Abstract
Two new sesquiterpene glycosides, namely massonside A (1) and massonside B (2), were isolated from the n-Bu extract of the fresh needles of Pinus massoniana Lamb. Their structures were established by 1D, 2D nuclear magnetic resonance and high-resolution mass spectrometry. Their biological activities were profiled by the anti-HBV and anti-HCV assays.
1. Introduction
Pinus massoniana Lamb. (P. massoniana), is distributed throughout southern China. The extract of fresh needles of P. massoniana is the main component of Songling Xuemaikang capsule. The leaves of P. massoniana are recorded in the Dictionary of Chinese Materia Medica, and have been used as a folk herbal medicine for centuries. Recent pharmacological studies revealed that the needles of P. massoniana possess antioxidant (Chen et al. Citation2014), antibacterial (Feng et al. Citation2010), cardiovascular protection (Wang et al. Citation2008), lipid regulation (Zheng et al. Citation2010), and antitumour (Zheng et al. Citation2009) properties. Up to now, volatile oil (Yatagai & Hong Citation1997), lignans (Lundgren et al. Citation1985), flavonoids (Shen & Theander Citation1985; Patel et al. Citation2016), shikimate (Ma et al. Citation2008), proanthocyanidins (Gao et al. Citation2011; Shen et al. Citation2010) and their derivatives were isolated from P. massoniana. In our phytochemical investigation, two new sesquiterpene glycosides, compounds 1 and 2, were isolated from the title plant. In their structures, a bullatantriol and a trihydroxy endesmane skeleton each connected a glucose via a C–O bond. This paper described the isolation and structure elucidation of the new compounds 1 and 2, along with their anti-HBV and anti-HCV activities.
2. Results and discussion
2.1. New compound structure elucidation
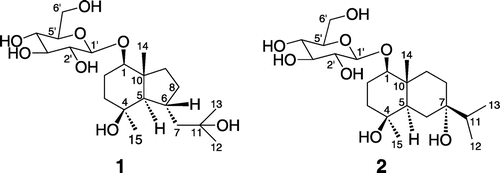
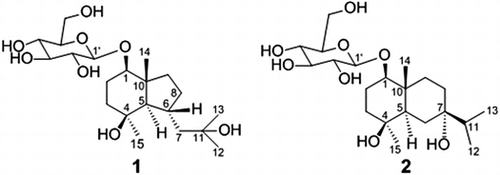
Purification of the EtOH-H2O (50 : 50, v/v) extract of the fresh needles of P. massoniana, using combination of silica gel, ODS, SBC MCI gel and Sephadex LH-20 column chromatography, gave two new sesquiterpene glycosides 1 and 2 (Figure ).
The fresh needles of P. massoniana were extracted with EtOH-H2O (50 : 50, v/v) two times. The combined extracts were concentrated by rotary evaporator and suspended in water, then partitioned with petroleum ether (PE), AcOEt and n-Bu successively. The n-Bu part was subjected to repeated macroporous resin D101, silica-gel, Sephadex G25, Sephadex LH-20, ODS and SBC MCI gel column chromatography to afford the compounds 1 and 2 (Figure ). The structures were established by various spectroscopic analyses and chemical studies.
Compound 1 was obtained as a white amorphous powder. The IR spectrum indicated the presence of OH (3411 cm−1). The molecular formula was determined as C21H38O8 based on the HR-ESI-MS data (m/z 441.2451 ([M + Na]+)), besides the HR-ESI-MS gave fragment ions at m/z 383.2408 [M + H-2H2O]+ and 221.1899 ([M + H-2H2O-C6H10O5]+), indicating the potential presence of one hexose unit.
The 13C NMR (Table S1) data combined with analysis of the heteronuclear multiple quantum coherence (HMQC) of 1 revealed the remaining 21 carbon signals due to four methyls, six methylenes, eight methines and three quaternary carbons, of which 15 were assigned to the aglycone, and the remaining six were ascribed to a glucopyranosyl unit at δC 101.65–61.77 ppm. Further assignments of all hydrogen and carbon signals were achieved by its HMQC, 1H–1H COSY and HMBC spectra (Figure S1(a)). The hexose was suggested to be a d-glucose by the comparison of data with those reported in the literature (Zhao et al. Citation2008). The coupling constant of H-1′ (δH 4.15, 1H, d, J = 7.8 Hz) indicated that the d-glucose was a β-linkage. The aglycone spectral features were closely related to those of bullatantriol (Xie et al. Citation2012), except that the chemical shift of C-1 shifted downfield for 7.9 ppm, and the correlation of δH 4.15 (H-1′) with C-1 (δC 86.13) and δH 3.30 (H-1) with C-1′ (δC 101.65) was observed, which suggested that C-1 of 1 should be glycosylated.
