 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Recently, a modulated formation behaviour of lath martensite in Fe–Ni(-based) alloys was observed, exhibiting a series of transformation-rate maxima. This peculiar transformation behaviour was explained on the basis of the hierarchical microstructure of lath martensite, minimising the net shape strain associated with martensite formation, by a block-by-block formation of martensite packages occurring simultaneously in all packages. In the present work, the martensitic transformation upon slow cooling of two Fe–Ni alloys, containing 22 and 25 at.% of Ni, respectively, was investigated by high-resolution dilatometry with the aim of identifying the influence of alloy composition on the modulated transformation behaviour. The differences observed for the two alloys, a more rapid sequence of the transformation-rate maxima and a narrower temperature range in case of Fe-25 at.% Ni, can be explained consistently as a consequence of the lower transformation temperatures in Fe-25 at.% Ni, highlighting the role of temporary accommodation of the shape strain during formation of the lath martensite microstructure: the depression of the transformation toward lower temperatures leads to a higher strength of the austenite, hence resulting in a more elastic (less plastic) temporary accommodation of the shape strain upon block formation and thereby in a more effective mutual compensation of the shape strain by neighbouring blocks. A kinetic model on the basis of energy-change considerations is presented which is able to describe the observed modulated transformation behaviour.
1. Introduction; discontinuous variations in the martensite-formation rate; plate vs. lath martensite
One of the most prominent features of martensitic transformations in many ferrous alloy systems is the so-called ‘burst phenomenon’ of plate martensite upon cooling [Citation1,Citation2]: the formation of a single martensite plate (which grows very fast, e.g. 0.7∙103–1.8∙103 m s−1 for Fe 30 wt% Ni [Citation3]) induces ‘auto-catalytically’ nucleation and growth of secondary plates, which themselves induce formation of further plates, and so on. The first, very fast formation of martensite, a ‘burst’, at the martensite start temperature M s is often followed by repeated bursts during further cooling. Due to the usually very fast formation of martensite plates, i.e. the rapid spread of the plates through the specimen, this type of autocatalytic formation of martensite plates leads to abrupt, large increases of (i.e. jumps in) the overall degree of transformation, especially in the early stages of the transformation. A similar (and possibly identical) phenomenon is the occurrence of so-called ‘avalanches’ upon martensitic transformations in Cu–Al–X and Ni–Mn–Ga shape memory alloys (e.g. [Citation4–6]); a related phenomenon, with respect to the kinetics, is the emergence of transformation-rate maxima in the massive austenite-ferrite transformation [Citation7,Citation8].
The above-described abrupt, pronounced increases in the transformed fraction of plate martensite as observed upon cooling are not generally reproducible for different specimens (although the overall increase in transformed fraction is more or less similar, indicating that the overall transformation kinetics are widely controlled by the provision of additional chemical driving force by continued cooling, i.e. indicating a so-called athermal transformation behaviour). This lack of reproducibility can be explained by the more or less statistical, random nucleation of plate martensite, depending on the availability of suitable nucleation sites [Citation9,Citation10].
Different from the irregular burst-type phenomena as observed for plate martensite, a regular series of well-defined maxima in the transformation rate was recently observed upon formation of lath martensite during (comparatively slow) cooling in different ferrous systems [Citation11,Citation12]. This phenomenon was fully reproducible for different specimens with regard to number and position of the rate maxima on the temperature scale [Citation11]. Although the martensite start temperature M s was, within the experimental accuracy, independent of the applied cooling rate, and the increase in the transformed fraction as function of temperature did not vary considerably for different cooling rates, the transformation-rate maxima disappeared above a specific upper critical cooling rate and became more distinctive with decreasing cooling rate [Citation12]. It thereby follows that a thermally activated process is involved in this peculiar martensite-formation process.
The appearance of a regularly modulated transformation rate, i.e. of a regularly accelerating and decelerating transformation on a macroscopic (time/temperature) scale requires (i) a practically simultaneousFootnote1 formation of a large number of martensite units and (ii) a mechanism which leads to the initiation/acceleration and deceleration/termination of this simultaneous transformation strictly and reproducibly dependent on the degree of undercooling.
These two prerequisites are explained on the basis of the special, hierarchical lath-martensite microstructure observed in many iron-based alloys (also see [Citation11] and [Citation12]): these systems typically show a martensite–austenite orientation relation (OR) close to Kurdjumov–Sachs (KS, and also close to Nishiyama–Wassermann, NW) and a crystallographic habit plane close to {5 5 7}γ [Citation13–15]. One original austenite grain is subdivided into several martensite packages built of parallel blocks, which consist of sub-blocks composed of individual laths [Citation15–17]. All building units follow strict crystallographic ORs in order to minimise the overall shape strain [Citation18,Citation19]: given in terms of the KS-OR, a package exhibits one of the four possible {1 1 1}γ/{0 1 1}α′ parallel lattice-plane variants of the (KS-) OR, for which only six different OR variants can occur. Each block within one package consists of two types of sub-blocks, each type featuring one of these orientation variants (thus there are three pairs of different variants per package; one pair per block). All laths within one sub-block thus are of the same OR variant with only slight mutual misorientation [Citation18,Citation17].
The formation of the hierarchical microstructure is proposed to proceed as follows: nucleation of the first laths of a certain (sub-)block generally appears to occur at (or close to) the austenite-grain boundary [Citation20,Citation21], thus initiating formation of one martensite block. After that, (repeated) nucleation and growth of parallel laths of the same variant, or of the variant of the other type of sub-block of the concerned, developing block, takes place. One block thereby continues to grow in thickness, until the accumulated local transformation-induced plastic and elastic deformation impedes further formation of laths of its sub-block variants in view of the instantaneous available driving force (or until an obstacle is met). Upon (considerable) further cooling, i.e. provision of additional driving force, eventually the nucleation barrier for formation of laths of the next block (with a different set of two orientation variants) can be overcome and a new block of the package, adjacent to the earlier formed block, is formed.Footnote2 In order to relate the transformation on the above-described microstructural level to the macroscopically modulated transformation behaviour, in Refs. [Citation11] and [Citation12] it was proposed that, after the initial more or less random formation of the first block of each package, the formation of following blocks in all packages occurs at approximately the same degree of further undercooling, thus implying a concerted, simultaneous ‘next-block formation’, which leads in sum to well-defined maxima of the transformation rate. This simultaneity of the ‘next-block formation’ is attributed to realisation of same (energetic) conditions, by a thermally activated process, for the formation of a new block next to the previously formed block, so that the same amount of driving force is required for formation of all next blocks (this concept strongly contrasts with a statistical nature of nucleation as pertaining to plate martensite; see above).
