ABSTRACT
Introduction
Coronary artery calcification (CAC) is commonly encountered by interventional cardiologists. Severe CAC may impair stent delivery or result in stent underexpansion, stent thrombosis and/or in-stent restenosis (ISR). Multiple tools have been developed to help overcome the challenges associated with CAC and improve outcomes for these patients. Intravascular shockwave lithotripsy (IVL) is a novel therapy that uses acoustic pressure waves for the modification of CAC.
Areas covered
This review discusses the growing body of evidence to support the safety and efficacy of IVL in the setting of de novo severely calcified coronary arteries prior to stenting. We also discuss international real-world experience with the coronary IVL system. This includes the use of IVL in the setting of acute coronary syndrome (ACS), ISR and in combination with other tools for calcium modification.
Expert opinion
IVL is a safe and effective therapy that results in the fracture of coronary calcium and facilitates optimal stent delivery and expansion. Longer term follow-up is essential to shed light on the durability and late outcomes of an IVL strategy. Randomized control trials are warranted to compare IVL to alternative methods of calcium modification and to explore further the use of IVL for ACS.
1. Introduction
Coronary artery calcification (CAC) presents a challenge for interventional cardiologists throughout the world. It is particularly common in older patients and in patients with diabetes, chronic kidney disease (CKD) and previous coronary artery bypass grafting (CABG) [Citation1,Citation2]. In a study by Mintz et al. CAC was detected in 38% of target lesions on angiography and 73% using intravascular ultrasound (IVUS) [Citation3].
Inflammation is the key driver of CAC. The process is triggered by apoptosis of smooth muscle cells and inflammatory cells within the atherosclerotic plaque [Citation4]. Cholesterol deposits beneath the endothelium set an inflammatory response in motion, involving chondrocyte-like cells, macrophages, foam cells and inflammatory mediators such as cytokines. This leads to the development of microcalcifications within the atherosclerotic plaque [Citation4]. It is a complex process involving many activators and inhibitors and in many ways resembles osteogenesis elsewhere in the body [Citation5].
On the topic of CAC, the statin paradox describes the phenomenon whereby statin therapy lowers risk of cardiovascular events; however, paradoxically increases CAC, which is associated with increased cardiovascular risk [Citation6]. A 2021 study supports the theory that statins may alter the microarchitecture of calcific deposits resulting in plaque stabilization. This was demonstrated in an in vivo model of aortic calcification in mice and may not be directly translatable to the coronary vasculature [Citation6].
Calcified lesions account for 25–30% of coronary interventions, of which 8–10% are severely calcified. Calcified lesions are associated with poor clinical outcomes [Citation7]. Patients with severe CAC undergoing PCI have a notably higher all-cause mortality than those without [Citation8]. Bourantas et al. report mortality rates of 10.8% and 4.4%, respectively. CAC can lead to difficulty with stent delivery, requiring adjunctive lesion preparation and use of alternative interventional techniques to enable stent delivery. CAC predisposes to stent underexpansion and consequently stent thrombosis and in-stent restenosis (ISR) [Citation9–11]. It has been shown that in patients with ACS, those with moderate to severely calcified lesions had a 62% increased risk of stent thrombosis and a 42% increased risk of target lesion failure [Citation12].
It has been shown that for drug-eluting stents (DESs), CAC can result in disruption of the polymer, which may lead to nonuniform drug distribution, neointimal hyperplasia, local inflammation, and thrombosis [Citation13]. As greater pressures are required to dilate balloons in calcified vessels, this can lead to dissection of the vessel, particularly in the setting of eccentric calcification [Citation2]. There are currently many dedicated tools available to enable calcium modification and/or de-bulking. These include cutting and scoring balloons, orbital, rotational, and laser atherectomy, OPN balloons, and intravascular lithotripsy (IVL) [Citation14–16]. These devices are selected depending on the distribution of calcium within the particular lesion and indeed collectively constitute the armamentarium of equipment available for CAC. The remainder of this review will focus primarily on IVL.
With many options now at our disposal for the preparation of CAC, selecting the most appropriate strategy becomes a challenge. In a 2019 review on the topic, Sorini Dini et al. suggested an algorithm to aid with decision-making [Citation4]. Optical coherence tomography (OCT) or IVUS is first used to evaluate the extent and features of the CAC. Based on these findings, the steps in the algorithm () are followed. Alternative devices can be used in the event of an unsuccessful or partially successful outcome with the first-choice agent.
Figure 1. Decision algorithm for the treatment of calcified coronary lesions [Citation4].
![Figure 1. Decision algorithm for the treatment of calcified coronary lesions [Citation4].](/cms/asset/b0e5e105-5141-4b1f-a29c-bbac94c1403e/ierk_a_2069561_f0001_oc.jpg)
2. Beginnings of IVL
Shockwave lithotripsy (SWL) was first introduced to the world of medicine for the treatment of urinary stones in the 1980s [Citation17]. Up to this point, open surgery had been the primary method of stone retrieval and was associated with a high burden of potential complications. Therefore, the advent of this novel, noninvasive technique for stone removal was received with much enthusiasm and revolutionized the field of urology. Many papers have been published on the safety and efficacy of extracorporeal SWL over the years [Citation18]. However, complications such as vessel rupture, acute renal injury (due to tubular damage) and inflammation and scarring with permanent loss of renal function soon came to light. The SWL technique was then honed to reduce the likelihood of complicationsfor example, by using a slower shock-wave rate and pausing between each step of the procedure [Citation17].
Given the ability of the pulsatile sonic pressure waves to disrupt and fracture calcium, the technology was modified for use within the vasculature. IVL was initially utilized safely in the peripheral circulation [Citation19]. Balloon angioplasty of calcified peripheral vessels is associated with dissection, residual stenosis, and need for re-vascularization. In 2017, Brodmann et al. published a prospective trial of 35 patients with de novo calcified femoropopliteal lesions. The lesions had ≥70% stenosis, were ≤ 150 mm in length and had moderate-to-severe calcification. A minimum of 60 pulses of IVL were delivered to the target lesion. Procedural success, defined as residual stenosis of <50%, was achieved in all patients with no immediate vascular complications. There were no target vessel revascularisations (TVRs) reported at 6 months post-procedure. Pre-dilation was used in 8.6% of cases and post-dilation in 14.3%. No stents were implanted [Citation19].
The Disrupt PAD II trial followed with a greater number patients and a longer follow-up period [Citation20]. This was a non-randomized, multicentre trial of 60 patients with complex, calcified stenotic peripheral lesions. The rate of major adverse events at 30 days was 1.7%. The patency rate at 1 year was 54.5% and the TVR rate was 20.7%. The authors report that when balloon sizing was correct and therapeutic miss was avoided, primary patency increased and the need for revascularisation decreased. This suggested that unfamiliarity of the operators with this new technology may have influenced the results. None-the-less, this trial delivered in demonstrating a convincing safety profile for IVL in the setting of complex calcified lesions.
Disrupt PAD III enrolled 306 patients and aimed to compared IVL to percutaneous transluminal angioplasty (PTA), making it the first randomized trial in the series [Citation21]. In both groups, this was followed by definitive treatment with a drug eluting balloon (DEB) or a stent. Procedural success (residual stenosis ≤30% without flow limiting dissection) was higher in the IVL group (65.8% vs 50.4%). In those randomized to the IVL group there were lower rates of flow-limiting dissection and provisional stent placement, as well as lower maximal inflation pressures required. At 30 days there was no difference between the groups with regard to major adverse events or TVR.
In the current practice, IVL is used for iliac, femoral, femoral popliteal and infrapopliteal lesions. Given that the challenges associated with calcified coronary vessels mimic those in the peripheral vasculature, the application of the IVL technology was expanded for use in the coronary setting.