The NOESY (Figure S1(b)) correlations of H-5/H-1, H5/H-15 and H-6/H-14 suggested that the H-1, H-5 and Me-15 were of α-orientation, H-6 and Me-14 were of β-orientation. Thus, the structure of compound 1 was established as bullatantriol-1-O-β-d-glucopyranoside.
Compound 2 was obtained as a white amorphous powder. The IR spectrum suggested the presence of OH (3427 cm−1). The molecular formula was determined to be C21H38O8, based on the HR-ESI-MS data (m/z 441.2446 ([M + Na]+)), besides the HR-ESI-MS gave fragment ions at m/z 383.2423 [M + H-2H2O]+ and 221.1904 ([M + H-2H2O-C6H10O5]+), indicating the potential presence of one hexose unit.
The 1H, 13C NMR, HMQC and HMBC data of 2 (Table S1 and Figure S2 (a)) showed the presence of four methyls, six methylenes, eight methines and three quaternary carbons. Identical to compound 1, compound 2 also contained one β-d-glucopyranosyl by comprehensive analysis of NMR spectrum evidence.
The 13C NMR (Table S1) data combined with analysis of the HMQC of 2 revealed the remaining 21 carbon signals due to four methyls, six methylenes, seven methines and four oxygenated carbons, of which 15 were assigned to the aglycone, and the remaining six were ascribed to a glucopyranosyl unit at δC 100.57–60.85 ppm. Identical to compound 1, compound 2 also contained one β-d-glucopyranosyl by comprehensive analysis of NMR spectrum evidence. Further assignments of all hydrogen and carbon signals were achieved by its HMQC, 1H–1H COSY and HMBC spectra (Table S1 and Figure S2(a)). The aglycone spectral features were closely related to those of 1β, 4β, 7α-trihydroxyeudesmane (De Menezes et al. Citation2004), except that the chemical shift of C-1 shifted downfield for 6.6 ppm and the chemical shift of C-2 shifted upfield for 7.1 ppm, and the correlations of δH 4.42 (H-1′) with C-1 (δC 86.96) and δH 3.37 (H-1) with C-1′ (δC 100.57) were observed, which suggested that C-1 of 2 should be glycosylated.
The NOESY (Figure S2(b)) correlations of H-5/H-1and H5/H-15 suggested that the H-1, H-5 and Me-15 were of α-orientation, OH-7 and Me-14 were of β-orientation. Thus, the structure of compound 2 was established as 1β, 4β, 7α-trihydroxyeudesmane-1-O-β-d-glucopyranoside.
2.2. Anti-HBV and anti-HCV activities
The two compounds identified in the present study were examined for their anti-HBV and anti-HCV activities. Entecavir was used as a standard in the anti-HBV assay on HepG 2.2.15 cell line in vitro and both of compounds 1 and 2 exhibited no activity. Sofosbuvir was used as a standard in the study of anti-HCV 1b replicon cell in vitro, and compound 2 showed weak activity with EC50 at 145.2 μM, while compound 1 exhibited no activity.
3. Experimental
3.1. General experimental procedures
NMR Spectra: Bruker AV-400 spectrometer (MA, USA), at 100 (13C) MHz (probe (5 mm PABBO BB-), pulse width (17.42 μsec), acquisition time (1.36 s), spectral width (24038.5 Hz), decoupling mode (completely decoupling), digital resolution (0.74 Hz)), Bruker AV-600 spectrometer (MA, USA), at 600 (1H) MHz (probe (5 mm PABBO BB-), pulse width (12.88 μsec), acquisition time (2.67 s), spectral width (12536.6 Hz), digital resolution (0.37 Hz)), resp.; (D6) DMSO and D2O solns; δ in ppm, J in Hz. HR-ESI-MS: Bruker micrOTOF-Q mass spectrometers (MA, USA) (The Q-TOF (ESI+) mass spectrometer was running at 4.5 kV, at a desolvation temperature of 180 °C. The Q-TOF MS instrument was calibrated in the m/z range from 100 to 1000. All data were processed via Agilent MassHunter Qualitative Analysis software version B.04.00.); in m/z. ELSD-LT-II (Shimadzu Corporation, Kyoto, Japan). All solvents used were of anal. grade (Kelong Chemical, Chengdu, P.R. China). Column chromatography (CC): Macroporous resin D101 (Kelong Chemical, Chengdu, P.R. China), Silica gel (SiO2, 200–300 mesh; Qingdao Ocean Chemical Industry Co., P.R. China), SBC MCI gel (Chengdu Sci-Bio-Chem Co.Ltd., Chengdu, P.R. China), Sephadex G25 medium (GE Healthcare Life Sciences, MA, USA), ODS (GP-C18, 50 μm, Sepax Technologies, Inc, Newark, USA) and Sephadex LH-20 (GE Healthcare Life Sciences, MA, USA). TLC Spots were visualised under UV light (254 nm) and by spraying Vanillin-10% H2SO4 in alcohol followed by heating. Optical rotation: Perkin-Elmer-241 polarimeter (MA, USA). IR Spectra: Vector 22-FTIR spectrometer (MA, USA) with KBr pellets; in cm−1.
