migration and effector activity. J Immunol. 2006;177(9):5890–5901. 1.
- Rangachari M, Mauermann N, Marty RR, et al. T-bet negatively regulates autoimmune myocarditis by suppressing local production of interleukin 17. J Exp Med. 2006;203(8):2009.
- Dolfi DV, Mansfield KD, Polley AM, et al. Increased T-bet is associated with senescence of influenza virus-specific CD8 T cells in aged humans. J Leukocyte Biol. 2013;93(6):825–836.
- Lee KA, Shin KS, Kim GY, et al. Characterization of age-associated exhausted CD8⁺ T cells defined by increased expression of Tim-3 and PD-1. Aging Cell. 2016 Apr;15(2):291–300.2016.
- Sun Y, Li L, Wu Y, et al. PD-1/PD-L1 in cardiovascular disease. Clin Chim Acta. 2020;505:26–30.
- Ma F, Zhao M, Song Z, et al. T-bet interferes with PD-1/PD-L1-mediated suppression of CD4+ T cell inflammation and survival in crohn’s disease. Clin Exp Pharmacol Physiol. 2019;46(9):798–805.
- Kao C, Oestreich KJ, Paley MA, et al. Transcription factor T-bet represses expression of the inhibitory receptor PD-1 and sustains virus-specific CD8+ T cell responses during chronic infection. Nat Immunol. 2011;12(7):663–671. 29.
- David P, Megger DA, Kaiser T, et al. The PD-1/PD-L1 pathway affects the expansion and function of cytotoxic cd8+ t cells during an acute retroviral infection. Front Immunol. 2019;10:54.
- Pirozzi F, Poto R, Aran L, et al. Cardiovascular toxicity of immune checkpoint inhibitors: clinical risk factors. Curr Oncol Rep. 2021;23(2):13. 7.
- Tarrio ML, Grabie N, Bu DX, et al. PD-1 protects against inflammation and myocyte damage in T cell-mediated myocarditis. J Immunol. 2012;188(10):4876–4884. 15.
- Polanczyk MJ, Hopke C, Vandenbark AA, et al. Estrogen-mediated immunomodulation involves reduced activation of effector T cells, potentiation of Treg cells, and enhanced expression of the PD-1 costimulatory pathway. J Neurosci Res. 2006;84(2):370–378. 1.
- Karpuzoglu E, Phillips RA, Gogal RMJ, et al. IFN-gamma-inducing transcription factor, T-bet is upregulated by estrogen in murine splenocytes: role of IL-27 but not IL-12. Mol Immunol. 2007;44(7):1808–1814.
- Mathew D, Giles JR, Baxter AE, et al., Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science. 369(6508): eabc8511. 2020.