3. CAC and IVL
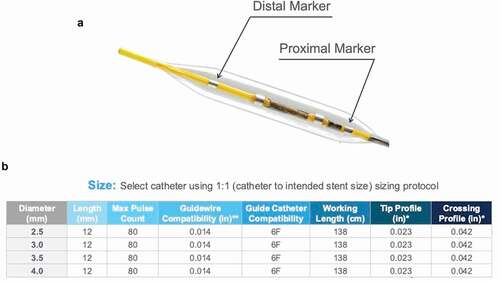
The Shockwave Medical coronary IVL catheter is designed and manufactured by Shockwave Medical Inc. based in Fremont, California, United States of America. The IVL system consists of an angioplasty balloon catheter with a 12 mm fluid-filled balloon (). Balloons range from 2.5 to 4.0 mm in diameter (). The catheter is delivered on a standard 0.014” coronary wire with a rapid exchange platform. IVL pulses are delivered from 2 lithotripsy emitters in the shaft of the balloon [Citation1]. These emitters initiate the formation of an acoustic pressure wave within the balloon. This is followed by the formation of a cavitation bubble that expands and collapses, creating secondary shock waves. The shock waves travel through the soft tissue of the vasculature. Once they encounter calcium, a fracturing effect occurs, due to the compression stress [Citation22]. OCT has demonstrated this fracturing of the intravascular calcium as the primary mechanism of calcium modification [Citation23]. Both intimal and medial calcification are impacted. Ultimately, this improves the compliance of the vessel, allowing for controlled balloon dilatation and allowing easier delivery and expansion of stents [Citation1]. The balloon is fluid-filled to help mitigate thermal injury to the vessel wall, while the multiple emitters along the shaft allow delivery of shock waves along the length of the lesion. The positive and negative peak pressures are reduced when compared to extracorporeal SWL to avoid excessive tensile stress to the vessel wall [Citation22].
Figure 2. Coronary IVL system; (A) generator, (B) connector cable and (C) catheter. (modified images with permission from shockwave medical Inc.).
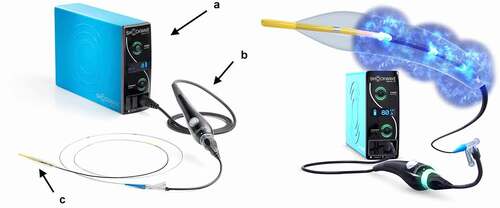
Figure 3. A = Proximal and distal markers of IVL balloon. B = IVL catheter sizes and specifications (modified images with permission from shockwave medical Inc.).
To use the system, a guidewire and guide catheter are placed within the target vessel. An appropriately sized balloon is selected based on the reference vessel diameter (1:1 sizing). The balloon and indeflator device are prepared using standard technique with a 50:50 mix solution of contrast dye and saline. After flushing the guidewire, the hydrophilic coating is activated by wetting the balloon and distal shaft with saline. The connector cable is attached to the IVL generator on one end and the catheter on the other end (). Once the IVL balloon is in position at the treatment site, aided by proximal and distal markers (), it is inflated to a pressure no greater than 4.0 atm. There should be full apposition of the balloon to the vessel wall. When the button on the connector cable is pressed, 10 pulses of IVL treatment are delivered over a 10 second period. The balloon is inflated to 6.0 atm or more prior to deflation (as per the balloon compliance chart) and then deflated to allow blood flow to be reestablished. These steps can be repeated to deliver multiple rounds of IVL to the lesion, up to a maximum of 80 pulses per treatment segment. A minimum of 10 seconds pause must follow each round of 10 pulses to allow for adequate distal perfusion. The balloon can be repositioned along the lesion between rounds [Citation24].
Specific risks associated with the coronary IVL system (as reported by the manufacturer) include allergic reactions to any component of the device, vessel dissection, device embolization and atrial or ventricular capture/extrasystole [Citation24]. This is in addition to known risks associated with catheter-based interventions and coronary interventional procedures in general.
Coronary IVL is currently licensed for balloon dilatation of severely calcified, stenotic de novo coronary arteries prior to stenting. Caution is advised when using IVL for lesions within 5 mm of previous stenting [Citation24]. Off-label use of the IVL system includes cases of ISR, stent underexpansion and for lesions post CABG.
4. Evidence for CAC and IVL
4.1. DISRUPT CAD I
The Disrupt CAD I trial was designed as a single arm, prospective, pre-market study of the Shockwave Coronary Lithoplasty System [Citation25]. Published in early 2019, this was the first study to demonstrate the feasibility of coronary IVL for modification of calcified plaques prior to stenting. It enrolled 60 patients with coronary lesions ≤32 mm in length and with ≥50% stenosis in a native vessel. The presence of heavy calcification was determined by the operator.
The primary safety endpoint was defined as freedom from major adverse cardiovascular events (MACE) at 30 days (a composite of cardiac death, myocardial infarction (MI), or target vessel revascularization (TVR)). At 30 days, the rate of MACE was 5%, climbing to 8.3% at 6 months. There were 3 peri-procedural MIs. MI was defined as creatine kinase-muscle/brain >3× the upper limit of normal. This definition was used in similar studies of CAC modification (for example, the ORBIT II study [Citation14]) and so allows for more accurate comparisons to be drawn. There were 2 deaths reported at 6-month follow-up. These were deemed unlikely to be related to the IVL technology [Citation25].
The primary efficacy endpoint (defined as residual diameter stenosis <50% after stenting, without in-hospital MACE) was achieved in 95% of patients. There were no reported instances of residual dissection, perforation, slow flow, no re-flow or embolization. Stent delivery was achieved in all 60 patients with a median of 1 stent used. Median diameter stenosis was reduced from 72.5% to 12.2% [Citation25].
4.2. DISRUPT CAD II
The Disrupt CAD II study followed in 2019 [Citation26]. This was a post-market, prospective, single-arm study with a larger cohort of 120 patients. The primary end point, in-hospital MACE, was 5.8%. MACE was 7.6% at 30 days. Procedural success was achieved in 100% of cases. One death, likely due to stent thrombosis, was reported. Of note, this patient was later found not to have met the inclusion/exclusion criteria for the study [Citation26]. There was 1 type B and 1 type C dissection reported, both of which were managed conservatively. Mean vessel stenosis was 60 ± 12%. Following IVL, residual luminal stenosis was 32.7 ± 10.4%. This decreased to 7.8 ± 7.1% after stenting [Citation26].
A sub-study was performed in 48 patients using OCT to assess the mechanism of action of IVL and to quantify plaque fracture (). This demonstrated that IVL significantly increased lumen area and decreased calcium angle. Calcium fracture was seen in 78.7% of lesions. Multiple fractures were present in 55.3% of lesions. The mean number of fractures per lesion was 3.4 ± 2.6 [Citation26].
Figure 4. OCT images pre and post-IVL of a severely calcified lesion. Calcium fractures, identified with white arrows in the post-PCI images above, allow for optimal stent expansion [Citation26].
![Figure 4. OCT images pre and post-IVL of a severely calcified lesion. Calcium fractures, identified with white arrows in the post-PCI images above, allow for optimal stent expansion [Citation26].](/cms/asset/549455bc-d695-48fc-8017-f91c112acd8b/ierk_a_2069561_f0004_oc.jpg)
4.3. DISRUPT CAD III
At this point, following the publication of the DISRUPT CAD I and II studies, the application of coronary IVL showed promising outcomes for patients. However, these initial studies were single-arm, non-randomized and were limited by small sample sizes [Citation1]. The success of these studies stimulated further interest in the technology and prompted the initiation of larger-scale trials.
When published in October 2020, DISRUPT CAD III became the largest and most rigorous trial in the series to date [Citation1]. This sponsor-funded, prospective, multicenter, single-arm study enrolled an impressive 431 patients from 47 centers across the USA and Europe. It was primarily designed to further evaluate both the safety and effectiveness of IVL prior to stent implantation.
All patients had severely calcified de novo coronary artery lesions which were <40 mm in length and in vessels with a reference diameter of 2.5–4 mm. Patients with an acute MI within the last 30 days, renal failure, or New York Heart Association (NYHA) class III or IV symptoms were excluded, as were patients with complex lesions. This included extremely tortuous, ostial, true bifurcations and unprotected left main lesions. Alternative methods of calcium modification such as atherectomy devices and cutting or scoring balloons were not permitted to be used. High pressure post-dilatation following stenting was mandated for all lesions. OCT was used in 100 patients to assess calcium burden and to evaluate the effect of IVL on the calcific plaque. OCT was preformed pre and post IVL and immediately following stent deployment [Citation1].
