?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Taiwan is currently in the process of demonstrating the feasibility and capability of deep geological disposal of the country’s spent nuclear fuel. The current concept is based on the Swedish KBS-3 approach, suitably modified for Taiwanese conditions. A key component of the analysis is an assessment of the long-term corrosion behaviour of the copper canister. Here, the extent of corrosion due to the effects of radiation, the initially trapped oxygen, and sulphide is assessed and the canister lifetime predicted.
This paper is part of a supplement on the 6th International Workshop on Long-Term Prediction of Corrosion Damage in Nuclear Waste Systems.
Introduction
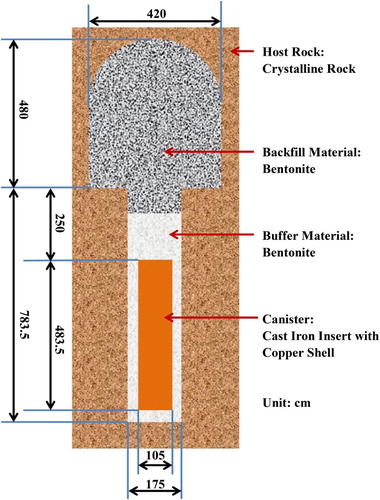
The Taiwan Power Company (Taipower) currently has three nuclear power plants in operation with a total installed capacity of 5.1 MW (as of 2015). Taipower is assessing the feasibility and capability for deep geological disposal in Taiwan based on the Swedish KBS-3 design () [Citation1,Citation2]. In collaboration with the Institute of Nuclear Energy Research (INER), a reference case (SNFD2017) is being developed to demonstrate the feasibility of the concept. The assessment is based on the assumption of the need to dispose of 4900 tonnes of U (a total of approximately 23000 BWR and PWR spent fuel assemblies) in 2500 copper canisters.
In the overall multi-barrier concept, the canister is the first important barrier for the disposal of spent nuclear fuel (SNF). The main purpose of the canister is to prevent or delay contact of the SNF by ground water and the resultant release of radionuclides. However, in a geological disposal environment, the canister will be subject to corrosion and it is necessary to assess the rate and extent of corrosion over long periods of time with the aim of confirming the primary safety function of the canister.
The main causes of canister corrosion can be divided into the following [Citation3]:
Effect of radiation
During the repository saturation phase, moist air may be present in the canister-buffer gap. Gamma irradiation of moist air leads to the formation of nitric acid, which could dissolve in the pore-water of the buffer material and in thin moisture films on the canister surface resulting in corrosion.
After the buffer material has saturated, radiolysis of the bentonite pore-water near the canister is possible, leading to the formation of H2 and oxidising radiolysis products.
| (2) | Effect of oxygen | ||||
During handling, emplacement and before repository closure, the canister will be exposed to atmospheric conditions for a short period of time leading to the formation of copper oxide corrosion products.
During the early stages after the closure of the deposition tunnel, oxygen trapped initially in the pores of the buffer and backfill materials will be the major source of oxidant in the repository.
| (3) | Effect of sulphide | ||||
In the presence of sulphide (and some other sulphur-containing species [Citation4]), copper corrodes accompanied by the evolution of hydrogen [Citation5]. Three separate sources of sulphide are considered in the assessment:
It is conservatively assumed that pyrite in the buffer and backfill materials dissolves in ground water as sulphide after the buffer material has saturated.
Sulphate-reducing bacteria (SRB) can reduce the sulphate in buffer and backfill materials and groundwater to produce sulphide, which can then be transported to the canister surface to support corrosion.
Ground water at the proposed repository depth in Taiwan contains sulphide, which can be transported to the canister surface by diffusion and/or advection.
Assessment methods
A well-established approach was used to develop the corrosion assessment and comprised the following steps:
Inclusion of all corrosion processes: a formal review of all features, events and processes (FEPs) was conducted which, along with expert judgement and other information, was used to define all corrosion mechanisms that needed to be addressed in the assessment.
Establishment of knowledge base: for each of the corrosion mechanisms identified in the previous step, fundamental physical and chemical mechanistic information was reviewed and documented.