The above-indicated thermally activated process leading to the appearance of the modulated transformation behaviour, for sufficiently low cooling rates, could be the thermally activated relaxation of the deformed austenite in the direct vicinity of an existing block, causing the establishment of local (strain) conditions similar for all (just before developed) blocks in all packages [Citation12].
Until now, the modulated transformation behaviour was observed in a Fe–Ni–Co–Mo maraging steel [Citation11] and in a binary Fe-22 at.% Ni alloy [Citation12]. However, a systematic characterisation of the influence of alloy composition on the transformation behaviour is still lacking. Therefore, in the present study two Fe–Ni alloys of different Ni content, 22 and 25 at.% of Ni, have been investigated with respect to their martensitic transformation behaviour upon slow cooling. Both alloys form bcc lath martensite, but show distinctly different chemical driving forces, and hence different temperature ranges, for the martensitic transformation, as well as different mechanical properties, allowing to investigate the role of chemical driving force and shape-strain evolution in a systematic way.
2. Experimental
Two iron-nickel alloys with 22 at.% of Ni and 25 at.% of Ni, respectively, were prepared by melting of corresponding amounts of solid elemental Fe (4N) and Ni (4N6) in an inductively heated furnace. The melt was cast into cylindrical moulds with a diameter of 12 mm. The two resulting alloy rods were then encapsulated under argon atmosphere and homogenised for 24 h at 1323 K. Subsequently, the rods were hammered down to a diameter of 6 mm. A second heat treatment at 1323 K for 24 h was then performed to recrystallise the specimens. Finally, cylindrical dilatometer specimens with a length of 10 mm and a diameter of 5 mm were prepared by turning. The nickel contents of these specimens were determined by inductively coupled plasma optical emission spectrometry and the amount of carbon contamination by a combustion method (results have been summarised in Table ).
Table 1. Chemical composition of the two Fe–Ni alloys.
For dilatometric measurements, a high-resolution differential dilatometer DIL 802 (Bähr Thermoanalyse GmbH) was used, employing a quartz measurement system under flowing Ar atmosphere (7.0 l/h). The length-change resolution of this instrument is ±10 nm [Citation24]. In this type of instrument, the specimen and the reference specimen, as well as the entire quartz measurement system is heated up and (passively) cooled down via a surrounding tube furnace. A corundum specimen with a length of 10 mm and a diameter of 5 mm served as a reference. Starting from room temperature, the specimens were heated up to 1073 K at 10 K min−1 and annealed for 10 min in order to ensure complete transition into the austenite phase, and to remove any (deformation) effects from previous treatment. Afterwards, the specimens were cooled with 5 K min−1 to a temperature right above the martensite-start temperature M s: i.e. down to 563 K for the Fe-22 at.% Ni specimen and down to 423 K for the Fe-25 at.% Ni specimen. Further cooling to room temperature was then performed with a cooling rate of 0.1 K min−1. The actual temperature in the dilatometer furnace is measured by a thermocouple which is located right above the specimen. The accuracy of the temperature measurement upon cooling, checked by measuring the length change of pure Ni (which shows a discontinuous change of the thermal expansion coefficient at its Curie temperature [Citation25]), is better than ±2 K. The cooling rates derived from the actual temperatures agree quite well with the ones set by the temperature programme, e.g. for a set cooling rate of 0.1 K min−1,the rate determined from the actual furnace temperature amounts to (0.094 ± 0.003) K min−1.
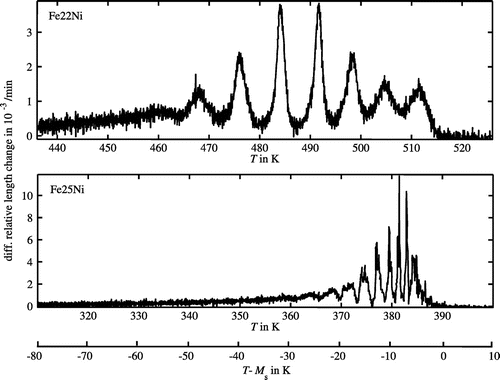
For microstructure investigations, cross sections of the dilatometer specimens perpendicular to their length axis were prepared, ground and polished with an oxide polishing suspension as final stage. Electron back-scatter diffraction (EBSD) from these cross sections was performed with a Zeiss LEO 438 VP SEM (acceleration voltage 20 kV) equipped with a high-speed camera. For each specimen, three different areas with lateral dimensions of 800 μm × 800 μm were recorded with a 1-μm step size. Quantitative image analysis was performed using the analysing software OIM 7 (Ametek EDAX/TSL) [Citation26]. For extracting the dimensions of the martensite blocks, the area of one block was defined as a group of data points with mutual misorientation angles less than 15°. For characterisation of the block shape, blocks were approximated by elliptical shapes providing average block minor- and major-axis lengths which can serve as a measure for the block width and the block length, respectively.Footnote3
3. Results and discussion
Due to the difference in specific volume of the austenitic (fcc) phase and the martensitic (bcc) phase, the austenite(γ)-to-martensite(α′) transformation is associated with considerable increase in length for both the Fe-22 at.% Ni and the Fe-25 at.% Ni dilatometric specimens during the austenite-to-martensite transformation (Figure (a)). In both cases, the onset of the transformation is characterised by a sharp initial increase in the relative length; upon proceeding transformation for both alloys a discontinuous, stepwise increase in relative length occurs, corresponding to a series of regularly spaced (on the temperature scale) transformation-rate maxima (Figures (a) and ; note that in Figure , the rate of relative length change instead of the rate of transformation is shown: for Fe-25 at.% Ni, a full transformation to martensite, i.e. f = 1, could not be attained, because the instrumental set-up of the employed high-resolution dilatometer allows controlled cooling with 0.1 K min−1 only down to approx. 300 K, where the martensitic transformation is only about to finish, cf. Figure (a). The specimens were completely martensitic after removal from the dilatometer.