3.2. Plant material
The fresh needles of P. massoniana were collected from Sichuan province, P.R. China, in March 2014, and identified by Prof. Zhu-Yun Yan (College of Pharmacy, Chengdu University of Chinese Medicine, Chengdu, P.R. China). A voucher specimen (no. 20140401) was deposited in the Chengdu Kanghong Pharmaceutical Co. Ltd, P.R. China.
3.3. Extraction and isolation
The fresh needles of P. massoniana (24 kg) were extracted with EtOH–H2O (50 : 50, v/v). The crude extract was mixed with H2O (1 L) to form a suspension, then partitioned successively with PE, AcOEt and n-BuOH. The n-BuOH part (300 g) was subjected to CC (Macroporous resin D101; EtOH/H2O, 0 : 1 → 1 : 0, then SBC MCI; EtOH/H2O, 0 : 1 → 1 : 9 → 2 : 8 → 3 : 7 → 1 : 0) to afford five fractions. Fr.3 (EtOH/H2O, 2 : 8) was purified by repeated CC over Sephadex G25 (H2O), ODS (EtOH/H2O, 1 : 9), Sephadex LH-20 (EtOH/H2O 2 : 8) and SiO2 (AcOEt/EtOH 5 : 1, PE/Acetone 1 : 2), to afford 1 (50 mg), 2 (25 mg).
Massonside A ((1R, 4S, 5R, 6R, 10R)-octahydro-4-hydroxy-6-(2-hydroxy-2-methyl-propyl)-4, 10-dimethyl-1-indenyl-β-d-glucopyranoside, 1). White amorphous powder. (c = 0.21, MeOH). IR: 3411, 2964, 2920, 2871, 1635, 1460, 1380, 1269, 1188, 1160, 1188, 1076, 1022. 1H NMR (600 MHz, DMSO-d6) δ: 4.15 (1H, d, J = 7.8, H-1′), 3.65 (1H, dd, J = 12.0, 6.0, H-6′a), 3.46 (1H, m, H-6′b), 3.30 (1H, dd, J = 11.4, 4.2, H-1), 3.12 (1H, dt, J = 8.4, 4.8, H-3′), 3.05 (2H, m, H-4′, 5′), 2.90 (1H, dt, J = 8.4, 4.8, H-2′), 2.17 (1H, dq, J = 10.2, 2.4, H-6), 1.93 (1H, m, H-8a), 1.92 (1H, d, J = 13.2, H-7a), 1.68 (1H, dq, J = 12.6, 4.2, H-2a), 1.60 (1H, m, H-2b), 1.50 (2H, m, H-3a, 9a), 1.30 (2H, m, H-3b, 8b), 1.21 (1H, dd, J = 13.2, 10.2, H-7b), 1.18 (1H, m, H-9b), 1.17 (3H, s, H-15), 1.12 (3H, s, H-12), 1.10 (3H, s, H-13), 0.96 (3H, s, H-14), 0.80 (1H, d, J = 10.2, H-5). 13C NMR (100 MHz, DMSO-d6) δ: 101.65 (C-1′), 86.13 (C-1), 77.45 (C-5′), 77.09 (C-3′), 74.03 (C-2′), 70.71 (C-4′), 70.54 (C-4), 70.30 (C-11), 61.77 (C-6′), 59.36 (C-5), 51.76 (C-7), 46.39 (C-10), 41.30 (C-3), 39.29 (C-9), 32.45 (C-8), 32.02 (C-15), 31.75 (C-6), 30.56 (C-12), 30.53 (C-13), 24.78 (C-2), 15.90 (C-14). HR-ESI-MS (pos.): 441.2451 ([M + Na]+, C21H38NaO8+; Calcd 441.2459), 859.5011, 519.1871, 457.2200, 441.2451, 383.2408, 221.1899, 203.1789, 163.1479, 147.1166.