Decision of the essential parameters and the corrosion processes: based on these analyses, the corrosion mechanisms and associated parameters that were to be handled quantitatively in the corrosion assessment were selected.
Calculation of the corrosion: appropriate methods of calculation were selected based on the corrosion mechanisms involved.
All corrodents present in the disposal environment, and the associated corrosion mechanisms were identified, the most important being O2, HS−, and radiolysis products resulting in uniform (general) corrosion of the canister. General corrosion (by O2 and HS−) was handled using mass-balance or mass transport arguments. Localised corrosion and stress corrosion cracking (SCC) were excluded from the quantitative analysis based on literature evidence that these processes would either not occur or would be of limited extent in the disposal environment [Citation5,Citation6]. In the case of localised corrosion, the pore-water composition is expected to promote active dissolution during the early aerobic transient period [Citation6,Citation7]. In the absence of passivity, any localised attack will take the form of surface roughening rather than discrete pitting [Citation5]. For SCC, a combination of (i) the absence from the repository environment of species known to cause cracking (such as ammonium, nitrite, and acetate ions), (ii) corrosion potential and interfacial pH values below the threshold for SCC, and (iii) the generally compressive stress regime is considered to result in a low probability of cracking [Citation5]. These arguments against pitting and SCC apply generally to repository designs in which bentonite backfill is used, be it a repository in Taiwan or one in Scandinavia. Thus, the assessment is based on a combination of reasoned arguments for the exclusion of some corrosion processes and a quantitative analysis for other processes considered likely to occur in the repository.
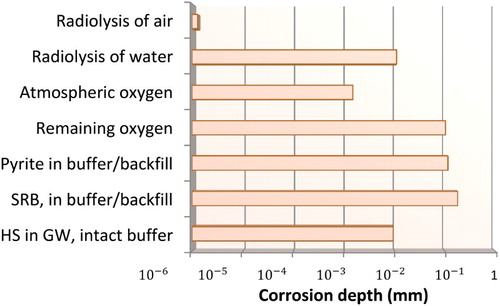
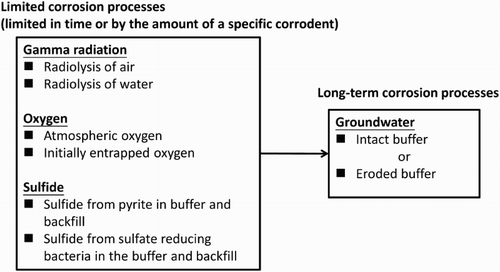
The processes considered quantitatively in the assessment are summarised in , where they are characterised as either ‘limited corrosion processes’ or ‘long-term corrosion processes’. Processes such as the radiolysis of water, radiolysis of air, and atmospheric corrosion are considered to be limited corrosion processes as they occur for a short period of time. The extent of radiolytic corrosion is estimated based on the integrated absorbed dose and an effective G-value for the production of HNO3 for the radiolysis of air or radiolytic oxidants for the radiolysis of water [Citation3]. Atmospheric corrosion was assumed to occur at a rate of 0.5 µm/y [Citation3].
Figure 2. Corrosion assessment methodology dividing the various forms of corrosion into limited or long-term processes.
Other processes, such as corrosion due to the initially trapped O2 or the transport of sulphide produced by the dissolution of pyrite or SRB activity, are also considered to be limited because either the amount (in the case of O2 and HS− from pyrite dissolution) or the rate of production (in the case of HS− produced by SRB activity) is limited. The extent of corrosion due to the trapped O2 was estimated based on mass-balance arguments. The extent of corrosion due to HS− from anaerobic pyrite dissolution was estimated based on the rate of diffusive transport and an assumed equilibrium pore-water [HS−] of 3.8 × 10−9 mol L−1 [Citation3]. Finally, SRB activity in the buffer and backfill material was assumed to result in the formation of Cu2S at a rate equivalent to 3.4 × 10−12 mol cm−2 d−1 [Citation3].