Figure 1. (a) Relative length change vs. temperature as determined by high-resolution dilatometry upon the austenite → martensite transformation in a Fe-22 at.% Ni specimen and a Fe-25 at.% Ni specimen, both annealed for 10 min at 800 °C and cooled with 0.1 K min−1, (b) Difference of the chemical energies of the fcc and bcc phases as a function of temperature. The martensite start temperature is 516 K for Fe-22 at.% Ni and 390 K for Fe-25 at.% Ni, and the T 0 equals 580 K for Fe-22 at.% Ni and 470 K for Fe-25 at.% Ni, respectively.
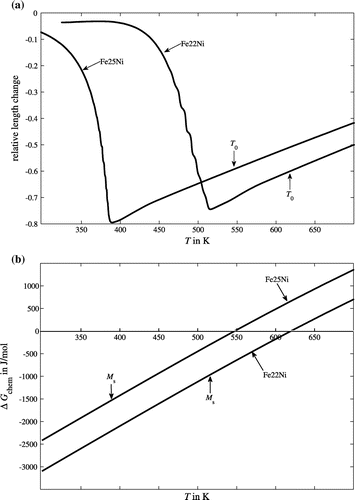
Figure 2. Austenite → martensite transformation rate (length-change rate) vs. temperature as obtained by high-resolution dilatometry of a Fe-22 at.% Ni specimen and a Fe-25 at.% Ni specimen, both annealed for 10 min at 800 °C, upon cooling with 0.1 K min−1. In order to allow a direct comparison of the transformation behaviour in the two alloys, a second temperature axis, showing the temperature relative to the specific martensite start temperature, has been added. The transformation-rate maxima follow in more rapid succession for higher Ni content.
3.1. The M s temperature
The martensite start temperature shows a considerable shift toward lower temperature with increasing Ni content: from M s ≈ 516 K for Fe-22 at.% Ni to M s ≈ 390 K for Fe-25 at.% Ni.Footnote4 This shift can, at first glance, be readily explained by the stabilisation of the austenite phase by Ni: the chemical driving force −∆G chem = −(G α′ − G γ), where G α′ and G γ denote the Gibbs energies per mole of martensite(α′) and austenite (γ), respectively, for the (partitionless) transition from fcc Fe–Ni to bcc Fe–Ni considerably decreases with increasing Ni-content (see Figure (b), calculated using the recent CALPHAD assessment of the Fe–Ni system in [Citation28]). For both alloys, the observed M s temperatures are considerably lower than the temperatures T 0 at which G α′ and G γ are equal (i.e. ∆G chem = 0): T 0 ≈ 620 K for Fe-22 at.% and T 0 ≈ 550 K for Fe-25 at.% Ni. Hence, the formation of martensite is suppressed well below the equilibrium temperature where (bulk) austenite and (bulk) ferrite are in equilibrium.
Nucleation of martensite in (virginal) austenite is generally described as a mechanism mediated by defects within the austenite phase [Citation29–31]. Following the approach for martensite nucleation by a faulting process derived from an existing defect [Citation29], the Gibbs energy change ΔG
net associated with a planar fault having a height of n atomic planes can be expressed as [Citation29,Citation31,Citation32]:(1a)
(1a)
per mole of the nucleus, where ΔG chem is the change in chemical Gibbs energy per mole and ΔG strain is the increase in strain energy per mole associated with the formation of the nucleus. σ is the interface energy associated with the fault per unit area of (in-plane) interface, l denotes the spacing of the atomic planes and V m is the molar volume of the nucleus phase. The perhaps most straight-forward applications of this model of planar faults as potential nuclei in martensitic transformations pertain to allotropic transformations of Laves phases [Citation33] and the hcp–fcc transformation in Co [Citation34].
Equation (Equation1a(1a)
(1a) ) implies that, if ΔG
net < 0, an existing fault can become an active nucleation site from an energetic point of view. However, for movement of the austenite–martensite interface, an additional, kinetic barrier, the frictional work w
f per mole nucleus phase, has to be overcome [Citation32]. Accordingly, the total Gibbs energy change ΔG
net, act associated with the activation of a nucleus can be defined as
(1b)
(1b)
Hence, according to this combined thermodynamic and kinetic consideration, the fault becomes activated when ΔG net, act becomes negative upon cooling, i.e. upon decreasing ΔG chem (the larger n, the smaller the necessary chemical driving force = −ΔG chem). This is often termed athermal nucleation.
In the here presented approach, the term can be conceived as a measure for the potency P of a nucleation site, determined by the characteristic fault parameters n and l. In a macroscopic specimen, these potencies of the nucleation sites follow a certain distribution [Citation35] which strongly depends on the austenite microstructure (e.g. correlated with the austenite grain size [Citation36,Citation37]). Hence, the M
s temperature is the temperature at which the most potent nuclei (faults of largest height n), at whatever location in the bulk of the specimen, become active upon cooling.
Setting ΔG
net, act = 0, the chemical Gibbs energy change ΔG
chem at T = M
s can serve as a measure for the quantities ΔG
strain, and w
f obstructing the activation of an existing fault of highest potency to become a nucleus: at the corresponding M
s, ΔG
chem assumes a value of approximately −1500 J/mol in case of Fe-25 at.% Ni, whereas it only amounts to −1000 J/mol in case of Fe-22 at.% Ni (see Figure (b)), i.e. a considerably smaller amount of chemical driving force −ΔG
chem is required to initiate the martensitic transformation in the latter case (these values are well compatible with the value of ∆G
chem at M
s of approximately −1300 J/mol reported for Fe–Ni alloys in Refs. [Citation31,Citation38]). Since both alloys underwent exactly the same heat treatment (see Section 2) and also show similar (comparatively large) average austenite grain sizes, the potency (size) distributions of the potential nucleation sites (=faults) may be quite comparable. A quantitative assessment of the difference in ΔG
strain (also with regard to establishment of the invariant-plane condition [Citation39]) appears difficult since data for lattice parameters and single crystal elastic constants, especially for the fcc phase at the respective composition and M
s can be indicated only roughly by extrapolation (see Table ):
| (1) | With increasing Ni-content (above 20 at.% Ni) the lattice parameter of the austenite phase increases and the lattice parameter of the ferrite phase decreases [Citation40], implying reduction of the dilatational components, but increase of the deviatoric components of the shape strain, and hence to a similar total magnitude of the shape strain, and of the resulting strain energy contribution ΔG strain for both Ni-contents. | ||||
| (2) | Fcc Fe–Ni alloys in this composition range (22–25 at.%) show considerable softening of elastic constants with increasing Ni-content ([Citation41,Citation42], note the markedly different effect of Ni for lower Ni-content and in ternary Fe–Ni–C alloys [Citation32]). A similar trend also holds for the bcc phase in this composition range [Citation43,Citation44]. Thus, the strain energy contribution ΔG strain is expected to decrease with increasing Ni content. | ||||
| (3) | With decreasing temperature, so for decreasing M s, the stiffness,Footnote5 and therefore ΔG strain increases [Citation41,Citation42]. | ||||
Table 2. Lattice parameters of Fe-22 at.% Ni and Fe-25 at.% Ni at RT and the respective M s temperatures. The lattice parameters of the fcc phase of the Fe–Ni alloys at RT were determined by extrapolation from higher Ni contents using data given in Ref. [Citation40]. The lattice parameters at M s were extrapolated from RT using thermal expansion coefficients of Fe-16 wt% Ni (bcc) and Fe-24 wt% Ni (fcc) as given in Ref. [Citation46].
