ABSTRACT
Introduction
Pathogen-focused, randomized, controlled trials (PF-RCT) are important in the fight against carbapenem-resistant (CR) Gram-negative infections. Some recently approved antibiotics and older generic antibiotics with activity against CR Gram-negative bacteria were investigated in PF-RCTs in a variety of infections.
Areas covered
We searched Pubmed, Cochrane database and international clinical trial databases for PF-RCTs for the period between 2005 and 2020 and compared the study designs, patient populations, infection types, pathogens, and Day-28 all-cause mortality (ACM).
Expert opinion
PF-RCTs are particularly challenging to quantitatively assess and compare due to the heterogeneity in infection types, pathogens, CR mechanism, inclusion/exclusion criteria, and endpoints. Data interpretation is further complicated by lack of formal statistical analysis plans and/or non-inferiority design, and limited power across most PF-RCTs. The studies with new antibiotics (i.e. plazomicin, meropenem/vaborbactam, cefiderocol) ranked lower regarding feasibility, with relatively small sample sizes (analyzed: 37–118) versus the comparative effectiveness studies of older generic drugs (analyzed: 94–406). ACM ranged between 11.8% and 40% for CR Enterobacterales, 17.7% and 57.4% for CR Acinetobacter spp., and 20.0% and 30.8% for CR Pseudomonas aeruginosa. The information gathered must be considered carefully alongside the study limitations and caution should be exercised when making direct comparisons across trials.
PLAIN LANGUAGE SUMMARY
New antibiotics to treat multidrug-resistant Gram-negative bacterial infections are needed because antimicrobial resistance has become a global threat. In recent years, several pathogen-focused, randomized, controlled clinical trials were conducted to test new antibiotics or combinations of older generic antibiotics in the fight against resistant bacteria. However, these trials were exceptionally challenging and most of them enrolled relatively few patients. These studies were highly heterogeneous in terms of species, antibiotics, infection site, mechanism of resistance, endpoints and patient factors. In these trials, all-cause mortality at Day 28 or Day 30 were numerically lower with the new antibiotics in infections caused by carbapenem-resistant (CR) Enterobacterales. However, in the trials which investigated CR Acinetobacter spp. infections, there was no reduction in all-cause mortality at Day 28 or Day 30 with combinations of older generic antibiotics compared with colistin monotherapy. Limited information was available for CR Pseudomonas aeruginosa. More pathogen-focused, randomized, controlled clinical trials with more feasible design and higher patient numbers are needed to demonstrate clinical benefit in drug-resistant infections.
1. Introduction
Carbapenem-resistant (CR) Gram-negative pathogens, including Enterobacterales and non-fermenters such as Acinetobacter baumannii and Pseudomonas aeruginosa, have spread alarmingly in recent years, leading to their inclusion on the World Health Organization (WHO) global critical priority list of antibiotic-resistant bacteria [Citation1]. These CR pathogens carry a higher risk of morbidity and mortality relative to their carbapenem-susceptible counterparts [Citation2–5].
About 10 years ago, initiatives in the USA and Europe set out plans to stimulate the development of new antimicrobial agents, with a particular focus on drug-resistant pathogens [Citation6–10]. The traditional approach to the investigation of new antibiotics utilized non-inferiority clinical trials, which centered on a specific infection site such as urinary tract, intra-abdominal or lung, rather than a specific pathogen or group of pathogens defined by antibiotic susceptibility phenotype (e.g. carbapenem resistance) [Citation11]. Despite the increased public health burden of CR pathogens, patients with suspected or documented CR pathogens at randomization have been excluded from recent infection site-specific trial designs due to the limited activity of the control drugs, which were mainly carbapenems [Citation12–20]. As set out by guidance, these Phase 2 or Phase 3 registrational, randomized, non-inferiority studies enrolled a large number of patients over a 2–3-year time frame because the burden of these infections is significant worldwide [Citation21–23]. However, they often excluded patient populations with severe illnesses or comorbidities and drug-resistant infections [Citation12–20].
