Abstract
The fungus Aspergillus amoenus Roberg strain UP197 was shown to produce antibacterial tetramic acid based alkaloids. Two new compounds, pyranterreone I and J (1 and 2), were isolated and characterized, in addition to the known compounds cordylactam, 7-hydroxycordylactam, pyranterreone C, D, F and G. Neither the pyranterreones nor the cordylacctams had previously been tested for antimicrobial activity. Thus, all isolated compounds were tested against a panel of clinically important bacteria and fungi. Pyranterreone C was active against Gram-positive and Gram-negative bacteria, with minimal inhibitory concentrations (MIC) between 1 and 8 µg/mL, whereas the MICs for all other compounds were >32 µg/mL. Pyranoterreone C was cytotoxic towards HepG2 cells, and since pyranterreone C reacted rapidly with the nucleophile cysteine, it is likely that the observed antibacterial activity is due to the chemical reactivity rather than enzymatic affinity, making it unsuitable for development as an antibacterial drug.
1. Introduction
Aspergillus species have been found to produce a wide and structurally heterogeneous range of bioactive secondary metabolites (Bok et al. Citation2006; Lee et al. Citation2013), some with therapeutic significance like lovastatin and penicillin (Frisvad and Larsen 2015; Vadlapudi et al. Citation2017). The extensive set of secondary metabolite biosynthetic genes present in Aspergillus species, which have evolved in environments subjected to intense competition, makes the genus interesting as a potential producer of new antibiotic drug candidates (Bok et al. Citation2006; Frisvad and Larsen 2015).
A recent study used genomic mining as a tool to find a new hybrid polyketide synthetase – non-ribosomal peptide synthetase (PKS-NRPS) gene cluster (pyt) in Aspergillus terreus, which was found to be responsible for the production of several new tetramic acid based alkaloids (pyranterreone A-H) (Tang et al. Citation2020). In parallel to the work of Tang et al. (Citation2020), in our ongoing search for new antibacterial compounds in cultures of fungi and bacteria, we isolated a number of the same compounds but also other related compounds from Aspergillus amoenus Roberg strain UP197. In this paper we thus describe two further pyranterreones [pyranterreones I and J (1 and 2)], along with antibacterial activity and cytotoxicity data for several of the previously isolated pyranterreones and the related cordylactams. We also show that the antibacterial and cytotoxic properties of pyranterreone C is due to chemical reactivity, and not to specific interactions with target molecules.
2. Results and discussion
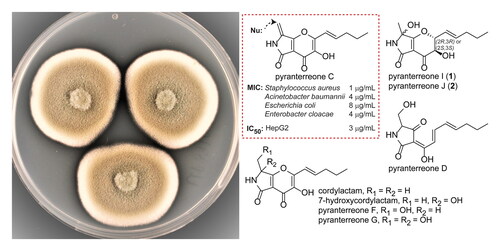
The fungus A. amoenus Roberg strain UP197 was found by UHPLC-MS, and subsequent database searches (AntiBase and Dictionary of Natural Products) to produce both putative novel and known compounds. Initial fractionation by preparative HPLC, followed by bioassays, suggested interesting antibacterial activity for some of these compounds. Culture upscaling, gradient and isocratic preparative HPLC, combined with bioassays and UHPLC-MS, resulted in the isolation of eight compounds. Structure analysis by NMR and MS, along with literature comparison, showed that two of the compounds were new (1 and 2), as described below, whereas six of the compounds were identified as cordylactam, 7-hydroxycordylactam, pyranterreone C, D, F and G () (Isaka et al. Citation2013; Shigemoto et al. Citation2018; Tang et al. Citation2020).
Figure 1. Structures of compounds 1, 2, cordylactam, 7-hydroxycordylactam, pyranterreone C, D, F, and G. The (2 R,3R,7S/R) isomers are shown for 1 and 2, but the configuration could equally well be (2S,3S,7S/R). For pyranterreone C and cordylactam, the proposed positions of addition of L-cysteine are indicated.