Similar arguments as (2) and (3) above can be brought forward for the amount of frictional work w f associated with moving the martensite–austenite interface: whereas elastic softening with increasing Ni-content will reduce w f, the increase in stiffness, and thus also in strength, with decreasing M s will lead to an increase in w f.
Overall, the increase in stiffness of both austenite and martensite with decreasing temperature appears to dominate over the softening effect brought about by an increased Ni-content. Hence, a higher amount of chemical driving force to activate martensite nucleation is required for Fe-25 at.% Ni as compared to Fe-22 at.% Ni.
It can be concluded that the observed decrease in martensite start temperature with increasing Ni content is largely due to a shift on the temperature scale of the chemical energy difference ∆G chem of fcc and bcc Fe–Ni (see Figure (b)), serving as driving force for the transformation. This effect is enhanced by an (overall) increase in stiffness at lower transformation temperatures, requiring an additional amount of chemical driving force −∆G chem to initiate the martensitic transformation.
3.2. The modulation period of the transformation rate; role of mechanical strength
Shortly after the onset of martensite formation at M s, both Fe-22 at.% Ni and Fe-25 at.% Ni show a regular series of clearly distinguishable maxima in the transformation rate as function of temperature upon isochronal cooling (Figure ). However, in case of Fe-25 at.% Ni the maxima follow in a more rapid succession on the temperature scale as compared to Fe-22 at.% Ni: the distance between the rate maxima in case of the Fe-25 at.% Ni alloy is approximately 2−3 K, whereas for the Fe-22 at.% Ni alloy the maxima are separated by approximately 7–8 K. These temperature intervals correspond to an additional increaseFootnote6 of chemical driving force upon cooling: an additional increase in 18.6 J/mol in case of Fe-25 at.% Ni and of 61.8 J/mol in case of Fe-22 at.% Ni (cf. Figure (b)).
These observations are now discussed on the basis of the transformation mechanism proposed in Ref. [Citation11] (see Section 1), in which the occurrence of each of the transformation-rate maxima is attributed to the simultaneous formation of a new block in all packages of the specimen due to realisation of same energetic conditions for formation of the new block in all packages. This can be realised by a thermally activated relaxation process that runs fast as compared to the further provision of chemical driving force, which is controlled by the cooling rate. The block grows in width whereby the continuous accumulation of deformation energy introduced by the misfit/shape strain into the adjacent material reduces the net driving force and slows down further growth of a block. Upon continuous cooling, i.e. upon continuous increase in the chemical driving force, growth of a block can continue (slowly), whereas upon isothermal holding, block growth would eventually come to a halt. Formation of a new block of another OR variant set (out of the three possible sets of two OR variants; cf. Section 1) of the package at this location can partially compensate the shape strain (see [Citation18] and [Citation19]; note that for the best possible partial compensation of the misfit/shape strain, all six KS-type OR variants/three OR variant sets pertaining to one pair of {1 1 1}γ||{1 1 0}α′ planes should be present in the package [Citation18], and packages of all four {1 1 1}γ||{1 1 0}α′ plane variants should be present in one grain [Citation19], all of equal volume fraction). Thus, at one point upon further cooling, it is more preferable for the transformation to proceed via formation of a new block of different OR variant set directly adjacent to the previous block than by continued growth (thickening) of the current block ([Citation11,Citation45], Section 1).
In view of the only minor changes of the lattice parameters with varying Ni-content (see Table , [Citation40,Citation46]), the magnitude of the shape strain associated with the formation of martensite according to the phenomenological theory of martensite crystallography (assuming one lattice-invariant shear on a (1 0 1)γ plane in the [−1 0 1]γ direction; [Citation47])Footnote7
assumes almost identical values for both alloys (Table , also see Section 3.1). Thus, for formation of a block of certain thickness d, the amount of introduced deformation energy is similar for both alloys. However, for the Fe-25 at.% Ni alloy the transformation temperatures are lower which implies a higher stiffness (Section 3.1). Consequently, a higher degree of subsequent undercooling to generate next-block formation is expected for the Fe-25 at.% Ni alloy. This contradicts the observation of a smaller temperature interval ∆T between two maxima and the correspondingly smaller changes in −∆G
chem () in case of the Fe-25 at.% Ni alloy. On the other hand, for smaller ∆T (i.e. smaller change in −∆G
chem), as observed in case of the Fe-25 at.% Ni alloy, a considerably narrower block thickness should result. This contradicts the observation of approximately the same block thickness for both alloys, with an average block minor-axis (lateral) length of 2.2 × 10−6 for Fe-22 at.% Ni and of 1.9 × 10−6 for Fe-25 at.% Ni (see Section 2 and Figure ).
Figure 3. (colour online) EBSD orientation maps of a Fe-22 at.% Ni specimen (left) and a Fe-25 at.% Ni specimen (right) after martensitic transformation realised by applying a cooling rate of 0.1 K min−1. The martensite microstructure of Fe-22 at.% Ni shows a larger ‘ruggedness’ than that of Fe-25 at.% Ni.