Approximately 92.2% of patients met the primary safety endpoint of 30-day freedom from MACE. This was driven by peri-procedural MIs in 6.8% of patients. Two deaths occurred in the 30-day period (including 1 in-hospital death) and there were 3 cases of stent thrombosis. The primary effectiveness endpoint, procedural success, was achieved in 92.4% of patients. Both the primary safety and efficacy endpoints exceeded the pre-specified performance goals [Citation1].
At 30 days post-PCI 72.9% of patients reported Canadian Cardiovascular Society (CCS) class 0 angina. This was a significant improvement from 12.6% pre-procedure (p < 0.001). Balloon loss of pressure occurred in 6.3% of patients. This was associated with a numerical increase in vessel dissection, which did not reach statistical significance. Longer calcific lesions were associated with higher rates of balloon loss of pressure and dissection.
Post-IVL OCT imaging demonstrated multiplanar and longitudinal calcium fractures in 67.4% of lesions. Notably, impressive residual stenosis and stent expansion outcomes were achieved regardless of the rate of calcium fracture on OCT. This suggests that perhaps OCT, as an imaging modality, may lack the sensitivity to detect the minute changes in the calcium architecture [Citation1].
When comparing the first 3 trials in the series, DISRUPT CAD III, which enrolled by far the largest number of patients (and was statistically powered), reported the highest rate of MACE at 30 days (7.8%). In this study, the lesion length was greatest (26 ± 11.7 mm), as was the calcified length (47.9 ± 18.8 mm) [Citation1]. The results of these studies must be interpreted in the context of a high-risk patient group known to have worse outcomes than patients with non-calcified lesions [Citation7].
At the TCT (Transcatheter Cardiovascular Therapeutics) 2021 meeting the 1-year data from Disrupt CAD III was presented [Citation27]. Of the 384 patients originally included in the intention-to-treat (ITT) analysis, 373 were available for follow-up. The MACE rate was 13.8%, which was an increase from 7.8% at the 30-day mark. For comparison, the 1 year MACE rate following orbital atherectomy was 16.4%, as reported in the follow-up data from the Pivotal ORBIT II trial [Citation28]. Taking each component separately; cardiac death increased from 0.5% to 1.1%, TVR from 1.6% to 6.0% and MI from 7.3% to 10.5% [Citation27].
On subgroup analysis, there was a significant association between MACE and lesions >25 mm in length. This was driven by peri-procedural MIs. Multi-variable analysis showed a positive association between MACE and prior MI (p = 0.048), bifurcation lesions (p = 0.006) and smoking history (current or former smoker) (p = 0.045). A higher rate of TVR was seen in patients with a history of prior MI (p = 0.024). There were no significant differences in MACE and TVR rates in the other subgroups analyzed [Citation27]. The 2-year outcome data from this trial are awaited, to further strengthen the evidence for the long-term safety and efficacy of IVL.
4.4. DISRUPT CAD IV
The results of the DISRUPT CAD IV study published in 2021 were designed for regulatory approval of the IVL system in Japanese hospitals [Citation29]. Japanese patients have been shown to have lower rates of CAC when compared to other ethnic groups [Citation30].
The trial enrolled 72 patients and 64 of these were included in the ITT analysis. The inclusion and exclusion criteria were similar to those of DISRUPT CAD III and the primary safety and efficacy endpoints were identical. Bifurcation or trifurcation lesions were included in this study, and constituted 34.4% of lesions. Non-inferiority analysis was carried out for the primary endpoints comparing the study group to a propensity-score matched historical IVL control group (from the DISRUPT CAD III study) [Citation29].
MACE at 30 days was 6.2% in the IVL group and 8.8% in the control arm. Four patients in the IVL group had non-Q-wave MIs and 1 had a type D dissection, managed successfully with a DES. Mean diameter stenosis improved from 65.8% to 9.9% following IVL and stenting.
This study concluded that the clinical outcomes in Japanese patients treated with IVL were in keeping with those demonstrated for western populations. It is noteworthy that the safety and efficacy of IVL held up in a trial that included patients with more complex coronary lesions [Citation29]. However, it must be kept in mind that Japanese patients tend to have smaller coronary arteries and shorter lesions on angiography [Citation31]. Furthermore, outcomes post coronary revascularisation are not equal between Japanese and western populations [Citation32]. Follow-up data will be collected for this patient group for 2 years post
treatment.
5. IVL in the real world setting
In addition to the studies described above (summarized in ), which were designed with approval of the IVL system in mind, other investigators have published observational and prospective data from real-world use of the technology. These types of studies add to our knowledge base and bring to our attention perhaps previously unforeseen challenges or complications. Since the introduction of IVL, multiple-case reports and case series have also been published on various uses of the technology, outside of the licensed indication (ie. de novo calcific lesions in native vessels prior to stenting).
Table 1. Summary of DISRUPT CAD studies values are n, percentage (%), median (Q1,Q3) or mean±SD
ACS = acute coronary syndrome, CAD = coronary artery disease, HR = heart rate, MI = myocardial infarction, NSTEMI = non-ST elevation myocardial infarction, LM = left main, CCS = chronic coronary syndrome, MACE = major adverse cardiac events.
Values are n, percentage (%), median (Q1,Q3) or mean ± SD.
5.1. IVL for acute coronary syndrome (ACS)
In 2019, Wong et al. published a study on their real-world experience with the IVL system, expanding on the initial patient groups enrolled in the DISRUPT CAD series. In this study, IVL was used in patients with ACS (53.8%) (targeting the culprit lesion), staged PCI to non-culprit lesions after ACS, stable coronary artery disease (CAD) and at elective angiography [Citation9]. In contrast to DISRUPT CAD, there were no angiographic exclusion criteriafor example, length of lesion. IVL was used as both an initial strategy and as a bail-out strategy following poor expansion of the predilating balloon.
This manuscript differs from its predecessors in its description of angiographic success. Wong et al. used the definition of residual stenosis <20% following IVL and many subsequent studies on the topic used this same definition. Never-the-less angiographic success was reported in all 26 cases. Of the 26 cases reported, the most commonly used balloons were the 2.5 mm and 3.5 mm. In this study of real-world practice, the LAD was the most common coronary artery targeted (50%) [Citation9]. This is in keeping with the DISRUPT CAD studies in which 47% [Citation25], 62.5% [Citation26], 56.5% [Citation1] and 75% [Citation29] of patients had IVL for LAD lesions.
In 2020, the same group published their 1 year follow-up data of 44 patients who were treated with IVL in their center, 56.8% of whom had ACS [Citation33]. Again successful stent delivery was achieved in 100% of cases. There were no in-hospital complications or adverse outcomes. At 1 year, there were one cardiovascular death, three non-cardiovascular deaths and three non-ST elevation MIs (NSTEMIs). The cardiovascular death occurred secondary to a hemorrhagic stroke in a patient on triple therapy post PCI. Of the three patients who had NSTEMIs, two patients required TVR for ISR and 1 patient had PCI to non-IVL target vessel.
Aksoy et al. used IVL for 71 patients as a primary, secondary, or tertiary strategy [Citation34]. The primary endpoints were strategy success and safety outcomes. ACS was the indication for PCI for 19.7% of patients, the remainder having stable or unstable angina. Patients were divided in three groups based on strategy. Strategy success (residual stenosis <20% with TIMI flow three and without stent failure) was 84.6% for primary IVL strategy (for de novo calcific lesions), 77.3% for secondary IVL strategy (when NC balloon dilation failed), and 64.7% for tertiary IVL strategy (when IVL was used in patients with stent underexpansion after previous stenting). From a safety point of view, there was four type B coronary dissections and seven instances of balloon rupture (with no subsequent adverse events). There was 1 episode of MACE at 30 days (1.4%). This patient presented with unstable angina but at angiography there was no evidence of target lesion failure.