2.1. Structural elucidation
Compound 1 was obtained as a pale yellow amorphous solid with the molecular composition C13H17NO5 based on the [M + H]+ ion peak at m/z 268.1182 (calcd. for C13H18NO5+, 268.1179) in HRESIMS. The 1H and 13C NMR spectra of compound 1 (Table S1) were similar to the spectra of cordylactam, 7-hydroxycordylactam and pyranterreone C, D, F, and G in general, and to 7-hydroxycordylactam in particular (Table S2 and S3), indicating a closely related structure, and compared to the formula of 7-hydroxycordylactam (C13H15NO5), compound 1 had two additional hydrogen atoms. The origin of this difference was located through COSY and HSQC NMR experiments, which identified a -CH(3)-CH(2)-CH(1′)=CH(2′)-CH2(3′)-CH2(4′)-CH3(5′) spin system in 1, compared to the shorter -CH(1′)=CH(2′)-CH2(3′)-CH2(4′)-CH3(5′) spin system in 7-hydroxycordylactam, indicating that the difference between 1 and 7-hydroxycordylactam is that the C-2/C-3 double bond in 7-hydroxycordylactam has been reduced in 1. An HMBC NMR correlation was observed from the H-3 at the end of the spin system to the C-4 carbonyl, confirming the position of the spin system. However, no HMBC cross-peak was observed between H-2 and C-7a, which would have supported the presence of a pyran ring. According to energy minimization in Chem3D (data not shown), the dihedral angle H-2/C-2/O-1/C-7a is ca 80°, which probably explains the absence of this HMBC correlation. The similarity of 1 and 7-hydroxycordylactam was verified by HMBC NMR correlations between methyl H3-8 [δH = 1.44 ppm (s), δC = 23.2 ppm] and carbons at 82.0 ppm (C-7) and 185.9 ppm (C-7a, Figure S1), and from the amide proton NH-6 to C-7 and C-7a as well as to the C-5 carbonyl at 164.9 ppm and to the olefinic carbon at 102.6 ppm (C-4a). Together with the similarity in shifts between the remaining NMR signals of compound 1 and reported shifts for 7-hydroxycordylactam, this resulted in the proposed structure of compound 1, which was given the name pyranterreone I. Compound 1 was determined to have the specific rotation [α]D +104.
Compound 2 was isolated as a pale yellow amorphous solid, with the molecular formula C13H17NO5 based on the [M + H]+ ion peak at m/z 268.1183 (calcd. for C13H18NO5+, 268.1179) in HRESIMS. The 1H and 13C NMR spectra of compound 2 (Table S1) were almost identical to those of compound 1, with only small chemical shift variations. 2 D NMR data (COSY, HSQC, and HMBC) of compound 2 displayed the same correlations as observed for compound 1, hence concluding compound 2 to be a diastereomer of compound 1. Compound 2 was subsequently named pyranterreone J. Compound 2 was determined to have the specific rotation [α]D +68.
Compound 1 and 2 were not stable in conditions required for formation of Mosher’s esters for subsequent determination of absolute configuration by NMR (Dale et al. Citation1969). Instead, based on biosynthetic reasoning as outlined below, the compounds were proposed to have 2S,3S,7S/R or 2 R,3R,7S/R configuration.