This disparity can be understood by taking into account more specific consequences of the increased strength of the austenite and the martensite at lower transformation temperatures as holds for the Fe-25 at.% Ni alloy (see Section 3.1): upon formation of a martensite block, the corresponding transformation strain must, at least temporarily,Footnote8 be accommodated in some way. This accommodation can occur elastically, by elastically straining the martensite and the surrounding austenite, as well as plastically, by inducing plastic flow (especially in the softer austenite [49]). If the accommodation is of more elastic nature, as is to be expected for the Fe-25 at.% Ni alloy due to the increased strength at lower T, formation of a new block of a different OR variant set can decrease the amount of deformation energy, being stored elastically (see [Citation18,Citation19,Citation45] and above); the newly forming block thus strongly benefits from the previously formed block, reducing the amount of additional driving force necessary for the block to nucleate. By contrast, if the accommodation occurs more plastically, as is to be expected for the softer Fe-22 at.% Ni alloy at higher T, a smaller share of the deformation is accommodated elastically (to be, at least partly, released upon formation of the new block). Hence, a comparatively larger degree of undercooling is required for formation of the next block in the Fe-22 at.% Ni alloy. As a consequence, the larger amount of energy dissipated by plastic deformation processes upon martensite formation in case of Fe-22 at.% Ni leads to a more extended temperature interval (larger increase in chemical driving force) before emergence of the next transformation-rate maximum, as is indeed observed experimentally (Figure ).
Plastic dissipation of the energy associated with the transformation strain counteracts the tendency of the alloy to form the highly hierarchical microstructure of lath martensite as the outcome of shape strain minimisation as described above [Citation18,Citation19,Citation45]: indeed, the martensite microstructure of Fe-22 at.% Ni showed a much higher ‘ruggedness’, i.e. a less defined block and package microstructure, as compared to Fe-25 at.% Ni (see Figure ).
A similar influence of the mechanical strength on martensite formation was also proposed for different rapidly quenching Fe–C alloys [Citation18], ranging from relatively soft low-carbon alloys, to high-carbon alloys with a relatively higher stiffness of the austenite (also due to a decreasing M s): with increasing stiffness, an increasing refinement of the martensite microstructure (i.e. a decreasing block and package size) was observed and it was suggested that a more efficient elastic self-accommodation occurs for the alloys with larger stiffness, whereas significant plastic accommodation occurs for the soft low carbon alloys [Citation18]. This refinement of the microstructure with increasing stiffness is different from the observation of similar block and package size in the present study of Fe–Ni alloys of different stiffness. This difference may be attributed to martensite formation occurring in the Fe–C alloys [Citation18] upon rapid quenching, whereas the martensite formation in the present Fe–Ni alloys occurs upon very slow cooling.
Hence, the period of the transformation-rate modulations upon isochronal cooling of Fe-22 at.% Ni and Fe-25 at.% Ni differs mainly because of the lower temperature range of martensite formation in Fe-25 at.% Ni, which involves a higher strength of martensite and austenite and thus leads to a smaller amount of energy dissipation by plastic processes and a more rapid succession of transformation-rate maxima.
3.3. Model for the stepwise transformation behaviour
On a microscopic scale, the modulated transformation behaviour originates from the successive, stepwise formation of single blocks of a package upon cooling, which was the focus of the discussion in Section 3.2. On a macroscopic scale, the concerted formation of all k-th blocks in all packages then leads to the train of transformation-rate maxima as observed here by dilatometry (see Figure ). In the following, in order to visualise the basic mechanism for the modulated transformation behaviour, first a simple phenomenological kinetic model on the package scale is presented which considers the subsequent nucleation and growth of blocks in one representative package.
Referring to the general model of martensite nucleation (see Section 3.1, Equation (Equation1a(1a)
(1a) ) and (Equation1b
(1b)
(1b) )), the nucleation of a block, by activation of the nucleus of its first lath is thermodynamically and kinetically possible if ∆G
net,act ≤ 0. Equation (Equation1b
(1b)
(1b) ) pertains to the activation of a nucleus within virginal austenite, i.e. to the nucleation of the first block of a package. As soon as the first block forms, the nucleation condition for the next block is modified by the introduction of deformation energy associated with the formation of the first block in the austenite, and so on. Following the concepts introduced in Sections 3.1 and 3.2, this deformation energy is subdivided into two contributions:
| (1) | ∆G 1, the part of the elastic deformation energy, stored in the material upon growth of the currently growing k-th block, which, by shape strain accommodation due to the formation of the (k + 1)-th, next block of another OR variant set type (see Sections 3.2 and 1), can be, released upon formation of that next block.Footnote9 This contribution is assumed to scale with the current thickness d (k) of the previously nucleated and currently growing k-th block, i.e. ∆G 1 = −∆g 1 · d (k) with ∆g 1 > 0. | ||||
| (2) | ∆G
2, the part of the deformation energy which is introduced into the material and which cannot be released upon the formation of the next block(s) (i.e. the energy dissipated by plastic deformation processes; see Section 3.2). This contribution is assumed to scale linearly with the total amount of martensite which has already formed. Introducing d (i) as the thickness of the i-th block, the instantaneous thickness d
p (k) of the package is given by | ||||
Hence, in order to describe the activation of a fault to become the nucleus of the next, (k + 1)-th block, Equation (Equation1b(1b)
(1b) ) is modified to:
(2)
(2)
where ∆G
barrier,act represents the (positive) energy contribution counteracting the activation of a fault to become a nucleus, including the potency P of a nucleation site (cf. Equation (Equation1a(1a)
(1a) )), the increase in strain energy ∆G
strain upon nucleation of this block (i.e. additional to the strain energy already present due to the formation of the previous blocks), as well as the amount of frictional work required to move the martensite–austenite interface w
f (cf. Equation (Equation1b
(1b)
(1b) )).
Following the above treatment for nucleation, the net driving force −∆G
net,g (k) for growth of the current, k-th block can be expressed as:(3)
(3)
This equation results from the following consideration: the growth of the current k-th block is impeded (1) by the deformation energy ∆g 2 · d p (k) resulting from the already formed blocks, including the currently growing one (see (2)), and (2) by the deformation energy ∆g 1 · d (k) which develops upon growth of this block, is stored in the surrounding material, and will be released upon formation of the next, (k + 1)-th block. Finally, the term ∆g 1 · d final (k − 1) is the deformation energy stored in the material by formation of the previous, (k − 1)-th block with final thickness d final (k − 1), to be released upon growth of the current block which forms with another OR variant set. This stored energy is totally consumed if the current block reaches the final thickness of the previous block, i.e. ∆g 1 · d (k) − ∆g 1 · d final (k − 1) = 0 for d (k) = d final (k − 1).
The growth velocity of the block is given as [Citation50,Citation51]:(4)
(4)
where v 0 is a pre-exponential factor of growth and Q 0 is an activation energy associated with the movement (‘mobility’) of the interface, with R as the gas constant and T as the temperature [Citation51].