Aziz et al. collected data on 190 patients in 6 centers around the UK and Italy, who had undergone IVL [Citation35]. Of these 190, 47.8% presented with ACS. Procedural success was achieved in all but one case. The complication rate was 3%. There were six coronary perforations, none of which were directly related to IVL treatment. One of these patients died in hospital. After a median of 222 days follow-up there were 2 cardiac deaths, 1 MI and 3 TVRs, giving a MACE rate of 2.6%.
In a recent publication on IVL from January 2022, a German study of 134 patients, 17.2% of patients had ACS [Citation36]. Of note 29.9% were cases of ISR, while the remainder where de novo lesions. Procedural success was seen in 88.1% of cases. MACE at 1 month was 3%, driven by 4 deaths, 2 of which were cardiovascular in nature. There was 1 episode of stent thrombosis. Importantly, in terms of complications there were 13 IVL balloon ruptures (9.7%) (with no adverse sequelae), 1 dissection that required stenting and 1 coronary artery perforation that resulted in death.
A Polish registry of 52 patients with ‘un-dilatable lesions’ (82.7% of which had ACS) demonstrated highly favorable results [Citation37]. Clinical success was achieved in an impressive 98.1% of cases. In terms of adverse events 1 patient had a ventricular arrhythmia (that was thought to be due to ongoing ischemia rather than related to the IVL therapy). There was 1 case of balloon rupture and 1 case of residual stenosis >50% (despite 100 IVL pulses). One in-hospital stroke was recorded, as were 2 major bleeding events. The complexity of this patient cohort should be appreciated when putting these complications into context.
The only large study to look at STEMI patients in isolation was that published by Cosgrove et al. in 2021 [Citation38]. This retrospective analysis of 72 patients reported a 18% rate of MACE at 1 month follow-up this was driven by 12 deaths, 1 MI and 1 TVR. Complications encountered included 1 episode of intra-procedural stent thrombosis, 3 cases of no-reflow (4%) and a 4% rate VF/VT.
In a more acute patient cohort than that enrolled by the DISRUPT CAD investigators, the procedural complication and MACE rates are more variable. Despite this, overall the safety and efficacy of IVL holds up in these studies, that are growing in number year by year. Lessons can be learned from each case of unexpected dissection, perforation or death.
5.2. IVL for stent underexpansion
It is well documented in the literature that a sub-optimally expanded stent is associated with an increased risk of ISR and stent thrombosis [Citation39,Citation40]. In cases of where CAC limits stent expansion, IVL is a potential solution.
A case report from 2021 describes a 66 year-old man who was noted to have a severe proximal LAD lesion at elective angiography [Citation41]. PCI with a DES was the primary strategy. Despite extensive pre and post-dilation with non-compliant (NC) balloons, OCT imaging showed severe stent underexpansion. An OPN NC balloon was then considered. However, the risk of perforation was deemed too high. A 2.5 mm IVL balloon was positioned in the underexpanded segment and 50 pulses of IVL were delivered. Following post-dilation with a NC balloon, repeat OCT imaging showed complete stent expansion.
IVL has also been used for stent underexpansion as a bail-out strategy, weeks after the index procedure. Włodarczak et al. described the case of a 58 yo man with a history of MI, who had PCI with a DES to a critical RCA stenosis [Citation42]. There was a significant stent underexpansion post-procedure. This was despite attempted optimization with NC and OPN-NC balloons (with aggressive post-dilation pressures). The patient continued to experience CCS class III angina on review 1 month later. Repeat angiography with OCT revealed a massive burden of calcification. At this point, IVL was utilized and, following optimization, resulted in no appreciable residual stenosis.
Yeoh et al. described a case series of 13 patients who had IVL for stent underexpansion [Citation43]. The average minimal stent area gained was 238%. There were no incidences of MACE during or after the procedures, even up to 30 days later.
One of the largest registries in the literature focusing on stent underexpansion is the SMILE registry, comprising of 34 patients (39 underexpanded stents) [Citation44]. A median of 13 months had passed since the original stent implantation. This multicenter study showed a success rate of 87.1% for lesion dilatation with IVL. There was one case of non-fatal peri-procedural MI following an IVL balloon rupture. There were no incidences of TLR, stent thrombosis, vessel perforation or cardiac death at 30 days.
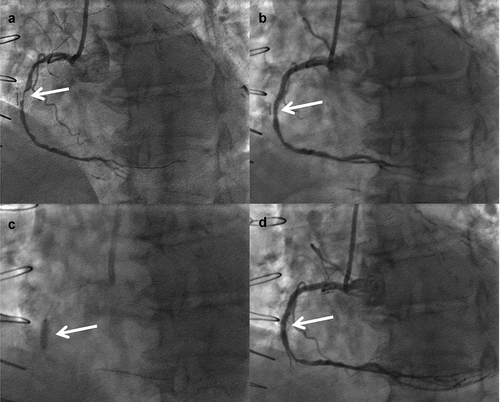
An example of IVL for stent underexpansion can be seen in . This patient presented with unstable angina in the setting of a prior CABG with an occluded graft to the posterior descending artery. A stent was implanted in the mid native RCA. However, severe CAC resulted in stent underexpansion. Thirty pulses of IVL were delivered with an excellent final result. A decision was made by the operators not to stent the distal vessel.
5.3 IVL for ISR
A retrospective study of 6 patients who received IVL for ISR in a German center was the first to address this group of patients specifically [Citation45]. These lesions had underexpanded stents secondary to CAC and/or calcified neointimal proliferation. IVL was used following initial failed therapy with NC, cutting or scoring balloons. In 5 of the 6 cases, IVL resulted in successful stent delivery with angiographic success. There were no procedural complications or in hospital adverse events. Patients were followed for a median of 141.5 days. Two patients required TVR in the follow-up period.
A case report from Poland describes the case of a 67 year old man with prior PCI to the LAD in 2005 (with a bare metal stent) and in 2007 (with a DES for ISR) [Citation46]. He continued to experience CCS class II angina. IVUS confirmed the presence of ISR in the proximal LAD due to stent underexpansion and heavy calcification. Fractional flow reserve measurement was positive at 0.70. Patient preference was for PCI rather than CABG. Multiple attempts with NC balloons failed to adequately predilate the lesion. The operators decided to use IVL and delivered 80 pulses to the calcified segment. This facilitated full expansion of the balloon and success deployment of a DES. The result was <10% residual stenosis and there were no complications post-procedure.
Salazar et al also published a similar case report of a 73 year old male patient with recurrent ISR in a diagonal artery [Citation47]. In this case, OCT was used to demonstrate severe calcific neoatherosclerosis and stent underexpansion. Similar to the above case, excellent results were achieved post-IVL and post-dilation with an NC balloon.
In both of these cases, ISR recurred despite to use of contemporary DESs, which are designed to prevent such outcomes. The likely explanation for the recurrence of ISR is the heavy calcification, preventing adequate stent expansion. As described above, the German study by Aksoy et al. included 20 patients who received IVL for underexpanded stents that were previously inserted, achieving 64.7% strategy success [Citation34].
A successful case of IVL in the setting of in-stent chronic total occlusion (CTO) can be found in the literature. A 75 year old gentleman, with 2 prior BMSs to the LAD, had successful stent delivery following IVL therapy. The case was complicated by a distal wire perforation (managed with coil embolization) but an otherwise uneventful hospital course [Citation48].
Of interest, in the 2022 study by El Jattari et al. mentioned above, the rate of procedural success was 92.6% for de novo lesions and 77.5% for ISR lesions [Citation36]. Laser atherectomy has been proposed as an alternative potential therapy for calcific ISR, but has yet to be supported by evidence in the setting severely calcified lesions [Citation49].
5.4. IVL and rotablation (RA)
Until recently, rotational atherectomy (RA) was the principal tool for tackling high-grade CAC [Citation50]. The RA system is composed of an elliptical, diamond-coated burr mounted on an advancer drive-shaft and this is connected to a motor. The burr rotates at high speeds and effectively drills into the exposed surface of the calcified plaque. It preferentially targets the fibrocalcific component of the plaque. The microscopic debris that results ultimately travels into the distal vessel [Citation50]. Therefore, slow-flow or no-reflow can occur, at rates of up to 24% of cases [Citation51].