2.2. Biosynthetic considerations
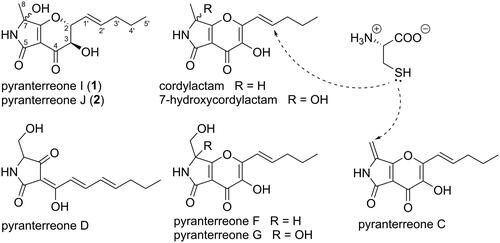
The pyt PKS-NRPS gene cluster in A. terreus has been described to be responsible for the production of the pyranterreones (Tang et al. Citation2020), and the presence of a very similar set of compounds in A. amoenus suggests the involvement of the pyt gene cluster. 7-Hydroxycordylactam is the C-2/C-3 unsaturated analogue of 1 and 2, and the biosynthesis of 1 and 2 may be very similar to the biosynthesis of 7-hydroxycordylactam (Figure S41). If the ring-closed pyranterreone D escapes PytD oxidation to pyranterreone F, the resulting intermediate may be modified to 1 and 2 in analogy with the processing of pyranterreone F to 7-hydroxycordylactam (Figure S41). In this scenario, the C-2 and C-3 configurations of 1 and 2 would be set to R,R or S,S, resulting in the 2S,3S,7S/R or 2 R,3R,7S/R configuration for 1 and 2. Assuming a pseudo-equatorial position for the pentenyl group at C-2, these configurations both result in pseudo-axial/axial relationships for H-2 and H-3, in line with the corresponding coupling constants 8.1 and 9.7 Hz recorded for 1 and 2, respectively. The alternative 2 R,3S and 2S,3R configurations would both lead to pseudo-axial/equatorial relationships associated with smaller coupling constants.
Tang et al. (Citation2020) did not report the formation of 1 and 2, or associated intermediates, in a pytD deletion strain, which may disagree with the here proposed formation of 1 and 2. On the other hand, the data presented by Tang et al. (Citation2020) does not exclude this possibility since 1, 2 and intermediates may be among the unidentified compounds in their metabolic profiling of deletion strains. Furthermore, Yamamoto et al. (Citation2015) investigated the biosynthesis of the very similar pyranonigrins by the pyn gene cluster in A. niger and detected the direct intermediate resulting from oxidative ring-closure of the pyranonigrin I (analogue to pyranterreone D). Thus, there may be differences between A. amoenus investigated here and the A. terreus and A. niger with respect to the involved pyt PKS-NRPS gene cluster and the regulation of these genes.
When storing cordylactam and pyranterreone F over prolonged periods of time, we observed a small production of 7-hydroxycordylactam and pyranterreone G, respectively, indicating that the formation of these compounds by C-7 oxidation, is a spontaneous rather than enzymatic process. To further test this hypothesis cordylactam was dissolved in water or 1 M NaOH and then analysed by HRESIMS. The experiments showed the formation of 7-hydroxycordylactam from cordylactam suggesting that it is a degradation product rather than an enzymatic product. This process did only occur slowly in water solution with most of the starting material remaining after 1 week in 50 °C, but in alkaline solution all starting material was gone within 24 h in room temperature. This finding strongly suggests that also compounds 1 and 2 are formed by spontaneous oxidation of a saturated cordylactam analogue.
2.3. In vitro antibacterial and cytotoxicity assays
Pyranterreone I and J (1 and 2) were tested for antimicrobial activity against a panel of microorganisms consisting of Gram-positive and Gram-negative bacteria, as well as against two fungi. Since none of the cordylactams or the pyranterreones were previously tested for antibacterial activity, also the cordylactams and pyranoterreone C, D, F and G, were included in the antimicrobial tests. Of all tested compounds, only pyranterreone C displayed potent antibacterial activity, with minimal inhibitory concentration (MIC) 1 µg/mL against the Gram-positive Staphylococcus aureus and MIC 4 µg/mL, 8 µg/mL and 4 µg/mL, respectively, against the Gram-negative Acinetobacter baumannii, Escherichia coli and Enterobacter cloacae. For all other compounds the MICs were >32 µg/mL. The 50% inhibitory concentration (IC50) against the HepG2 cell line was determined to be 3.2 µg/mL for pyranterreone C, resulting in a 1:3 effect toxicity ratio against S. aureus. This is too toxic for the compound itself to be interesting as a candidate for further development as an antibacterial drug.