Hence, as the contribution ∆g
1 · d (k) introduces a competition between the growth of the current block and the nucleation of a new block (by impeding the growth of the current block (Equation Equation3(3)
(3) ) and supporting the nucleation of the next block (Equation Equation2
(2)
(2) ), it leads to the switching between blocks of different orientation upon progress of the martensitic transformation in the package concerned: after nucleation of the first block, growth of this block continues whereby, upon increasing d (k), the net driving force for growth of this block −∆G
net,g (k) decreases (see Equation Equation3
(3)
(3) ), the speed of growth slows down and becomes eventually dominated by the change of ∆G
chem = ∆G
chem (T) upon continued cooling (see Equations Equation3
(3)
(3) and Equation4
(4)
(4) ). Simultaneously, with increasing block thickness, at one point ∆G
net, act (k + 1) becomes zero, i.e. nucleation of the next block is activated.
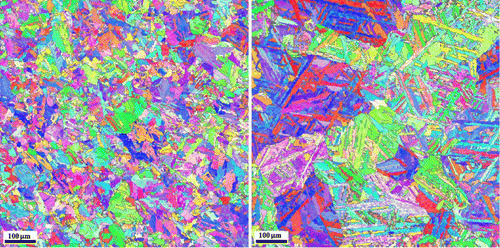
Adopting appropriate values for the model parameters, this relatively simple kinetic model for the martensitic transformation on the package level can reproduce the prominent features of experimental results quite well (Figure ): after nucleation of the first block at T = M s, a series of (rapid) accelerations and decelerations of the package formation rate, caused by successive block nucleation-and-growth events, is obtained, corresponding to the train of transformation-rate maxima experimentally found by high-resolution dilatometry. This strongly supports the present theory that the macroscopically observed modulated transformation behaviour is indeed caused, on the microscopic level, by the subsequent formation of blocks.
Figure 4. (a) Austenite → martensite transformation rate vs. temperature as obtained by high-resolution dilatometry of a Fe-22 at.% Ni specimen applying a cooling rate of 0.1 K min−1and (b) the growth velocity (Equation Equation4(4)
(4) ) of a package upon the austenite → martensite transformation as obtained from the model presented in Section 3.3 (with ∆g
1 = 5057 J/mol, ∆g
2 = 72,427 J/mol, v
0 = 210 μm/min, Q
0 = 53,673 J/mol, ∆G
barrier, act (first block) = 1000 J/mol and ∆G
barrier, act (following blocks) = 1650 J/mol as model parameters).
For observation of the modulated transformation behaviour on the macroscopic level, a simultaneous formation of all k-th blocks in all packages is required (see Section 1). It was proposed that the simultaneity condition is established by a thermally activated process, e.g. relaxation of the surrounding austenite, leading to energetically similar conditions for all next blocks in all packages at the same temperature (see Section 1, Refs. [Citation11,Citation12]). Hence, following this hypothesis, for fast cooling rates, (almost) no relaxation can occur and more or less dissimilar local conditions for formation of the next blocks (in the various packages) prevail, leading to disconcerted block formation, which is compatible with the results presented in Refs. [Citation11] and [Citation12]. The process of thermal relaxation (i.e. its time- and temperature dependencies) can be included into the above model for instance by assuming a certain distribution of the values of ∆g 1 for the different packages, which converge (relax) with time and temperature upon continuous cooling to a common value. For fast cooling, i.e. fast provision of chemical driving force and little time for relaxation, the distribution of ∆g 1 would then lead to asynchronous formation of blocks in different packages and vanishing of the macroscopically visible modulation, and vice versa.
4. Conclusions
| • | Both in Fe-22 at.% Ni and Fe-25 at.% Ni alloys, lath martensite formation is associated with a train of transformation-rate maxima which is attributed to the concerted formation of blocks in all packages. | ||||
| • | A lower martensite start temperature M s for Fe-25 at.% Ni was found and explained by (i) the austenite-stabilising nature of Ni, which decreases the chemical driving force −∆G chem for formation of bcc phase and thereby shifts M s towards lower temperatures for higher Ni contents, and (ii) the larger strength of the austenite with increasing Ni content, requiring additional chemical driving force, thereby further shifting M s towards lower temperature. | ||||
| • | The number of transformation-rate maxima appears to be independent of Ni content, but the single maxima follow in more rapid succession in the case of Fe-25 at.% Ni. This can be explained by the larger mechanical strength of Fe-25 at.% Ni at its lower transformation temperatures which leads to a more elastic nature of shape strain accommodation. Hence, the next block, arising with another orientation variant set, can benefit to a larger extent from the stored elastic energy in Fe-25 at.% Ni. In case of the softer Fe-22 at.% Ni, shape strain accommodation occurs more plastically, less elastic energy is available for the formation of the next block and thus a larger degree of undercooling is needed for formation of the new block, i.e. the temperature intervals between the maxima are larger for Fe-22 at.% Ni. | ||||
| • | A simple, straightforward kinetic model is presented which is able to explain the observed modulated transformation behaviour on a microscopic level by the successive formation of blocks within one representative package. Block-by-block formation, associated with an acceleration and deceleration of the package-formation rate, results from the release of elastic energy upon formation of a block. This elastic energy, pertaining to the elastically accommodated part of the shape strain, is stored in the austenite upon formation of the preceding block, and is released upon formation of a new block of a different orientation-variant set. This leads to a preferred nucleation of a new block over continued growth of the previously nucleated block. The macroscopically visible modulation of the transformation rate indicating the concerted, simultaneous formation of blocks in all packages is attributed to a thermally activated process, such as relaxation of deformation energy, which leads to similar energetic conditions for block formation in all packages. | ||||
Disclosure statement
No potential conflict of interest was reported by the authors.
Acknowledgements
The authors would like to thank Dr E. Bischoff (Max Planck Institute for Intelligent Systems) for performing the EBSD measurements and H. Göhring for the thermodynamic calculations.
Notes
1. at least within the instrumental time-resolution.
2. In some works, formation of individual parallel martensite blocks of one package still separated by retained austenite was observed [Citation13,Citation22,Citation23]. It is assumed that such spatially separated formation of blocks, which only eventually form one package, is rather exceptional (e.g. imposed by the limited degree of freedom of variant selection for a given austenite grain boundary) and less frequent than formation of blocks directly adjacent to existing ones since separated blocks do not yet profit from the effect of mutual shape strain minimisation (cf. Ref. [Citation15]).