With regard to the literature, the DISRUPT CAD studies did not allow for the use of adjunctive tools for calcium modification [Citation1,Citation25,Citation26,Citation29]. However, in theory, an approach using both IVL and RA strategies may have a synergist effect and result in more optimal outcomes. Perhaps the greatest barrier with the use of IVL is the issue of crossability [Citation50]. In these cases, RA may be utilized to debulk luminal calcium and facilitate IVL balloon crossing [Citation52].
Jurado-Román et al. published a case report in 2019 of a 76-year-old man with severe diffuse calcific disease of the proximal-mid LAD [Citation53]. This included multiple areas of 360 degree calcification on IVUS. An initial PCI approach with NC balloons and RA was attempted. The lesion remained stubbornly un-dilatable. Therefore, the operators elected to use a 3 mm IVL balloon and 30 pulses were applied at the site. A further 50 pulses were delivered along the course of the target lesion. OCT confirmed deep fractures of the circumferential plaques. Also, visible in the OCT images were intimal microdissections as a result of the RA. Two DESs were then implanted, with OCT demonstrating excellent results. This case report illustrates a role for RA in allowing the IVL balloon to cross a tight calcified lesion and therefore facilitates the delivery of IVL shock waves to fracture deep-lying circumferential calcium.
Chen et al. also describe a case of combined rota-ablation and IVL for the treatment of ISR with severe neointimal calcification [Citation54]. The patient was a 61 year old man with a history of CABG and repeated PCI to the RCA. OCT demonstrated neoatherosclerosis and severe calcification between the first and the second overlapping stents. RA was performed in the first instance, allowing for the passage of the IVL balloon and delivery of shock wave therapy to good effect.
On a larger scale, the Aziz et al. study of 190 IVL treated patients included 34 cases of this combined RA and IVL approach, now termed ‘Rotatripsy’ [Citation35]. However, a subgroup analysis was not carried out for these patients.
Buono et al. published a retrospective study of 34 ‘Rotatripsy’ patients [Citation52]. RA was used prior to IVL in all cases, either during the index procedure (79%) or as a staged procedure during the same hospital admission. Procedural success was achieved in all cases. All-cause of death at 1 year was 9%, and there were two target vessel MIs in this period. There was three coronary perforations (successfully managed with DES or balloon inflation) and no major in-hospital adverse events.
In cases where RA is used as the initial strategy, upgrading from a low to high rotational speed may mitigate the need for the additional use of the IVL system. In one study of 100 patients, high-speed RA (190,000 rpm) was not associated with a higher rate of slow/no reflow when compared to low-speed (140,000 rpm) [Citation51].
5.5. IVL for left main (LM) disease
A retrospective study of 31 patients who received IVL for CAD involving the LM reported 100% successful PCI rate with no in-hospital adverse events [Citation55]. A single- or two-stent PCI strategy was used in 97% of cases. For one patient, a DEB only was used. The 30-day MACE was 3.2%, on account of 1 NSTEMI due to a plaque rupture in a non-target vessel.
A case series of three patients published in 2019 also showed good angiographic results following IVL in the setting of severely calcified unprotected LM disease [Citation56].
Rola et al. described a group of 16 patients with calcified unprotected LM disease who were managed with IVL [Citation57]. This followed an initial unsuccessful first-line strategy with NC balloons or RA. These patients were a high-risk group with an average Syntax score of 24. The indication for PCI was ACS in 62.5% of cases. Clinical success with successful stent delivery and residual stenosis <20% was achieved in all cases. There were 2 deaths reported including 1 case of fatal acute stent thrombosis at day 5 post-procedure.
5.6. IVL for CTO PCI
CTO PCI is associated with a higher risk of complications when compared to non-CTO PCI, particularly so for complex lesions (for example heavily calcified lesions) [Citation58,Citation59]. Fifty-five CTO PCI cases using IVL were identified from a large international muticenter registry [Citation60]. CTO PCI technical success was achieved in 96% of cases and procedural success in 93%. 5% had main vessel perforations and 4% had procedural MIs.
In a 2021 case series that included 5 patients with un-dilatable calcific CTOs, all 5 procedures were successful [Citation61]. At 30 day follow-up there no incidences of stent thrombosis, TLR or MACE. During 1 case the IVL balloon ruptured. However this did not result in any negative sequelae for the patient.
5.7. IVL in special circumstances
IVL has been also used in special off-label high-risk cases. Buono et al describe a case of 67 year old lady with an LVEF of 25% and a history of multiple coronary interventions and CABG [Citation62]. PCI was attempted to a critical calcific ostial lesion of the native LCx, with protruding stent struts from the ostium of the ramus. The operators elected to use IVL in combination with a mechanical circulatory support device to reduce the risk of hemodynamic compromise during this high-risk and lengthy procedure. The result was a successful implantation of a DES without any significant complications.
6. 5.8 ‘Shocktopics’ with IVL
Use of IVL in the clinical setting led to reports transient ventricular capture during the delivery of IVL therapy. This was noted by Cicovic et al. who report a case of 73 year old lady who presented with a non-ST elevation MI [Citation63]. While delivering IVL to the culprit vessel, a spike was seen on the ECG tracing with each shockwave pulse delivered. PCI was preformed to both the LAD and RCA with no adverse clinical outcomes.
In 2020 Wilson et al. published a retrospective, single center study in Eurointervention which described 54 PCI cases using IVL in the Royal Victoria Hospital, Belfast [Citation64]. The authors reported a 77.8% incidence of IVL-provoked ventricular ectopics and asynchronous cardiac pacing. There were no associated adverse clinical events reported, including ventricular tachyarrhythmias. There was a statistically significant association between resting heart rate and propensity to experience ventricular capture. Patients with a resting heart rate of <65 bpm were more likely to experience capture (odds ratio 16.3, p = 0.004). Ventricular capture was less common with IVL used in the LCx artery, however this did not reach statistical significance.
With regard to ventricular capture, hemodynamic data was also reported from the DISRUPT CAD III study, which enrolled a much greater number of patients (N = 431) [Citation1]. IVL-induced capture was seen in 41.1% of cases. Again, patients with lower heart rates (<60 bpm) were more susceptible. An association between number of IVL pulses delivered and also male sex was noted. There were no episodes of sustained ventricular arrhythmias in patients with ventricular capture. There was 1 reported episode of sustained ventricular arrhythmia in the group without capture. In patients with IVL-induced capture, decreased systolic blood pressure during the procedure was more common. However there was no difference in clinically significant blood pressure drops between the two groups.
7. Conclusion
Overall, the four DISRUPT CAD studies included a total of 628 patients. The investigators were successful in demonstrating that IVL use in the coronary vasculature is both safe and effective. This is true when compared to alternative strategies of calcium preparation [Citation14,Citation16,Citation28].
In addition to these sponsored studies, many groups from across Europe [Citation65,Citation66], North America [Citation67], New Zealand [Citation9], Singapore [Citation68] and even India [Citation69] have published studies on their own experience with IVL in the real-world setting (studies are summarized in ). Their findings, on the whole, corroborate the results of the initial trials. Outside of the licensed indication for IVL, these studies along with many case reports and case series, have shown successful use of the technology in more challenging settings and in conjunction with other interventional tools. With regard to higher levels of evidence, a meta-analysis published in 2020 confirmed the findings of significant improvement in the target vessel diameter to help facility stent delivery [Citation70].
Table 2. Summary of worldwide IVL studies by date published ACS = acute coronary syndrome, CAD = coronary artery disease, HR = heart rate, MI = myocardial infarction, NSTEMI = non-ST elevation myocardial infarction, LM = left main, CCS = chronic coronary syndrome, MACE = major adverse cardiac events. Values are n, percentage (%), median (Q1,Q3) or mean±SD
The growing body of evidence for IVL is a highlight of recent research in the field of interventional cardiology. This technology holds particular promise for a group of high-risk patients [Citation7] and for their treating cardiologists alike.