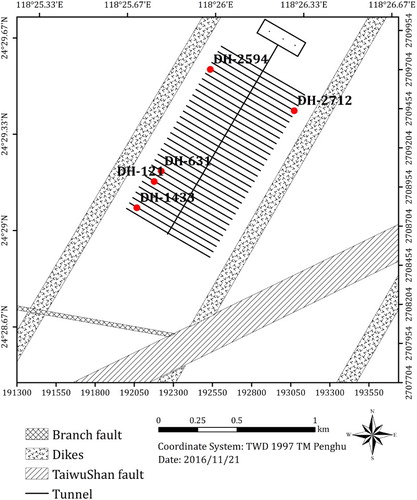
Long-term corrosion processes are defined by the duration over which corrosion may occur and, of those processes considered to occur in the repository, is limited here to the supply of HS− from the ground water. Two scenarios are considered: the diffusive transport of sulphide through intact bentonite and advective transport through bentonite that has undergone chemical erosion and a resulting loss of density. The treatment of the diffusive and advective transport regimes and the impact of the ground water flow rate and the size of the fracture aperture were similar to those developed by SKB for SR-Site [Citation3] and will not be repeated here. For the case of eroded bentonite, a discrete fracture network (DFN) model was developed for the SNFD2017 reference case using data from a suitable test site [Citation8] and used to predict the deposition hole locations within the repository with the highest Darcy velocities ().
Figure 3. Plan view of the conceptual repository layout highlighting the five deposition hole (DH) locations exhibiting the highest Darcy flux.
For these long-term corrosion processes, as well as for the limited supply of HS− from the dissolution of pyrite, the interfacial corrosion reaction is assumed to be fast with the rate limited by the rate of supply of sulphide to the canister surface. Thus, the corrosion rate was calculated from the interfacial flux of sulphide based on the overall stoichiometry
Assessment results and discussions
The assessment of the canister lifetime was conducted as follows:
Estimate the depth of corrosion for each of the limited processes (over a period of one million years for those processes not limited by the amount of corrodent).
Calculate the remaining canister wall thickness (based on an assumed corrosion allowance of 47 mm of the nominal canister wall thickness of 50 mm, corresponding to the minimum wall thickness from pilot canister fabrication studies [Citation3]).
Estimate the time to corrode the remaining wall thickness based on the rates of the long-term corrosion processes involving sulphide transport for intact and eroded bentonite.
The estimate of the depths of corrosion for each of the limited corrosion processes were:
Radiolysis of moist air: gamma irradiation of the 5 mm air gap around the canister was estimated to produce 0.0075 mol HNO3 (for an assumed half-life of 30 years and a G-value 0.02 molcules/eV), resulting in an estimated depth of corrosion of 1.3 nm for the assumed stoichiometry
Radiolysis of water: by analogy with the extent of oxidation of Fe(II) to Fe(III) due to irradiation in aqueous solution [Citation3], the amount of oxidant produced over 10 half-lives (approximately 300 years) is estimated to be approximately 28 mol, which would corrode an equal number of moles of Cu, resulting in a uniform corrosion depth of 0.011 mm.
Atmospheric oxygen: for a maximum exposure period of 3 years and the assumed atmospheric corrosion rate of 0.5 µm/y, the estimated depth of atmospheric corrosion is 0.0015 mm.
Initially trapped oxygen: based on the volumes of buffer and backfill material per canister and the respective initial degree of saturation, the inventory of trapped atmospheric O2 per canister was estimated to be 19.2 and 335 mol for the buffer and backfill materials, respectively. Consistent with the treatment in SR-Site [Citation3], it is argued that only 50% of the O2 in the deposition hole will diffuse to the canister surface, the remaining 50% diffusing towards the rock where it will be consumed. Similarly, it is likely that the vast majority of O2 in the backfill will be consumed by processes other than canister corrosion, such as pyrite oxidation and microbial aerobic respiration. It is assumed that only a fraction of the O2 in the backfill diffuses to the canister, with the proportion equivalent to the fractional cross-sectional area of the deposition hole compared with that of the disposal tunnel. If it assumed that corrosion from the O2 in the deposition hole is uniformly distributed over the entire canister surface, but that from the backfill is limited to the upper 10% of the canister surface, the predicted depths of corrosion are 0.0865 mm and 0.0155 mm for O2 from the backfill and buffer, respectively, giving a maximum total depth of corrosion of 0.102 mm.