To address the problem of drug-resistant organisms, modifications to clinical trial requirements for new antibacterial drugs were introduced by the US Food and Drug Administration and European Medicines Agency, which led to the possibility of pathogen-focused investigations [Citation24,Citation25]. As a result, several pathogen-focused, randomized clinical trials (PF-RCTs) have been conducted in an attempt to gauge the clinical efficacy of new and older generic antibiotics in specific CR pathogen-associated infections [Citation26–36]. In contrast to Phase 2 or Phase 3 registrational, randomized, non-inferiority studies, the pathogen-focused studies allowed for the enrollment of patients with more severe illnesses and previously excluded comorbidities to explore further the efficacy of the new agents and effectiveness of older approved agents in special patient populations [Citation23]. As a result, these CR pathogen-focused studies were highly heterogeneous and enrolled patients with a variety of clinical diagnoses, including bloodstream infections (BSI), sepsis, urinary tract infection (UTI), skin and soft tissue infection, and nosocomial pneumonia, representing the most common clinical illnesses. While the broad enrollment criteria in the CR pathogen-focused studies result in more heterogeneous study populations, limiting the data interpretation [Citation26,Citation37,Citation38], they are important in gaining a better understanding of the efficacy of new antibiotics, as well as the effectiveness of older approved agents, where they are intended to be used [Citation26,Citation37]. To gain an overview and understanding of the totality of the data generated by pathogen-focused, multicenter trials, we conducted a literature and database search in order to review data from randomized, prospective, interventional, pathogen-focused trials in patients with CR Gram-negative infections. Our evaluation included studies of approved older generics and new antibiotics, and considered baseline patient demographics, infection types, and outcome of Day 28 or Day 30 all-cause mortality (ACM).
2. Methods
2.1. Database and literature search strategy
We sought to identify prospective, randomized, controlled, interventional studies in hospitalized adult patients (≥18 years old) focusing on a single species or group of species based on their antibiotic resistance profiles. Phase 2, 3, and 4 studies, and those from which phase information was not available, in patients with a laboratory-confirmed CR Gram-negative pathogen (Enterobacterales, especially Klebsiella spp., Acinetobacter spp., or Pseudomonas spp.), registered from 2005 in clinical trial registries and with outcome data (including Day 28 or Day 30 ACM) reported from 2010, were included.
The following sources were searched: ClinicalTrials.gov registry database, European Union Drug Regulating Authorities Clinical Trials Database, WHO International Clinical Trials Registry Platform, Cochrane database, and PubMed. Infection-related search terms were: ‘carbapenem resistance,’ ‘carbapenem-resistant Acinetobacter,’ ‘carbapenem-resistant Enterobacteriaceae,’ ‘carbapenem-resistant Klebsiella,’ ‘carbapenem-resistant Pseudomonas,’ ‘randomized study,’ ‘prospective trial,’ and/or ‘pathogen-focused’ (Supplementary Table S1). The following types of study were excluded: 1) those investigating aerosolized, inhaled, nebulized, or dry-powder inhalation antibiotics; 2) pediatric, children studies; 3) prophylaxis antibiotic studies; 4) topical agent or scrub use studies; 5) oral, bowel, digestive, perioperative decontamination studies; 6) antiseptic agent or scrub use decolonization studies; 7) fecal transplantation studies; 8) outbreak reports; 9) infection site-specific studies; 10) retrospective studies (any); 11) prospective observational (case–control, cohort, cross-sectional) or non-interventional, non-randomized, or single-arm studies (any); 12) mixed Gram-negative/Gram-positive infections; 13) studies not reporting Day 28 or Day 30 ACM; 14) studies not reporting pathogen-focused Day 28 or Day 30 ACM; 15) fewer than 50 patients enrolled; 16) Phase 1 studies; 17) studies published on a non-English language platform. Clinical studies registered on the WHO register in English were reviewed for inclusion. International, multicenter trials were cross-checked in each clinical trial registry and were counted only once.
Reference titles and abstracts available before 1 December 2020, were screened and reviewed by the authors. Peer-reviewed publications of primary clinical study results, secondary publications, systematic reviews (to ensure that all randomized clinical trials had been included), and congress abstracts and/or posters were reviewed for availability of outcomes and patient characteristics and to confirm eligibility for inclusion.
2.2. Primary analysis outcomes
Each study identified was described according to a number of categories. Study design features recorded were: study dates; number of patients screened/enrolled and randomization ratio; number of study sites and geographical locations; antibiotics and dose regimens used; study design and development phase; treatment duration; target pathogens; key inclusion and exclusion criteria; study endpoints and the primary analysis population. Intervention treatments were described as either older generic or new antibiotics.