. Summary of worldwide IVL studies by date published
ACS = acute coronary syndrome, CAD = coronary artery disease, HR = heart rate, MI = myocardial infarction, NSTEMI = non-ST elevation myocardial infarction, LM = left main, CCS = chronic coronary syndrome, MACE = major adverse cardiac events.
Values are n, percentage (%), median (Q1,Q3) or mean±SD.
8. Expert opinion
As our population ages, it is likely that we will see a rise in the prevalence of complex calcific coronary lesions. In this older, generally more frail and co-morbid population, a strategy of minimally invasive PCI is typically preferred to surgery when feasible.
The simplicity of the IVL coronary system is a major advantage over its competitors. It comprises few components and is straightforward in its set-up and application (). The learning curve for techniques such as RA is certainly much steeper than that for IVL. The DISRUPT CAD III study revealed a procedural success rate of 92.4% despite reporting that most operators had no prior experience with the system [Citation1]. In any case, if IVL is to become standard practice for heavily calcified lesions, training in its use as well as comprehensive procedural guidelines need to be set out.
A striking advantage of IVL over other methods of calcium modification is that the acoustic shock waves selectively target calcium in the vessel wall with minimal damage inflicted to the more compliant, fibroelastic components. Both balloon angioplasty and atherectomy are subject to guidewire bias, which may result in a greater force (in the case of the former) or increased ablation of non-calcified segments of eccentric calcific lesions. Stent delivery may be improved but optimal stent expansion is limited due to deeper calcium deposits [Citation25]. In addition, higher inflation pressures naturally predispose to barotrauma and greater risk of vessel dissection. Therefore, when compared to these alternative methods of calcium modification, the ultrasonic energy waves of IVL can fracture deeper-lying calcium within the vessel wall while preserving the integrity of the soft tissue components [Citation1]. Lower inflation pressures also reduce the risk of vascular injury such as perforation.
As described above in relation to RA, slow flow and no-reflow are commonly observed with atherectomy techniques given their inherent mechanism of action [Citation50]. Incidences of atheromatous embolization are not frequently encountered in the IVL literature. Randomized controlled trials looking at the two strategies head-to-head will shed further light on this.
IVL remains a relatively novel technique in the field of interventional cardiology. Further research, particularly well-designed randomized control trials are needed going forward. A randomized study comparing a strategy of PCI with IVL versus PCI with atherectomy is the next logical step. As operators become more familiar with the armamentarium of tools that are now available for dealing with coronary calcium, adjunctive use of different tools will likely become commonplace.
Longer term follow-up, particularly of the larger trials, will provide us with more information regarding durability and late outcomes of IVL therapy, particularly in relation to ISR and need for revascularisation.
Further trials will help us to define which patients should be selected for IVL over an alternate approach to calcium modification. Thus far, trials have been largely limited to target lesions <32 mm (or <40 mm in DISRUPT CAD III) [Citation1]. IVL for complex lesions have not been evaluated to date. As mentioned above, the DISRUPT CAD III trial design excluded patients with extremely tortuous vessels, true bifurcation lesions, ostial lesions or unprotected left main lesions. Therefore, we cannot generalize the results of this trial to include these higher risk subgroups. DISRUPT CAD IV was the only trial in the series to include patients with bifurcation and trifurcation lesions (accounting for approximately one-third of patients) [Citation29]. Furthermore, IVL for the treatment of ISR has yet to be evaluated in a clinical trial setting, but with promising outcomes observed in real-world practice [Citation46,Citation47].
IVL in the setting of acute MI warrants further consideration. Stent underexpansion can prolong the primary PCI procedure and potentially place patients at risk of contrast-induced kidney injury and bleeding secondary to high levels of anticoagulants.
Patients with an LVEF of <25% were excluded from the DISRUPT CAD trials, and so we can not directly apply the results to this population. As these patients may not be suitable surgical candidates, it will be important to establish the safety and efficacy of IVL in this group. In instances where CABG carries too high of a risk, IVL guided PCI to severely calcific lesions would be an attractive alternative.
Going forward it would be prudent that studies use a more contemporary definition of MI than their predecessors. Understandably, the investigators of the DISRUPT CAD trials chose a definition using CK-MB to allow direct comparison to alternative strategies for calcium modification, for example, orbital atherectomy [Citation14]. However, in the modern era, we have access to more sensitive biomarkers of myocardial injury such as cardiac troponin I (cTnI) and T (cTnT). cTnI levels are elevated exclusively following myocardial injury, while cTnT assay can detect proteins expressed by injured skeletal muscle [Citation71]. An appropriate would ensure that the threshold level of biomarker elevation would have a strong evidence-based association with adverse clinical events [Citation72].
The fourth universal definition of a type 4a MI (PCI-related MI) was described in 2018, replacing the 2012 definition. It requires an elevation of cTn >5 times the 99th percentile URL in patients with a normal baseline level (or a > 20% rise in patients with raised pre-procedure levels that were stable or falling). This must be accompanied by ECG changes, evidence of ischemia on imaging or in cases when there was a known complication during the procedurefor example, vessel dissection [Citation71]. In DISRUPT CAD III, a sensitivity analysis was performed by the investigators using the contemporary definition [Citation1]. This analysis supported the results of the primary endpoint analyses. This definition should ideally be the universal standard used in all future major PCI trials.
Cost is a major factor that may limit the use of IVL in certain centers. The IVL coronary system is markedly more expensive than the likes of an NC balloon, and indeed cutting and scoring balloons. At the time of writing, an IVL unit retails at €1800.00. The cost of the generator is €12,000.00 (prices provided by Shockwave Medical Inc.). A comparison study in 2021 examined the costs and resource utilization of RA versus IVL [Citation73]. It is concluded that although the initial costs of IVL are substantial, these costs may be offset over time due to lower resource utilization. Fewer balloons, guidewires, guide catheters, guide extensions and DESs were used in IVL cases when compared to RA cases.
Kassimis et al., in a 2021 publication, compare the direct cost of the different devices used for calcified lesions [Citation74]. The NC balloon is reported as the cheapest option (although often multiple balloons are used for the one lesion) while OA, laser atherectomy and IVL are on the more expensive end of the scale. In addition to the direct cost of equipment, for a comprehensive cost analysis, indirect costs must also be factored in. These include the cost associated with complications, reinterventions, hospital stays and the impact on mortality. The savings made from devices not used (for example stents) is also an important consideration. IVL may prove to be superior from a cost perspective, given the relatively low complication rate of this therapy and the promising long-term outcomes.
The re-purposing of a technology that existed in the field of medicine for over 30 years, for an entirely new purpose, is ground-breaking and insightful. In addition to designing novel therapies, the field of interventional cardiology may benefit from our innovative researchers and industry partners looking elsewhere to discover unprecedented solutions to longstanding problems.
Article highlights
The DISRUPT CAD studies successfully demonstrated the safety and efficacy of intravascular lithotripsy (IVL) in the setting of de novo severely calcified coronary arteries prior to stent delivery.
These initial results have been backed-up by single and multicenter observational studies from around the world.
IVL has also been used outside of the licenced indication. Patients with acute coronary syndrome (ACS), stent underexpansion and in-stent restenosis (ISR) have received IVL therapy with good effect.
A complimentary approach, employing various strategies that target coronary artery calcification (CAC), may result in optimal patient outcomes.
Further evidence to support this novel therapy will likely result in it becoming commonplace in the cardiac catheterisation lab.
Declaration of Interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer Disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Hill JM, Kereiakes DJ, Shlofmitz RA, et al. Intravascular lithotripsy for treatment of severely calcified coronary artery disease. J Am Coll Cardiol. 76(22): 2635–2646. 2020.
- Allison MA, Criqui MH, Wright CM. Patterns and risk factors for systemic calcified atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24(2):331–336.
- Mintz GS, Popma JJ, Pichard AD, et al. Patterns of calcification in coronary artery disease. A statistical analysis of intravascular ultrasound and coronary angiography in 1155 lesions. Circulation. 1995;91(7):1959–1965.