The antibacterial and cytotoxic pyranterreone C shares many structural features with the other isolated compounds, all of which had MICs >32 µg/mL. In particular cordylactam is similar to pyranterreone C, the only difference is a methyl group instead of a methylene group linked to C-7, and IC50 was determined to IC50 >16 µg/mL for this compound. Thus, the presence of a terminal methylene instead of a methyl group makes pyranoterreone C both antibacterial and cytotoxic, suggesting the activity and cytotoxicity to be due to chemical reactivity rather than specific interactions with cellular targets. Cordylactam and pyranterreone C comprise almost identical systems of double bonds conjugated to carbonyl groups, suitable for Michael type additions of nucleophiles, but in pyranterreone C, even C-8 is included in this system, and C-8 is likely to be highly accessible to nucleophilic attack. To test the difference in reactivity between cordylactam and pyranterreone C, the compounds were subjected to the nucleophile L-cysteine. Pyranterreone C reacted very fast and formed an adduct (A, Figure S2) immediately after the addition of cysteine, likely through a Michael type addition of the thiol moiety on cysteine. Only one adduct was observed, which suggests addition to C-8 in pyranterreone C (), since addition to any of the other possible positions (C2’, C-2 and C-7a) would create a new stereogenic carbon atom and thus two diastereomeric adducts. For cordylactam, which is present as a racemic mixture due to the acidic H-7, cysteine addition to C-2′, C-2 or C-7a is expected to result in four stereoisomers. For steric reasons, C-2′ should be the most accessible of these positions for nucleophilic attack by cysteine (). Cordylactam reacted considerably slower with cysteine than pyranterreone C and four isomeric products were formed (B-E, Figure S2), likely by a Michael type addition of L-cysteine to C-2′ in cordylactam. However, MS-data showed that the main products were not the expected C-3/C-4 diols, but most likely the corresponding C-3/C-4 diketo compounds instead, supposedly formed by autooxidation of the C-3/C-4 diols. The results supports that the antibacterial activity of pyranterreone C as well as the cytotoxicity is due to the chemical reactivity of the C-7 linked methylene group. This makes it highly unlikely that chemical modification of pyranterreone C would result in derivatives suitable as potential new antibiotic drugs.
Similar pyrano(2,3-c)pyrrole bicyclic compounds i.e., pyranonigrins, curvupallides, cordylactam, and 7-hydroxycordylactam, have previously been described with mainly antioxidant activity i.e., superoxide radical scavenging activity (Miyake et al. Citation2008; Riko et al. Citation2014; Tang et al. Citation2020). Only a few have been measured for antibacterial effects and of these only pyranonigrin A and F have been described with potent antibacterial activity with MIC 0.5 µg/mL against e.g., S. aureus (Meng et al. Citation2015), but for these compounds, the antibacterial mechanism was not discussed. These compounds are very similar to 7-hydroxycordylactam, but the C-7 linked methyl group has been exchanged to a hydrogen atom in pyranonigrin A and F, so neither of the compounds has the reactive C-7 linked methylene group of the antibacterial pyranterreone C. Instead, they both have a C-7 α/β-unsaturated aldehyde group, masked as a hemiaminal, which may contribute to the antibacterial activity. Interestingly, 7-hydroxycordylactam possesses a masked C-7 α/β-unsaturated keto group, which apparently does not make this compound antibacterial (S. aureus MIC >32 µg/mL).
3. Conclusions
We report the isolation and characterization of the new pyranterreone I and J (1 and 2) from A. amoenus, along with the previously known pyranterreone C, D, F and G, cordylactam and 7-hydroxycordylactam. These tetramic acid based alkaloids were not previously investigated for antibacterial properties, and here we describe pyranterreone C to be a potent antibiotic compound. However, pyranterreone C was also found to be rather cytotoxic, making it unsuitable for further studies as a putative future antibiotic drug, in particular since both the antibiotic property and the cytotoxicity were found to depend on reactivity with cellular nucleophiles. Additionally, we show that 7-hydroxycordylactam is formed by autooxidation of cordylactam, which indicates that the analogous compounds pyranterreone I and J may be formed by autooxidation of an unknown intermediate.