Massonside B ((1R, 4S, 5R, 7S, 10R)-decahydro-4, 7-dihydroxy-7-(1-methylethyl)-4, 10-dimethyl-1-naphthalenyl-β-d-glucopyranoside, 2). White amorphous powder. (c = 0.23, MeOH). IR: 3427, 2960, 2920, 2932, 2877, 1637, 1459, 1369, 1304, 1265, 1198, 1163, 1077, 1021. 1H NMR (600 MHz, D2O) δ: 4.42 (1H, d, J = 7.8, H-1′), 3.82 (1H, dd, J = 12.0, 1.8, H-6′a), 3.61 (1H, dd, J = 12.0, 6.0, H-6′b), 3.41 (1H, m, H-3′), 3.37 (1H, d, J = 8.8, H-1), 3.33 (1H, ddd, J = 10.2, 6.0, 1.8, H-5′), 3.28 (1H, t, J = 9.0, H-4′), 3.17 (1H, t, J = 9.0, H-2′), 1.73 (1H, dt, J = 12.0, 1.8, H-2a), 1.68 (2H, m, H-2b, 3a), 1.63 (1H, dt, J = 13.2, 3.0, H-9a), 1.56 (1H, m, H-11), 1.52 (3H, m, H-6a, 8), 1.46 (1H, m, H-3b), 1.36 (2H, m, H-5, 6a), 1.26 (1H, dt, J = 13.2, 4.2, H-9b), 1.03 (3H, s, H-15), 0.88 (3H, s, H-14), 0.84 (6H, d, J = 6.8, H-12, 13). 13C NMR (100 MHz, D2O) δ: 100.57 (C-1′), 86.96 (C-1), 75.93 (C-5′), 75.93 (C-3′), 74.69 (C-7), 73.07 (C-2′), 71.49 (C-4), 69.90 (C-4′), 60.85 (C-6′), 44.92 (C-5), 38.57 (C-11), 38.33 (C-3), 37.79 (C-10), 34.17 (C-9), 28.51 (C-15), 28.35 (C-8), 27.72 (C-6), 22.65 (C-2), 16.47 (C-12), 16.38 (C-13), 11.90 (C-14). HR-ESI-MS (pos.): 441.2446 ([M + Na]+, C21H38NaO8+; Calcd 441.2459), 859.5011, 457.2203, 441.2446, 426.2894, 383.2423, 221.1904, 203.1790.
3.4. Acidic hydrolysis and HPLC-ELSD analysis
Each solution of compound 1 and 2 (5 mg) in 1 M HCl (2.0 mL) was heated at reflux for 1 h and then the reaction mixture was neutralised with an equal volume of 1 M NaOH and extracted with CH2Cl2 (6 mL). The sugar moiety of both 1 and 2 was identified as d-glucose by HPLC-ELSD analysis (column: Sepax Hp-Amino (250 × 4.6 mm, 5 μm); carrier: 80% ACN in H2O (1.0 mL/min); column temperature: 35 °C; drift tube temperature: 40 °C; N2 pressure: 350 kPa; retention time: 8.146 min) of the aqueous solution in comparison with an authentic d-glucose.
3.5. Anti-HBV and anti-HCV activity assays
40,000 cells/well of HepG 2.2.15 cells (Wuhan Institute of Virology, Chinese Academy of Sciences, Wuhan, P.R. China) were seeded in 96-well plates, and were incubated at 37 °C in a humidified incubator containing 5% CO2 and were allowed to attach overnight. After incubation, culture medium was replaced with fresh medium supplemented with either 0.5% DMSO alone (control) or varying concentrations of test compounds dissolved in DMSO; this was repeated on the fifth day. And on the eighth day, the cell supernatants were collected to extract total HBV DNA. The qPCR assay was used to detect the HBV DNA. Entecavir (Accela ChemBio Co., Ltd., Shanghai, P.R. China) was used as a positive control.