- Sorini Dini C, Nardi G, Ristalli F, et al. Contemporary approach to heavily calcified coronary lesions. Interv Cardiol. 2019;14(3):154–163.
- Sage AP, Tintut Y, Demer LL. Regulatory mechanisms in vascular calcification. Nat Rev Cardiol. 2010;7(9):528–536.
- Xian JZ, Lu M, Fong F, et al. Statin effects on vascular calcification: microarchitectural changes in aortic calcium deposits in aged hyperlipidemic mice. Arterioscler Thromb Vasc Biol. 2021;41(4):e185–e92.
- Vliegenthart R, Oudkerk M, Hofman A, et al. Coronary calcification improves cardiovascular risk prediction in the elderly. Circulation. 2005;112(4):572–577.
- Bourantas CV, Zhang YJ, Garg S, et al. Prognostic implications of coronary calcification in patients with obstructive coronary artery disease treated by percutaneous coronary intervention: a patient-level pooled analysis of 7 contemporary stent trials. Heart. 2014;100(15):1158–1164.
- Wong B, El-Jack S, Newcombe R, et al. Shockwave intravascular lithotripsy for calcified coronary lesions: first real-world experience. J Invasive Cardiol. 2019;31(3):46–48.
- Mori S, Yasuda S, Kataoka Y, et al. Significant association of coronary artery calcification in stent delivery route with restenosis after sirolimus-eluting stent implantation. Circ J. 2009;73(10):1856–1863.
- Kobayashi Y, Okura H, Kume T, et al. Impact of target lesion coronary calcification on stent expansion. Circ J. 2014;78(9):2209–2214.
- Généreux P, Madhavan MV, Mintz GS, et al. Ischemic outcomes after coronary intervention of calcified vessels in acute coronary syndromes. Pooled analysis from the HORIZONS-AMI (harmonizing outcomes with revascularization and stents in Acute Myocardial Infarction) and ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy) TRIALS. J Am Coll Cardiol. 2014;63(18):1845–1854.
- Wiemer M, Butz T, Schmidt W, et al. Scanning electron microscopic analysis of different drug eluting stents after failed implantation: from nearly undamaged to major damaged polymers. Catheter Cardiovasc Interv. 2010;75(6):905–911.
- Chambers JW, Feldman RL, Himmelstein SI, et al. Pivotal trial to evaluate the safety and efficacy of the orbital atherectomy system in treating de novo, severely calcified coronary lesions (ORBIT II). JACC Cardiovasc Interv. 2014;7(5):510–518.
- Kini AS, Vengrenyuk Y, Pena J, et al. Optical coherence tomography assessment of the mechanistic effects of rotational and orbital atherectomy in severely calcified coronary lesions. Catheter Cardiovasc Interv. 2015;86(6):1024–1032.
- Tang Z, Bai J, Su SP, et al. Cutting-balloon angioplasty before drug-eluting stent implantation for the treatment of severely calcified coronary lesions. J Geriatr Cardiol. 2014;11(1):44–49.
- Lingeman JE, McAteer JA, Gnessin E, et al. Shock wave lithotripsy: advances in technology and technique. Nat Rev Urol. 2009;6(12):660–670.
- Talso M, Tefik T, Mantica G, et al. Extracorporeal shockwave lithotripsy: current knowledge and future perspectives. Minerva Urol Nefrol. 2019;71(4):365–372.
- Brodmann M, Werner M, Brinton TJ, et al. Safety and performance of lithoplasty for treatment of calcified peripheral artery lesions. J Am Coll Cardiol. 2017;70(7):908–910.
- Brodmann M, Werner M, Holden A, et al. Primary outcomes and mechanism of action of intravascular lithotripsy in calcified, femoropopliteal lesions: results of Disrupt PAD II. Catheter Cardiovasc Interv. 2019;93(2):335–342.
- Tepe G, Brodmann M, Werner M, et al. Intravascular lithotripsy for peripheral artery calcification: 30-day outcomes from the randomized disrupt PAD III trial. JACC Cardiovasc Interv. 2021;14(12):1352–1361.
- Kereiakes DJ, Virmani R, Hokama JY, et al. Principles of intravascular lithotripsy for calcific plaque modification. JACC Cardiovasc Interv. 14(12): 1275–1292. 2021.
- Ali ZA, Brinton TJ, Hill JM, et al. Optical coherence tomography characterization of coronary lithoplasty for treatment of calcified lesions: first description. JACC Cardiovasc Imaging. 2017;10(8):897–906.
- Shockwave Medical I. Shockwave intravascular lithotripsy (IVL) system with the shockwave C2 coronary intravascular lithotripsy (IVL) catheter instructions for use (IFU). Food and Drug Administration; 2021. [cited 2022 Jan 22]. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf20/P200039C.pdf
- Brinton TJ, Ali ZA, Hill JM, et al. Feasibility of shockwave coronary intravascular lithotripsy for the treatment of calcified coronary stenoses. Circulation. 2019;139(6):834–836.
- Ali ZA, Nef H, Escaned J, et al. Safety and effectiveness of coronary intravascular lithotripsy for treatment of severely calcified coronary stenoses: the disrupt CAD II study. Circ Cardiovasc Interv. 2019;12(10):e008434.
- Hill J, Kereiakes D, Stone G. TCT-1 intravascular lithotripsy for treatment of severely calcified coronary lesions: one-year results from the disrupt CAD III study. J Am Coll Cardiol. 2021;78(19_Supplement_S):B1–B.
- Généreux P, Lee AC, Kim CY, et al. Orbital atherectomy for treating de novo severely calcified coronary narrowing (1-year results from the pivotal ORBIT II trial). Am J Cardiol. 2015;115(12):1685–1690.
- Saito S, Yamazaki S, Takahashi A, et al. Intravascular lithotripsy for vessel preparation in severely calcified coronary arteries prior to stent placement - primary outcomes from the japanese disrupt CAD IV study. Circ J. 2021;85(6):826–833.
- Hisamatsu T, Liu K, Chan C, et al. Coronary artery calcium progression among the US and Japanese men. Circ Cardiovasc Imaging. 2019;12(2):e008104.
- Bryniarski KL, Yamamoto E, Sugiyama T, et al. Differences in coronary plaque morphology between East Asian and western white patients: an optical coherence tomography study. Coron Artery Dis. 2018;29(7):597–602.
- Kohsaka S, Kimura T, Goto M, et al. Difference in patient profiles and outcomes in Japanese versus american patients undergoing coronary revascularization (collaborative study by CREDO-Kyoto and the texas heart institute research database). Am J Cardiol. 2010;105(12):1698–1704.
- Wong B, El-Jack S, Newcombe R, et al. Calcified coronary lesions treated with intravascular lithotripsy: one-year outcomes. J Invasive Cardiol. 2020;32(7):E200–E1.
- Aksoy A, Salazar C, Becher MU, et al. Intravascular lithotripsy in calcified coronary lesions: a prospective, observational, multicenter registry. Circ Cardiovasc Interv. 2019;12(11):e008154.
- Aziz A, Bhatia G, Pitt M, et al. Intravascular lithotripsy in calcified-coronary lesions: a real-world observational, European multicenter study. Catheter Cardiovasc Interv. 98(2): 225–235. 2021.
- El Jattari H, Holvoet W, De Roeck F, et al. Intracoronary lithotripsy in calcified coronary lesions: a multicenter observational study. J Invasive Cardiol. 2022;34(1):E24–E31.
- Rola P, Włodarczak A, Kulczycki JJ, et al. Feasibility of the intravascular lithotripsy in coronary artery disease. short-term outcomes of the lower-silesia shockwave registry. Kardiol Pol. 2021;79(10):1133–1135.
- Cosgrove C, Hanratty CG, Hill JM, et al. Intravascular lithotripsy for treatment of calcific coronary lesions in ST elevation myocardial infarction. Catheter Cardiovasc Interv. 2021;99(2):322–328.
- Prati F, Kodama T, Romagnoli E, et al. Suboptimal stent deployment is associated with subacute stent thrombosis: optical coherence tomography insights from a multicenter matched study. from the CLI foundation investigators: the CLI-THRO study. Am Heart J. 2015;169(2):249–256.