Patient populations were presented according to age, sex, baseline diagnosis, target pathogens involved, sites of infections, and severity of illness propensity scores (Charlson Comorbidity Index [CCI], Acute Physiology and Chronic Health Evaluation II, and/or intensive care unit [ICU] admission). The Day 28 (or Day 30) ACM rates reported for each study were collated and summarized by pathogen group (carbapenem-resistant Enterobacterales [CRE] or CR Acinetobacter spp. or CR P. aeruginosa [CRPA]).
Data summarized from the analyses were descriptive and no formal statistical analysis plan was utilized. Day 28 (or Day 30) ACM rates were analyzed by treatment arm; point estimate and within-arm 95% confidence intervals (CIs) calculated by the Clopper–Pearson test are presented.
3. Results
3.1. Trials included in the analysis
A total of 931 entries were identified in the search, of which 883 were excluded on the basis of duplication or non-relevant information (Supplementary Figure S1). After author screening of the study designs, titles, abstracts, and full articles, 41 of the remaining 48 studies were excluded because they contained data from retrospective or prospective, observational, or single-arm studies, systematic reviews or meta-analyses, lacked mortality data required for inclusion in the analysis, or lacked pathogen-specific Day 28 mortality data (Supplementary Figure S1, Supplementary Table S1, Supplementary Table S2). We identified eight PF-RCTs; however, pathogen-specific ACM data were available in seven studies. In one of these studies (RESTORE-IMI 1), pathogen-focused ACM data were not available to compare with other studies [Citation36]. For one small, single-center, open-label, randomized, controlled study in infections caused by Klebsiella pneumoniae carbapenemase (KPC)-producing CR K. pneumoniae, limited inclusion and exclusion criteria were available for comparison with the other studies [Citation35].
Seven trials meeting the inclusion criteria for mortality analysis were open-label studies [Citation29–35] (). Three were national trials, two involving one site [Citation34,Citation35] and one involving five sites [Citation33], and the remaining four trials were international, involving 5–95 sites in 3–16 countries (). In accordance with the inclusion criteria, all studies required patients to be hospitalized. Some of the studies specifically excluded patients with endocarditis [Citation29–31], meningitis and other central nervous system infections [Citation29–31], bone and joint infections [Citation29–31], and intra-abdominal infections [Citation30].
Table 1. Study characteristics and inclusion and exclusion criteria of studies investigating carbapenem-resistant Gram-negative infections
Three of the trials involved a new antibiotic: CARE (plazomicin) [Citation29], TANGO II (meropenem/vaborbactam) [Citation30], and CREDIBLE-CR (cefiderocol) [Citation31]. Two new antibiotics, plazomicin and meropenem/vaborbactam, were investigated against CRE infections only [Citation29,Citation30], whereas cefiderocol was investigated in infections caused by either CREs or CR non-fermenters, including CR Acinetobacter spp [Citation31]. The remaining four studies involved older generic antibiotics, including colistin monotherapy or colistin in combination with meropenem (AIDA) [Citation32], rifampicin [Citation33], or fosfomycin [Citation34], or cefepime combined with amoxicillin/clavulanic acid versus tigecycline-based therapy [Citation35].
In two trials, the control arm was not a specified antibiotic regimen but rather best available therapy (BAT). In the TANGO II study, BAT included any of the following as monotherapy or in combination: polymyxins, carbapenems, aminoglycosides, or tigecycline; or monotherapy with ceftazidime-avibactam [Citation30]. Treatment selection was confirmed by the investigator based on institutional standards of care, patient characteristics, and local regulatory approval. In the CREDIBLE-CR study, BAT comprised a maximum of three systemic antibiotics with Gram-negative activity, dosed according to the country’s label and local clinical practice, and most patients (65%) received colistin-based combination therapy [Citation31], primarily for CR Acinetobacter spp. infections (Supplementary Table S3, Supplementary Table S4). In the CARE study, colistin monotherapy was selected as the control antibiotic [Citation29].