0.5% DMSO alone (control) and varying concentrations of test compounds dissolved in DMSO were added in 96-well plates first, then 8000 cells/well of HCV 1b replicon cells (WuXi AppTec, Shanghai, P.R. China) were seeded in 96-well plates, and were incubated at 37 °C in a humidified incubator containing 5% CO2 for three days. And inhibition of HCV replication was measured as for the stable replicon cells using Bright-Glo (Promega Corporation, MA, USA). Sofosbuvir (Shanghai Haoyuan Medchemexpress Co., Ltd., Shanghai, P.R. China) was used as a positive control with EC50 at 0.12 μM.
4. Conclusion
In conclusion, two new sesquiterpene glucopryanosides (1 and 2) were isolated and characterised by spectrometric analysis (1 and 2D NMR, HR-MS). Compound 2 exhibited weak anti-HCV activity with EC50 at 145.2 μM. Therefore, we believe that this plant is an important source for the diverse structure of sesquiterpene glycosides and should be further investigated for other biological activities.
Funding
This work was supported by the National Standardization Project of Traditional Chinese Medicine.
Supplemental data
Supplemental data for this article can be accessed here at http://dx.doi.org/10.1080/14786419.2016.1239089.
Disclosure statement
No potential conflict of interest was reported by the authors.
1239089_Supplementary_material.pdf
Download PDF (1.8 MB)Acknowledgements
The authors thank the Analytical and Testing Center of Sichuan University and China measurement technology research institute for the spectral measurements. The authors thank WuXi AppTec for the anti-HBV and anti-HCV activity measurements.
References
- Chen F, Sheng LQ, Ma JL. 2014. Smashing tissue extraction of procyanidine from pine needles and its antioxidant activity. Chin J Pharm. 45:120–123.
- De Menezes JESA, Machado FEA, Lemos TLG, Silveira ER, Braz Filho R, Pessoa ODL. 2004. Sesquiterpenes and a phenylpropanoid from Cordia trichotoma. Z Naturforsch, C: Biosci. 59:19–22.
- Feng S, Zeng WC, Luo F, Zhao J, Yang ZR, Sun Q. 2010. Antibacterial activity of organic acids in aqueous extracts from pine needles (Pinus massoniana Lamb.). Food Sci Biotechnol. 19:35–41.10.1007/s10068-010-0005-2
- Gao ZP, Liu G, Liu YL, He XZ, Yin S, Rao ZW, Li Y. 2011. Study on extraction technology of catechin from pine needle of Pinus massoniana. Appl Chem Industry. 40:420–421.
- Lundgren LN, Shen Z, Theander O. 1985. The constituents of conifer needles. Dilignol glycosides from Pinus massoniana Lamb. Acta Chem Scand, Ser B. 39b:241–248.10.3891/acta.chem.scand.39b-0241
- Ma LJ, Liu X, Zhuo YJ, Yu LR. 2008. Study on the sorption and isolation of shikimic acid by 717 anion exchange resin. Zhong Yao Cai. 31:1065–1067.
- Patel NK, Jaiswal G, Bhutani KK. 2016. A review on biological sources, chemistry and pharmacological activities of pinostrobin. Nat Prod Res. 30:2017–2027.
- Shen ZB, Theander O. 1985. Flavonoid glycosides from needles of Pinus massoniana. Phytochemistry. 24:155–158.10.1016/S0031-9422(00)80826-2
- Shen XY, Wang YD, Wang F. 2010. Characterisation and biological activities of proanthocyanidins from the barks of Pinus massonian and Acacia mearnsii. Nat Prod Res. 24:590–598.10.1080/14786410903194472
- Wang W, Wang XH, Zhang XJ. 2008. Study on the active compounents of anti-platelet aggregation from pine needles of Pinus massoniana Lamb. Chin J Hosp Pharm. 28:190–194.
- Xie XY, Wang R, Shi YP. 2012. Sesquiterpenoids from the rhizomes of Homalomena occulta. Planta Med. 78:1010–1014.
- Yatagai M, Hong Y. 1997. Chemical composition of the essential oil of Pinus massoniana Lamb. J Essent Oil Res. 9:485–487.10.1080/10412905.1997.9700759
- Zhao ZX, Jin J, Ruan JL, Cai YL, Zhu CC. 2008. Two new flavan glycosides from Abacopteris Penangiana. Acta Pharm Sinica. 43:392–395.
- Zheng XK, Zhou W, Wang XL, Feng WS. 2009. Study on antitumor effect of different extract parts of pine needles in vitro. Lishizhen Med Mater Med Res. 36:1749–1754.
- Zheng XK, Wang XL, Feng WS. 2010. Effect of pine needle extract on liver lipids in ovariectomized rat. Lishizhen Med Mater Med Res. 21:368–370.