3. No additional stereographic correction (considering that only a cross section was investigated) was applied because only the difference between the two alloys is of interest for this study.
4. It should be noted that the M s value determined by dilatometry represents a slight underestimate of the true, microscopic martensite start temperature, i.e. the temperature at which the first martensite unit actually forms, because only a certain, minimum amount of martensite formed leads to a detectable signal recorded by macroscopic methods such as dilatometry (~1% [Citation27]); but note the high sensitivity of the dilatometer used in this work (cf. Section 2).
5. Additional lattice softening of the fcc phase upon cooling due to the invar effect [Citation41] can still be neglected in the current range of temperatures and compositions.
6. Due to the continued accumulation of deformation in the residual austenite upon formation of martensite the transformation requires continued cooling, i.e. further increase in the chemical driving force, in order to proceed.
7. Note that the phenomenological theory, upon considering only one lattice-invariant shear system, does not correctly predict the experimentally observed habit plane in case of lath martensite [Citation14,Citation48]. However, for the qualitative comparison of the two Fe–Ni alloys in the present study, this deficiency may be neglected.
8. Obviously, the dilatational components of the transformation strain eventually lead to the macroscopically observed dilatation of the sample (Figure (a)).
9. Note that for an optimum shape strain accommodation within one package blocks of all three possible OR variant pairs must occur. However, to investigate the principal effect of shape strain accommodation on the kinetics of package formation, consideration of only two successive blocks is sufficient.
References
- E.S. Machlin and M. Cohen , Burst phenomenon in martensitic transformation , Trans. AIME. 191 (1951), pp. 746–754.
- R. Brook and A. Entwisle , Kinetics of burst transformation to martensite , J. Iron Steel Inst. 203 (1965), pp. 905–912.
- Z. Yu and P. Clapp , Growth dynamics study of the martensitic transformation in Fe-30% Ni alloys: Part I. Quantitative measurements of growth velocity , Metall. Trans. A. 20 (1989), pp. 1601–1615.10.1007/BF02663194
- A. Amengual , F. Garcias , F. Marco , C. Segui , and V. Torra , Acoustic emission of the interface motion in the martensitic transformation of Cu–Zn–Al shape memory alloy , Acta Metall. 36 (1988), pp. 2329–2334.10.1016/0001-6160(88)90332-X
- M.C. Gallardo , J. Manchado , F.J. Romero , J. del Cerro , E.K.H. Salje , A. Planes , E. Vives , R. Romero , M. Stipcich , Avalanche criticality in the martensitic transition of Cu67.64 Zn16.71Al15.65 shape-memory alloy: A calorimetric and acoustic emission study , Phys. Rev. B. 81 (2010) pp. 174102/1–174102/8.
- R. Niemann , J. Kopeček , O. Heczko , J. Romberg , L. Schultz , S. Fähler , E. Vives , L. Manosa , A. Planes , Localizing sources of acoustic emission during the martensitic transformation , Phys. Rev. B. 89 (2014), 214118/1–214118/11.
- Y. Liu , F. Sommer , and E.J. Mittemeijer , Abnormal austenite-ferrite transformation behaviour in substitutional Fe-based alloys , Acta Mater. 51 (2003), pp. 507–519.10.1016/S1359-6454(02)00434-2
- Y.C. Liu , F. Sommer , and E.J. Mittemeijer , Kinetics of the abnormal austenite–ferrite transformation behaviour in substitutional Fe-based alloys , Acta Mater. 52 (2004), pp. 2549–2560.10.1016/j.actamat.2004.02.003
- R.E. Cech and D. Turnbull , Heterogeneous nucleation of martensite transformation , Trans AIME. 206 (1956), pp. 124–132.
- G. Olson , K. Tsuzaki , and M. Cohen , Statistical aspects of martensitic nucleation, MRS Proc. 57 (1985), pp. 129–148.10.1557/PROC-57-129
- S. Loewy , B. Rheingans , S.R. Meka , and E.J. Mittemeijer , Unusual martensite-formation kinetics in steels: observation of discontinuous transformation rates , Acta Mater. 64 (2014), pp. 93–99.10.1016/j.actamat.2013.11.052
- S. Loewy , B. Rheingans , S.R. Meka , and E.J. Mittemeijer , Modulated martensite formation behavior in Fe–Ni-based alloys; athermal and thermally activated mechanisms , J. Mater. Res. 30 (2015), pp. 2101–2107.10.1557/jmr.2015.175
- A. Marder and G. Krauss , Formation of low-carbon martensite in Fe–C alloys , Trans. ASM. 62 (1969), pp. 957–964.
- B.P.J. Sandvik and C.M. Wayman , Characteristics of lath martensite: Part I. Crystallographic and substructural features , Metall. Trans. A. 14 (1983), pp. 809–822.10.1007/BF02644284
- S. Morito , X. Huang , T. Furuhara , T. Maki , and N. Hansen , The morphology and crystallography of lath martensite in alloy steels , Acta Mater. 54 (2006), pp. 5323–5331.10.1016/j.actamat.2006.07.009
- K. Wakasa and C. Wayman , The morphology and crystallography of ferrous lath martensite. Studies of Fe-20%Ni-5%Mn—I. Optical microscopy , Acta Metall. 29 (1981), pp. 973–990.10.1016/0001-6160(81)90051-1
- H. Kitahara , R. Ueji , N. Tsuji , and Y. Minamino , Crystallographic features of lath martensite in low-carbon steel , Acta Mater. 54 (2006), pp. 1279–1288.10.1016/j.actamat.2005.11.001
- S. Morito , H. Tanaka , R. Konishi , T. Furuhara , and T. Maki , The morphology and crystallography of lath martensite in Fe–C alloys , Acta Mater. 51 (2003), pp. 1789–1799.10.1016/S1359-6454(02)00577-3
- L. Qi , A.G. Khachaturyan , and J.W. Morris , The microstructure of dislocated martensitic steel: Theory , Acta Mater. 76 (2014), pp. 23–39.