In terms of the target pathogens involved, three of the trials focused on CREs: the CARE trial (primary analysis population, n = 37 [randomized n = 69]) [Citation29], the TANGO II trial (n = 47 [randomized n = 77]) [Citation30] and the study by Ji et al. (n = 51 [randomized n = 62]) [Citation35]. Two trials enrolled patients with extensively drug-resistant and/or CR A. baumannii infections only: Durante-Mangoni et al. (n = 209 [randomized n = 210]) [Citation33] and Sirijatuphat et al. (n = 94 [randomized n = 99]) [Citation34]. The AIDA trial (n = 406 [randomized n = 406]) [Citation32] and the CREDIBLE-CR trial (n = 118 [randomized n = 150) [Citation31] included both CRE infections and CR non-fermenters, among which CR Acinetobacter spp. was the most frequent. In these two studies, CRPA was also investigated and analyzed [Citation31,Citation32].
In four trials, CARE, TANGO II, CREDIBLE-CR and Ji et al. study [Citation29–31,Citation35], the primary analysis population was the microbiological modified intention-to-treat (mITT) population, which included patients with a confirmed qualifying CR pathogen who received at least one dose of study drug. In these four trials 53.6% [CARE], 61.0% [TANGO II], 78.7% [CREDIBLE-CR] and 82.3% [Citation35] of randomized patients were included in the primary analysis. For the remaining trials, the primary analysis population was the ITT population [Citation32–34] and almost all enrolled patients were analyzed (e.g. patients were excluded in the AIDA [Citation32] study from the analysis if they died within 48 hours). Two of the trials, CARE and TANGO II, underwent significant protocol amendments in order to address enrollment feasibility [Citation29,Citation30]. For the original analyses, the AIDA and Durante-Mangoni trials had adequate enrollment to support a prior statistical hypothesis [Citation32,Citation33]; the remaining trials used descriptive statistics only [Citation29–31,Citation34,Citation35].
Definitions of carbapenem resistance used in the different trials are shown in Supplementary Table S5. The definition for carbapenem resistance differed to some extent between studies, but generally coincided with the consensus definition and minimum inhibitory concentration interpretive criteria available at the time of the studies and according to the geographical location [Citation39].
3.2. Patient demographics and baseline characteristics
Demographics and baseline characteristics of patients in the primary analysis populations are shown in (). A total of 962 patients were included (range 37–406) and the mean age ranged from 60.2 to 69.2 years. The studies enrolling patients with CR Acinetobacter spp. were larger than studies with CRE infections and enrolled primarily patients with pneumonia, whereas patients with BSI were more frequent in studies investigating CRE infections. Within studies, the most frequent infections were hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) in four studies (CREDIBLE-CR [Citation31], AIDA [Citation32], and trials reported by Durante-Mangoni et al. [Citation33] and Sirijatuphat et al. [Citation34]), and BSI in two studies (CARE [Citation29] and TANGO II [Citation30]). The TANGO II trial [Citation30] included a notable proportion of patients (i.e. 34%) with complicated UTI (cUTI), and the trial from Durante-Mangoni et al. [Citation33] included fewer patients with BSI relative to HAP/VAP; both factors might be expected to influence mortality ().
Table 2. Demographic and baseline details in the primary analysis populations of recent studies in adult patients with infections caused by carbapenem-resistant Gram-negative pathogens
A. baumannii was the predominant baseline pathogen in four studies [Citation31–34] (). K. pneumoniae was the predominant baseline pathogen in the CRE-focused CARE, TANGO II and Ji et al. studies [Citation29,Citation30,Citation35]. In terms of comorbidities, two of the trials reported that 60–93% of the patients had a CCI score ≥2 [Citation30,Citation33], with similar proportions of patients between treatment arms within each study. In CREDIBLE-CR [Citation31] and AIDA [Citation32], the median CCI scores in both treatment arms were around 5.0 and 2, respectively (). Where reported, the proportion of patients in ICU varied between studies, being highest in the study by Ji et al. (cefepime arm 73%, tigecycline arm 72%) [Citation35], the CREDIBLE-CR study (cefiderocol 65%, BAT 50%) [Citation31] and the study reported by Durante-Mangoni et al. (colistin + rifampicin 60%; colistin 62%) [Citation33] and lowest in the TANGO II trial (meropenem/vaborbactam 16%; BAT 20%) [Citation30].