Nord_et_al_Supplementary_materials_rev1.docx
Download MS Word (1.2 MB)Acknowledgments
We acknowledge Albina Bakeeva and Su-Lin Hedén (Swedish University of Agricultural Sciences, SLU) for help and advice with identification of A. amoenus UP197 as well as A. Bakeeva and Tomas Linder (SLU) for conidiophore and colony photographs, respectively, and Lina Johansson (Ultupharma AB) for the laboratory help. We thank Lena Lenhammar and Rolf Larsson (Uppsala University) for measuring the in vitro cytotoxicity. Ultupharma AB is acknowledged for funding of J.J.L., J.B., C.N., B.G., and A.B.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Bok JW, Hoffmeister D, Maggio-Hall LA, Murillo R, Glasner JD, Keller NP. 2006. Genomic mining for Aspergillus natural products. Chem Biol. 13(1):31–37.
- Dale JA, Dull DL, Mosher HS. 1969. α-Methoxy-α-trifluoromethylphenylacetic acid, a versatile reagent for the determination of enantiomeric composition of alcohols and amines. J Org Chem. 34(9):2543–2549.
- Frisvad C, Larsen TO. 2015. Chemodiversity in the genus Aspergillus. Appl Microbiol Biotechnol. 99(19):7859–7877.
- Isaka M, Chinthanom P, Rachtawee P, Somyong W, Luangsa-Ard JJ, Hywel-Jones NL. 2013. Cordylactam, a new alkaloid from the spider pathogenic fungus Cordyceps sp. BCC 12671. Phytochem Lett. 6(2):162–164.
- Lee YM, Kim MJ, Li H, Zhang P, Bao B, Lee KJ, Jung JH. 2013. Marine-derived Aspergillus species as a source of bioactive secondary metabolites. Mar Biotechnol (NY)). 15(5):499–519.
- Meng L-H, Li X-M, Liu Y, Wang B-G. 2015. Polyoxygenated dihydropyrano[2,3-c]pyrrole-4,5-dione derivatives from the marine mangrove-derived endophytic fungus Penicillium brocae MA-231 and their antimicrobial activity. Chin Chem Lett. 26(5):610–612.
- Miyake Y, Mochizuki M, Ito C, Itoigawa M, Osawa T. 2008. Antioxidative pyranonigrins in rice mold starters and their suppressive effect on the expression of blood adhesion molecules. Biosci Biotechnol Biochem. 72(6):1580–1585.
- Riko R, Nakamura H, Shindo K. 2014. Studies on pyranonigrins-isolation of pyranonigrin E and biosynthetic studies on pyranonigrin A. J Antibiot (Tokyo)). 67(2):179–181.
- Shigemoto R, Oinuma K-I, Masuo S, Takaya N. 2018. Novel antioxidant isolated from Warcupiella spinulosa JCM 2358, 7-hydroxycordylactam. Heterocycles. 96(6):1075–1079.
- Tang S, Zhang W, Li Z, Li H, Geng C, Huang X, Lu X. 2020. Discovery and characterization of a PKS-NRPS hybrid in Aspergillus terreus by genome mining. J Nat Prod. 83(2):473–480.
- Vadlapudi V, Borah N, Yellusani KR, Gade S, Reddy P, Rajamanikyam M, Vempati L, Gubbala SP, Chopra P, Upadhyayula SM, et al. 2017. Aspergillus secondary metabolite database, a resource to understand the Secondary metabolome of Aspergillus genus. Sci Rep. 7(1):7325.
- Yamamoto T, Tsunematsu Y, Noguchi H, Hotta K, Watanabe K. 2015. Elucidation of pyranonigrin biosynthetic pathway reveals a mode of tetramic acid, fused γ‑pyrone, and exo-methylene formation. Org Lett. 17(20):4992–4995. −