- G. Miyamoto , A. Shibata , T. Maki , and T. Furuhara , Precise measurement of strain accommodation in austenite matrix surrounding martensite in ferrous alloys by electron backscatter diffraction analysis , Acta Mater. 57 (2009), pp. 1120–1131.10.1016/j.actamat.2008.10.050
- S. Zhang , S. Morito , and Y. Komizo , Variant selection of low carbon high alloy steel in an austenite grain during martensite transformation , ISIJ Int. 52 (2012), pp. 510–515.10.2355/isijinternational.52.510
- J. Marder and A. Marder , The morphology of iron-nickel massive martensite , Trans. ASM. 62 (1969), pp. 1–10.
- G. Krauss and A. Marder , The morphology of martensite in iron alloys , Metall. Trans. 2 (1971), pp. 2343–2357.10.1007/BF02814873
- Instructions of first use of a BÄHR-Dilatometer DIL801, DIL801L, DIL802, DIL802L, DIL803, DIL803L, 2001.
- T.G. Kollie , Measurement of the thermal-expansion coefficient of nickel from 300 to 1000 K and determination of the power-law constants near the Curie temperature , Phys. Rev. B. 16(1977), pp. 4872–4881.
- EDAX-TSL , OIM analysis 7 user manual, 2013.
- M. Umemoto and W. Owen , Effects of austenitizing temperature and austenite grain size on the formation of athermal martensite in an iron-nickel and an iron-nickel-carbon alloy , Metall. Trans. 5 (1974), pp. 2041–2046.10.1007/BF02644497
- W. Xiong , H. Zhang , L. Vitos , and M. Selleby , Magnetic phase diagram of the Fe–Ni system , Acta Mater. 59 (2011), pp. 521–530.10.1016/j.actamat.2010.09.055
- G. Olson and A. Roitburd , Martensitic nucleation , in Martensite , ASM International, Metals Park, OH, 1992, pp. 150–174.
- G. Olson and M. Cohen , A general mechanism of martensitic nucleation: Part I. General concepts and the FCC → HCP transformation , Metall. Mater. Trans. A. 7 (1976), pp. 1897–1904.
- G. Olson and M. Cohen , A general mechanism of martensitic nucleation: Part II. FCC → BCC and other martensitic transformations , Metall. Mater. Trans. A. 7 (1976), pp. 1905–1914.
- G. Ghosh and G.B. Olson , Kinetics of F.C.C. → B.C.C. heterogeneous martensitic nucleation – I. The critical driving force for athermal nucleation , Acta Metall. Mater. 42 (1994), pp. 3361–3370.10.1016/0956-7151(94)90468-5
- W. Baumann , A. Leineweber , and E.J. Mittemeijer , The kinetics of a polytypic Laves phase transformation in TiCr2 , Intermetallics. 19 (2011), pp. 526–535.10.1016/j.intermet.2010.11.027
- R. Bauer , E.A. Jägle , W. Baumann , and E.J. Mittemeijer , Kinetics of the allotropic hcp–fcc phase transformation in cobalt , Philos. Mag. 91 (2011), pp. 437–457.10.1080/14786435.2010.525541
- M. Lin , G.B. Olson , and M. Cohen , Distributed-activation kinetics of heterogeneous martensitic nucleation , Metall. Mater. Trans. A. 23 (1992), pp. 2987–2998.10.1007/BF02646117
- H. Yang and H. Bhadeshia , Austenite grain size and the martensite-start temperature , Scr. Mater. 60 (2009), pp. 493–495.10.1016/j.scriptamat.2008.11.043
- J.R.C. Guimarães and P.R. Rios , Martensite start temperature and the austenite grain-size , J. Mater. Sci. 45 (2009), pp. 1074–1077.
- L. Kaufman and M. Cohen , Thermodynamics and kinetics of martensitic transformations , Prog. Met. Phys. 7 (1958), pp. 165–246.10.1016/0502-8205(58)90005-4
- C.M. Wayman , Introduction to the Crystallography of Martensitic Transformazions , The Macmillan Company, New York, NY , 1964.
- R.P. Reed , Lattice parameters of martensite and austenite in Fe–Ni alloys , J. Appl. Phys. 40 (1969), pp. 3453–3458.10.1063/1.1658218
- G. Hausch and H. Warlimont , Single crystalline elastic constants of ferromagnetic face centered cubic Fe–Ni invar alloys , Acta Metall. 21 (1973), pp. 401–414.10.1016/0001-6160(73)90197-1
- H.M. Ledbetter and R.P. Reed , Elastic properties of metals and alloys, i. iron, nickel, and iron–nickel alloys , J. Phys. Chem. Ref. Data. 2 (1973), pp. 531–617.10.1063/1.3253127
- D.K. Chaudhuri , P.A. Ravidran , and J.J. Wert , Comparative X-ray diffraction and electron microscopic study of the transformation-induced substructures in the iron–nickel martensites and their influence on the martensite properties , J. Appl. Phys. 43 (1972), pp. 778–788.10.1063/1.1661280
- H. Zhang , M.P.J. Punkkinen , B. Johansson , S. Hertzman , and L. Vitos , Single-crystal elastic constants of ferromagnetic bcc Fe-based random alloys from first-principles theory , Phys. Rev. B – Condens. Matter Mater. Phys. 81 (2010), pp. 184105/1–184105/14.
- S. Morito , H. Saito , T. Ogawa , T. Furuhara , and T. Maki , Effect of austenite grain size on the morphology and crystallography of lath martensite in low carbon steels , ISIJ Int. 45 (2005), pp. 91–94.10.2355/isijinternational.45.91
- E. Owen , E. Yates , and A. Sully , An X-ray investigation of pure iron-nickel alloys. Part 4: The variation of lattice-parameter with composition , Proc. Phys. Soc. 49 (1937), pp. 315–322.10.1088/0959-5309/49/3/313
- H.K.D.H. Bhadeshia , Worked Examples in the Geometry of Crystals , 2nd ed., Institute of Materials, London, 2001.
- P. Kelly , Crystallography of lath martensite in steels , Mater. Trans. JIM. 33 (1992), pp. 235–242.10.2320/matertrans1989.33.235
- S. Loewy , L. Hjordt , B. Rheingans , and E.J. Mittemeijer , Modulated formation of lath martensite; influence of uniaxial compressive load and transformation-induced plasticity , Acta Mater. 109 (2016), pp. 46–54.
- J.W. Christian , The Theory of Transformations in Metals and Alloys , Pergamon Press, Oxford, 2002.
- E.J. Mittemeijer , Fundamentals of Materials Science , Springer, Berlin, Heidelberg, 2011.10.1007/978-3-642-10500-5