- Kang SJ, Mintz GS, Park DW, et al. Mechanisms of in-stent restenosis after drug-eluting stent implantation: intravascular ultrasound analysis. Circ Cardiovasc Interv. 2011;4(1):9–14.
- Seif S, Kumar A, Arya S, et al. Intravascular lithotripsy to treat an underexpanded coronary stent during index procedure: a case report study. Avicenna J Med. 2021;11(1):54–57.
- Włodarczak S, Rola P, Barycki M, et al. Successful shockwave intravascular lithotripsy of an underexpanded stent after a month from primary implantation. Kardiol Pol. 2022;80(3):359–360.
- Yeoh J, Cottens D, Cosgrove C, et al. Management of stent underexpansion using intravascular lithotripsy-Defining the utility of a novel device. Catheter Cardiovasc Interv. 2021;97(1):22–29.
- Ielasi A, Moscarella E, Testa L, et al. IntravaScular lithotripsy for the management of undilatable coronary stEnt: the SMILE registry. Cardiovasc Revasc Med. 21(12): 1555–1559. 2020.
- Brunner FJ, Becher PM, Waldeyer C, et al. Intravascular lithotripsy for the treatment of calcium-mediated coronary in-stent restenoses. J Invasive Cardiol. 2021;33(1):E25–E31.
- Szolc P, Guzik B, Wiewiórka Ł, et al. Intravascular lithotripsy for the treatment of a heavily calcified recurrent in-stent restenosis in patient with chronic coronary syndrome. Kardiol Pol. 2021;79(10):1159–1160.
- Salazar C, Escaned J, Tirado G, et al. Intravascular lithotripsy for recurrent restenosis caused by severe calcific neoatherosclerosis. EuroIntervention. 2020;16(4):e351–e2.
- Yousif N, Bardooli F, Hussain T, et al. Precision percutaneous coronary intervention of a complex lesion. Rev Recent Clin Trials. 2021;16(2):220–224.
- Lee T, Shlofmitz RA, Song L, et al. The effectiveness of excimer laser angioplasty to treat coronary in-stent restenosis with peri-stent calcium as assessed by optical coherence tomography. EuroIntervention. 2019;15(3):e279–e88.
- De Maria GL, Scarsini R, Banning AP. Management of calcific coronary artery lesions: is it time to change our interventional therapeutic approach? JACC Cardiovasc Interv. 2019;12(15):1465–1478.
- Sakakura K, Funayama H, Taniguchi Y, et al. The incidence of slow flow after rotational atherectomy of calcified coronary arteries: a randomized study of low speed versus high speed. Catheter Cardiovasc Interv. 2017;89(5):832–840.
- Buono A, Basavarajaiah S, Choudhury A, et al. “RotaTripsy” for severe calcified coronary artery lesions: insights from a real-world multicenter cohort. Cardiovasc Revasc Med. 2021;37:78–81.
- Jurado-Román A, Gonzálvez A, Galeote G, et al. RotaTripsy: combination of rotational atherectomy and intravascular lithotripsy for the treatment of severely calcified lesions. JACC Cardiovasc Interv. 2019;12(15):e127–e9.
- Chen G, Zrenner B, Pyxaras SA. Combined rotational atherectomy and intravascular lithotripsy for the treatment of severely calcified in-stent neoatherosclerosis: a mini-review. Cardiovasc Revasc Med. 2019;20(9):819–821.
- Cosgrove CS, Wilson SJ, Bogle R, et al. Intravascular lithotripsy for lesion preparation in patients with calcific distal left main disease. EuroIntervention. 2020;16(1):76–79.
- Wong B, El-Jack S, Khan A, et al. Treatment of heavily calcified unprotected left main disease with lithotripsy: the First Case Series. J Invasive Cardiol. 2019;31(6):E143–E7.
- Rola P, Włodarczak A, Kulczycki JJ, et al. Efficacy and safety of shockwave intravascular lithotripsy (S-IVL) in calcified unprotected left main percutaneous coronary intervention - short-term outcomes. Postepy Kardiol Interwencyjnej. 2021;17(4):344–348.
- Riley RF, Sapontis J, Kirtane AJ, et al. Prevalence, predictors, and health status implications of periprocedural complications during coronary chronic total occlusion angioplasty. EuroIntervention. 2018;14(11):e1199–e206.
- Karacsonyi J, Karmpaliotis D, Alaswad K, et al. Impact of calcium on chronic total occlusion percutaneous coronary interventions. Am J Cardiol. 2017;120(1):40–46.
- Øksnes A, Cosgrove C, Walsh S, et al. Intravascular lithotripsy for calcium modification in chronic total occlusion percutaneous coronary intervention. J Interv Cardiol. 2021;2021:9958035.
- Rola P, Włodarczak A, Barycki M, et al. Shockwave intravascular lithotripsy as a novel strategy for balloon undilatable heavily calcified chronic total occlusion lesions. Cardiol J. 2021. https://doi.org/10.5603/CJ.a2021.0112.
- Buono A, Ielasi A, De Blasio G, et al. “Shock-pella”: combined management of an undilatable ostial left circumflex stenosis in a complex high-risk interventional procedure patient. Cardiol J. 2020;27(4):427–428.
- Cicovic A, Cicovic S, Wong B, et al. A quicker pace: shockwave lithotripsy pacing with electromechanical capture. JACC Cardiovasc Interv. 2019;12(17):1739–1740.
- Wilson SJ, Spratt JC, Hill J, et al. Incidence of “shocktopics” and asynchronous cardiac pacing in patients undergoing coronary intravascular lithotripsy. EuroIntervention. 2020;15(16):1429–1435.
- Iwańczyk S, Włodarczak A, Hiczkiewicz J, et al. Feasibility of intravascular lithotripsy for calcific coronary lesions: a multi-institutional experience. Catheter Cardiovasc Interv. 2021;98(4):E540–E7.
- Sinclair H, Fan L, Fahy E, et al. Intravascular imaging–guided intracoronary lithotripsy: first real-world experience. Health Sci Rep. 2021;4(3):e307.
- Wiens EJ, Sklar JC, Wei YH, et al. Real-world outcomes in treatment of highly calcified coronary lesions with intravascular shockwave lithotripsy. Indian Heart J. 2021;73(5):653–655.
- Umapathy S, Keh YS, Wong N, et al. Real-World Experience of Coronary Intravascular Lithotripsy in an Asian Population: a Retrospective, Observational, Single-Center, All-Comers Registry. J Invasive Cardiol. 2021;33(6):E417–E24.
- Rao RS, Sharma GN, Kunal S, et al. Safety and procedural outcomes of intravascular lithotripsy in calcified coronaries in Indian patients. Indian Heart J. 2022;74(2):91–95.
- Sattar Y, Ullah W, Mir T, et al. Safety and efficacy of coronary intravascular lithotripsy for calcified coronary arteries- a systematic review and meta-analysis. Expert Rev Cardiovasc Ther. 2021;19(1):89–98.
- Thygesen K , Alpert J , Jaffe AS et al. Fourth universal definition of myocardial infarction. Rev Esp Cardiol. 2018;72:1–72.
- Moussa ID, Klein LW, Shah B, et al. Consideration of a new definition of clinically relevant myocardial infarction after coronary revascularization: an expert consensus document from the Society for Cardiovascular Angiography and Interventions (SCAI). J Am Coll Cardiol. 2013;62(17):1563–1570.
- Rishad S, McENTEGART M, Ford TJ, et al. Comparative study of costs and resource utilisation of rotational atherectomy versus intravascular lithotripsy for percutaneous coronary intervention. Minerva Cardiol Angiol. 2021. https://doi.org/10.23736/S2724-5683.21.05681-7.
- Kassimis G, Ziakas A, Didagelos M, et al. Shockwave coronary intravascular lithotripsy system for heavily calcified de novo lesions and the need for a cost-effectiveness analysis. Cardiovasc Revasc Med. 2021;37:128–134 .