3.3. Day 28 or Day 30 ACM
Data for Day 28 (or Day 30) ACM rates were mainly available for CRE and CR Acinetobacter spp. infections, and only in two studies for CRPA infections.
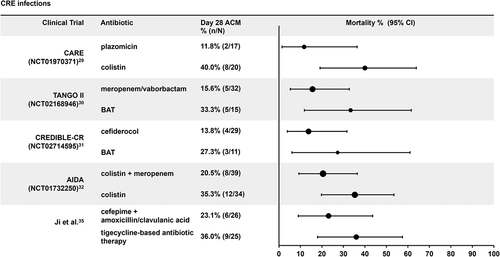
For CRE infections, the combined Day 28 ACM rate across the new and comparator arms of all studies was 25.0% (62/248). In three studies with the new antibiotics [Citation29–31], the Day 28 ACM was numerically lower in the new antibiotic arms (range: 11.8%–15.6%) compared with the comparator arms (range: 27.3%–40.0%) (). In the study conducted by Ji et al. [Citation35], the combination of cefepime with amoxicillin/clavulanic acid resulted in a numerically lower ACM versus tigecycline-based therapy (23.1% vs 36.0%) (). In the AIDA study, the combination of colistin with meropenem also resulted in numerically lower mortality than colistin monotherapy in CRE infections (combination 20.5% and monotherapy 35.3%) [Citation32]. The sample sizes were similar, but relatively small, across studies and treatment arms (range: 11–39 patients), which led to wide 95% CIs.
Figure 1. Day 28 all-cause mortality rates in carbapenem-resistant Enterobacterales infections. ACM: all-cause mortality, BAT: best-available therapy, CI: confidence interval, CR: carbapenem resistant. In the TANGO II and CREDIBLE-CR studies, patients were randomized 2:1 (investigational therapy:control).
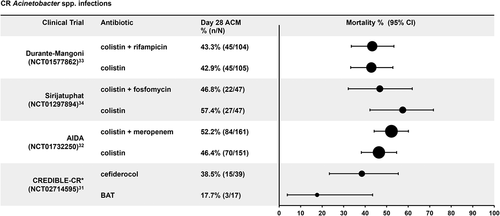
For CR Acinetobacter spp. infections [Citation31–34], the combined Day 28 ACM rate (46.3% [311/671]; ) across both arms of all studies was higher than for CRE infections (25.0%). Among studies of older generic antibiotics for CR Acinetobacter spp., the combination of colistin with rifampicin, fosfomycin or meropenem did not reduce the Day 28 ACM (range: 43.3%–52.2%) compared with colistin alone (range: 42.9%–57.4%). In the CREDIBLE-CR study [Citation31], ACM was 38.5% (15/39) with cefiderocol treatment and 17.7% (3/17) with BAT treatment (). The small sample size of A. baumannii infections in the CREDIBLE-CR study (39 patients in the cefiderocol arm and 17 patients in the BAT arm) resulted in 95% CIs that were wider than those seen in the larger AIDA [Citation32], Durante-Mangoni [Citation33], and Sirijatuphat [Citation34] studies, particularly for the BAT arm.
Figure 2. Day 28 all-cause mortality rates in carbapenem-resistant Acinetobacter spp. infections. ACM: all-cause mortality, BAT: best-available therapy, CI: confidence interval, CR: carbapenem resistant. In the CREDIBLE-CR study, patients were randomized 2:1 (cefiderocol:BAT). Acinetobacter spp. include: A. baumannii; A. nosocomialis.
ACM data for CRPA infections were limited in the studies due to small number of enrolled patients. In the AIDA study [Citation32], Day 28 ACM was reported for 30.8% (4/13) and 25.0% (2/8) for patients with CRPA in the colistin and colistin + meropenem treatment arms, respectively. In the CREDIBLE-CR study, among those with CRPA infections, 25.0% (3/12) of patients in the cefiderocol arm and 20.0% (2/10) of patients in the BAT arm died by Day 28 (unpublished data). The RESTORE-IMI 1 study was a pathogen-focused RCT to investigate the efficacy of imipenem/cilastatin/relebactam and imipenem/cilastatin + colistin against imipenem-non-susceptible infections () [Citation36]. In this study, patients with CRE or CRPA infections were enrolled and randomized (); however, pathogen-focused Day 28 (or Day 30) ACM was not reported (for either CRE or CRPA) [Citation36]. In this study, 50 patients were enrolled overall, and CRPA infections were reported for 24 of 31 patients across the two arms (11 with HAP/VAP, 3 with complicated intra-abdominal infections, and 10 with cUTI) in the primary analysis population, and only seven patients had CRE infections. Day 28 ACM overall was 9.5% (2/21) and 30% (3/10) in the imipenem/relebactam and colistin + imipenem arms, respectively [Citation36].