Sulphide from pyrite dissolution: the amount of sulphide produced by the anaerobic dissolution of pyrite that reaches the canister surface in one million years was estimated based on the effective diffusivity of HS− in saturated bentonite (1.2 × 10−10 m2 s−1) and the assumed solubility of pyrite (3.8 × 10−9 mol L−1) [Citation3]. Taking into account the finite amount of pyrite in the buffer (0.0374 wt-% for MX-80 bentonite), the estimated depth of corrosion from this source of sulphide is 0.114 mm in one million years.
Sulphide from SRB activity: based on an experimental rate of formation of copper sulphide of 3.4 × 10−12 mol cm−2 d−1 for coupons embedded in compacted bentonite (density 2 Mg m−3) and exposed to a source of organic C, the estimated depth of corrosion due to microbial activity in the buffer and backfill is 0.177 mm after one million years. This estimate assumes that microbial activity occurs at all times and is not limited by the availability of either organic nutrients or electron acceptors (sulphate ions).
The results of the above analyses of the limited corrosion processes are summarised in . Thus, a conservative estimate of the depth of corrosion due to these processes over a one million year period is <1 mm.
Table 1. Estimated depth of corrosion after one million years for the various limited corrosion processes considered in the assessment.
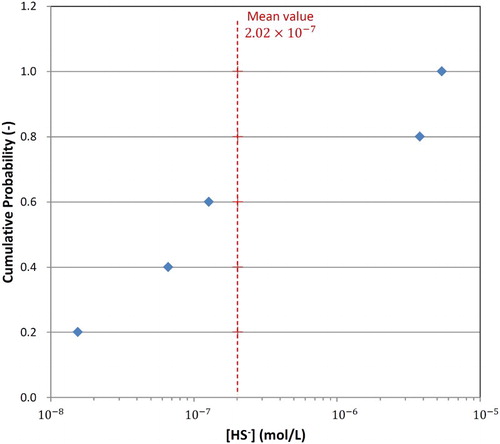
For the assessment of the extent of corrosion due to the long-term supply of HS− from the ground water (), we must first establish the likely concentration of sulphide in the ground water at repository depth. shows the cumulative distribution of sulphide concentrations measured at a depth of 400–500 m in five boreholes in a test area close to Taiwan. The mean sulphide concentration is 2 × 10−7 mol L−1, with a maximum measured concentration of 5.4 × 10−6 mol L−1, considered here to represent a conservative upper bound for the corrosion assessment.
Figure 4. Cumulative distribution of the ground water sulphide concentration based on borehole measurements at a depth of 400–500 m in a region close to Taiwan.
A DFN model was used to estimate the variation in Darcy velocity at various locations in the conceptual repository (). Deposition hole DH-631 was identified as the location with the highest Darcy flux (equivalent to a volumetric ground water flow rate of 0.0119 m3/y, ) and, therefore, the location with the highest corrosion rate.
Table 2. Predicted bentonite erosion times and resulting extent of corrosion in the case of eroded bentonite for the five deposition holes with the highest Darcy flux.
For the case of intact buffer, the flow of ground water through a fracture intersecting the borehole is translated to a corresponding sulphide diffusive flux at the canister surface using a concept developed by SKB based on equivalent flow rates [Citation3]. The same approach was used here and results in a depth of corrosion of only 0.00936 mm after one million years for the highest sulphide concentration of 5.4 × 10−6 mol L−1, largely because the 35-cm-wide highly compacted bentonite diffusion barrier is still in place.
Since the total amount of corrosion due to the limited corrosion processes and the long-term supply of HS− through intact buffer is <1 mm (), canister failure in the absence of eroded bentonite is unlikely to occur in a period of less than one million years.