4. Discussion
This analysis of seven pathogen-focused randomized, interventional trials provides important, largely descriptive information about antibiotic regimens used to treat CR Gram-negative infections from different infection sites. Only three studies were designed with inferential hypothesis testing (CARE, AIDA, and Durante-Mangoni) [Citation29,Citation32,Citation33]. Perhaps unsurprisingly, the analysis highlights considerable inter-study heterogeneity, in terms of the number of patients screened and enrolled, infection sites, the individual pathogens and pathogen types investigated and, consequently, the control antibiotics used, the proportion of patients in ICU, severity of illness (e.g. varying APACHE II scores across PF-RCTs), and the geographical location for patient enrollment. Due to the limited sample sizes and inclusion of multiple infection types, patients were not consistently stratified in PF-RCTs based on specific factors related to severity of illness, which further complicates inferences across PF-RCTs. There were also differences in the primary analysis populations. Three of the approved antibiotic studies consisted of older generic agents and allowed for a longer period of time prior to study drug treatment to confirm carbapenem resistance; thus, randomized patients with only confirmed CR pathogens were investigated [Citation32–34]. In the Ji et al. study, patients with confirmed CR K. pneumoniae were enrolled, which were also tested for the presence of blaKPC suggesting a 48–72-hour time window, although information was not available [Citation35]. Hence, their focus was on comparative effectiveness. In the new antibiotic studies, the focus was on efficacy; the study protocols following regulatory guidance were more restrictive in terms of the time window permitted for potentially effective antibiotics, and patients with infections due to suspected, but not confirmed, CR pathogens could have been randomized [Citation29–31]. The CRE-focused investigations of Day 28 ACM involved fewer patients in each treatment arm than the Acinetobacter spp. studies. Despite allowing more infection types for new treatments, the CRE studies often enrolled patients with BSI or cUTI. In contrast, pneumonia was more frequent in the CR A. baumannii studies.
Among the CRE-focused studies, mortality rates were high and consistent, and broadly match those reported in non-randomized cohort studies [Citation3,Citation40]. Although mortality was numerically lower in each study with new agents compared with standard-of-care regimens, patient numbers in all studies were small and 95% CIs overlapped. In the studies analyzed,Enterobacterales mainly expressed KPC in the TANGO II study [Citation30] and the Ji et al. study [Citation35], whereas in the CREDIBLE-CR study both KPC and metallo-beta-lactamases (MBLs) were identified [Citation31,Citation41]. Of note, TANGO II and RESTORE-IMI 1 excluded patients with confirmed MBL-expressing CREs, respectively [Citation30,Citation36]. No information was provided in the CARE or AIDA studies on mechanism of carbapenem resistance [Citation29,Citation32].
Studies investigating only CR Acinetobacter spp. infections assessed a relatively large number of patients (up to 161 patients per treatment arm) mainly with nosocomial pneumonia, representing a population at high risk of mortality. As with CRE, mortality rates were consistently high and are similar to those reported in prospective or retrospective observational, cohort studies [Citation40,Citation42,Citation43]. In the three large studies (AIDA, Durante-Mangoni, Sirijatuphat), the combination treatment arms did not reduce ACM [Citation32–34]. The exception to this was the mortality rate in the BAT arm of the smaller CREDIBLE-CR study, which was considerably lower than generally reported in larger studies using the same antibiotics (colistin monotherapy or combination therapy) [Citation31]. Most of the BAT regimens in the CREDIBLE-CR study used to treat CR A. baumannii were colistin-based combinations and nearly all 17 patients had an individual antibiotic regimen. In the CREDIBLE CR study, imbalances were found at randomization in some demographic parameters and patient factors for patients with CR A. baumannii infection (e.g. age, renal function, ICU location at randomization, and prior/ongoing shock) [Citation31]. This highlights the need for additional large RCTs to investigate the role of cefiderocol in patients with CR A. baumannii. Of note, the recent APEKS-NP Phase 3 non-inferiority RCT, which compared cefiderocol with high-dose, extended-infusion meropenem for patients with nosocomial pneumonia caused by a Gram-negative pathogen, included 36 patients with CR A. baumannii as their baseline Gram-negative pathogen [Citation20]. In APEKS-NP, carbapenem resistance was based on EUCAST high-dose susceptibility breakpoint (i.e. resistant with meropenem MIC >8 µg/mL) [Citation20]. No difference in ACM at Day 28 was observed in the subgroup of patients with CR Acinetobacter spp. between cefiderocol or high-dose, extended-infusion meropenem (cefiderocol 33%, meropenem 39%) [Citation20].
The treatment patterns observed across these trials are reflective of current practice. A recent Europe-wide survey highlighted that physicians consider three main factors when selecting antibiotics for the treatment of CR infections: in vitro susceptibility, severity of illness, and infection site [Citation44]. Patients with CR cUTI are usually treated with monotherapy, whereas patients with pneumonia, BSI, or intra-abdominal infections are treated mainly with combination therapy with the anticipation of antibiotic synergism against CR Gram-negative pathogens, despite the lack of rigorous clinical evidence [Citation44]. In the PF-RCTs with BAT as the comparator arm [Citation30,Citation31], a high variability in antibiotic agents was found and these agents could have been given as either monotherapy or combination therapy. Although extreme caution should be exercised when making direct comparisons across trials due to heterogeneity in patients, infection sites, and pathogens, our current analysis suggests that monotherapy and combination therapy in severely ill patients resulted in similar Day 28 ACM rates. ACM rates were numerically lower with the newer antibiotics relative to colistin-based therapies for CRE infections [Citation29–31]. A post-hoc analysis of the AIDA study highlighted a favorable trend in ACM for colistin plus meropenem combination in patients with CRE but not with CR A. baumannii infections [Citation32]. However, ACM rates were largely equivocal, as reflected in the overlapping 95% CIs, across all combination and monotherapy regimens assessed for CR A. baumannii infections. The lack of mortality benefit with any studied combination or monotherapy regimen in CR Acinetobacter spp. infections potentially reflects the fact that patient factors substantially contribute to the observed ACM rates, and these are more difficult to balance when designing PF-RCTs of limited sample size.
It should be noted that the efficacy of the various new antibiotics was driven in part by their activity against different carbapenem resistance mechanisms. The mechanisms of carbapenem resistance are variable and frequently mixed in Gram-negative pathogens. Carbapenem resistance in Enterobacterales is driven mainly by the production of carbapenemase enzymes [Citation45]. Among the non-fermenter species, carbapenemases are rarely expressed in CRPA, and carbapenem resistance is often the result of porin channel mutations or upregulated efflux pumps as well as overexpression of AmpC, whereas CR A. baumannii often expresses Ambler Class D carbapenemases such as oxacillinases OXA-23, OXA-24, or OXA-151 [Citation46].
Several additional items should be considered when reviewing the findings across studies. The findings of our analysis are limited by the minimal information on CRPA for which the search retrieved suitable data from the AIDA study, and information was subsequently obtained for the CREDIBLE-CR study. In the RESTORE-IMI 1 PF-RCT, ACM data for patients with CRPA infections were not reported separately, despite 77% of the patients having CRPA infections [Citation36]. The small size and descriptive nature of the trials included in our analysis precludes a formal meta-analysis and the drawing of definitive conclusions. Interpretation of the results can be challenging when these small studies are conducted with high degree of heterogeneity and risk of bias [Citation37,Citation38]. Of note, the degree of heterogeneity and risk of bias were not assessed in this review as it was not a full systematic review, and it was not registered in the PROSPERO database. Instead, a comprehensive review of the registered studies and the available literature to identify studies with Day 28 ACM result in CR Gram-negative infections was conducted. However, two recently published review papers included quality appraisal of six of the analyzed studies included in our review. Five of the analyzed studies in this review were assessed in a systematic literature review conducted by Savoldi et al. [Citation38]. According to their assessment, the AIDA study had low risk of bias in all six domains, the Durante-Mangoni study had moderate or critical risk of bias, the Sirijatuphat, TANGO II, and CARE studies had low, moderate and critical risk of bias. The second review paper by Hsueh et al. provides a meta-analysis of randomized, controlled studies investigating cefiderocol and assessed the risk of bias [Citation47]. The assessment of the CREDIBLE-CR study found low or critical risk of bias across all six domains.