Higher sulphide fluxes, and shorter canister lifetimes, are possible if the bentonite is chemically eroded by the presence of dilute ground waters. Chemical erosion of the bentonite by waters with a low ionic strength can result in a loss of material and reduction in density, with a corresponding increase in hydraulic conductivity and the possibility of advective transport in the buffer [Citation1]. SKB have developed a procedure for estimating the time required to erode the bentonite and the resulting increase in sulphide flux in the eroded buffer, details of which can be found elsewhere [Citation3]. The same procedure was followed here to predict the erosion time and canister lifetime, with results for the five deposition holes exhibiting the highest Darcy flux summarised in . The onset of advective transport in the bentonite is estimated to occur after approximately 200 000 years, with an estimated depth of corrosion of 1.2–1.7 mm after one million years for the upper bound ground water sulphide concentration. Thus, even in the event of buffer erosion, no canister corrosion failures are predicted in times less than 106 y. This conclusion is in line with that reached by SKB for the SR-Site assessment [Citation3]. In that case, failure of the canister was only predicted to occur for a small subset of the possible range of conditions involving eroded bentonite. In particular, canister failure in times less than 106 y was predicted only for the highest sulphide concentration of 1.2 × 10−4 mol L−1 (a factor of 20 times higher than that considered here) and/or the highest ground water flux of 0.251 m3/y (also a factor of 20 times higher than that considered here).
Conclusions
A corrosion assessment has been conducted for copper canisters in a reference case for a deep geological repository in Taiwan. The repository design and assessment methodology are based on those developed by SKB for the KBS-3 concept and the SR-Site safety assessment.
Various corrosion processes have been taken into account in the assessment, focussed on general corrosion due to different radiolytic species, the initially trapped O2 in the buffer and backfill, and due to sulphide formed by the anaerobic dissolution of pyrite, microbial activity in the bentonite, and transport from the ground water through either intact or eroded bentonite. The methodologies developed for SR-Site have been adapted for the Taiwanese repository design, supplemented by information on local geochemical and hydrogeological conditions. It is concluded that copper canisters disposed of according to the KBS-3 concept will not fail by corrosion in periods less than one million years.
The assessment has demonstrated the feasibility and capability of deep geological disposal of spent nuclear fuel in Taiwan based on the KBS-3 concept.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Swedish Nuclear Fuel and Waste Management Company. Longterm safety for the final repository for spent nuclear fuel at Forsmark; 2011. (Main Report of the SR-Site project, Swedish Nuclear Fuel and Waste Management Company Report, SKB TR 11-01).
- Swedish Nuclear Fuel and Waste Management Company. Fuel and canister process report for the safety assessment SR-Site; 2010. (Swedish Nuclear Fuel and Waste Management Company Report, SKB TR-10-46).
- Swedish Nuclear Fuel and Waste Management Company. Corrosion calculations report for the safety assessment SR-Site; 2010. (Swedish Nuclear Fuel and Waste Management Company Report, SKB TR 10-66).
- Macdonald DD, Sharifi-Asl S. Is copper immune to corrosion when in contact with water and aqueous solutions? 2011. (Swedish Radiation Safety Authority Research Report 2011:09).
- King F, Lilja C, Pedersen K, et al. An update of the state-of-the-art report on the corrosion of copper under expected conditions in a deep geologic repository; 2010. (Swedish Nuclear Fuel and Waste Management Company Report, SKB TR-10-67).
- King F, Lilja C. Localised corrosion of copper canisters. Corros Eng Sci Technol. 49:420–424. doi: 10.1179/1743278214Y.0000000182
- Qin Z, Deljeet R, Ai M, et al. The active/passive conditions for copper corrosion under nuclear waste repository environment. Corros Eng Sci Technol. 2017. doi:10.1080/1478422X.2016.1274088.
- Industrial Technology Research Institute, The Taiwan Reference Case of the SNFD2017 Report – Table-2. Geological conceptual models and characteristic data; 2015. (ITRI report for Taiwan Power Company, SNFD-ITRI-TR2015-0001).