5. Conclusion
Pathogen-focused trials are vital to provide clinicians with guidance on the efficacy and safety of new antibiotics in their critically ill patients, for whom limited treatment options are available due to antimicrobial resistance. Understanding how to utilize new antibiotics once they are approved is of paramount importance to preserve their efficacy against difficult-to-treat and/or CR Gram-negative pathogens. Our analysis of seven recent randomized trials confirmed the value of these types of studies in generating clinically useful information.
6. Expert opinion
There is still a clear need for well-designed, larger pathogen-focused clinical studies [Citation48]. PF-RCTs are challenging but necessary to gain a better understanding on the efficacy or effectiveness of antibiotics against the WHO priority pathogens. Although several antibiotics have recently been approved with activity against CR bacteria, they have varying in vitro activity against CR pathogens and stability against common carbapenemases. Of the new treatment options reviewed, cefiderocol has the broadest spectrum of activity against CR lactose- and non-lactose fermenters, such as A. baumannii or P. aeruginosa. To move away from the polymyxin-based therapies with increased toxicity, new treatment options are still needed, supported by prospective RCTs enrolling the type of patients in whom these agents are likely to be utilized.
In principle, pathogen-focused studies can either support regulatory approval of new antibiotics or provide complementary information on comparative effectiveness for antibiotics already used in clinical practice. Ideally, a single, prospective PF-RCT could address both objectives. The new antibiotics that are being developed to treat multidrug-resistant pathogens will certainly be restricted for use in specific patients infected by the target pathogens. The currently approved new beta-lactam/beta-lactamase inhibitors or the single agents plazomicin and cefiderocol can be selected, depending on the target species and mechanism of resistance, as control agents in future PF-RCTs. Notably, no uniform standard of care is available against CR infections [Citation37].
Both pathogen-focused and double-blind, non-inferiority RCTs investigating new antibiotics with in vitro activity against the WHO priority pathogens are currently ongoing [Citation45,Citation48–51] and face many challenges. Undoubtedly, the design and execution of pathogen-focused studies can be (and should be) improved to facilitate enrollment and increase the sample size as studies may require a large number of investigation sites to enroll a limited number of patients for new antibiotics, particularly for pathogens with low and variable prevalence [Citation29,Citation50,Citation51]. However, it will require a combined and maximized effort from regulatory agencies, diagnostic device manufacturers, and clinical investigators. Within the context of regulatory clinical trial requirements, rapid diagnostic tests, especially direct specimen polymerase chain reaction (PCR), could greatly facilitate the screening to randomization process. Rapid diagnostic tests are particularly useful in confirming MBL-producing pathogenic bacteria, which have very few treatment options. However, incorporation of rapid diagnostics in PF-RCTs is logistically challenging as the commercially available rapid diagnostic products are not approved for use in all the countries and hospitals that participate in PF-RCTs, and currently these tools are not part of routine microbiological testing. Target pathogen-focused studies are important to understand the usefulness of any new antibiotic being developed to address antimicrobial resistance, but these studies are often too confounded to be suitable for regulatory approval. Furthermore, it needs to be considered that the current reimbursement model for new antibiotics is insufficient to support robust post-marketing, comparative effectiveness studies. Government-funded agencies should be involved to fund RCTs in order to support organized efforts by expert clinicians.
The key to improvement in efficiency of pathogen-focused clinical trials is the utilization of rapid diagnostics during screening for the desired pathogen. Direct specimen (e.g. blood, respiratory specimen, or urine) or culture-derived PCR or other nucleic acid amplification test, as well as easy biochemical tests, can identify the specific genes or phenotype for carbapenem resistance within a few hours. Unfortunately, such tests may have their own regulatory hurdles and, depending on the country and investigation site, these tests may be considered experimental requiring an additional time-consuming informed consent prior to screening of patients. Alternatively, expanding the use of local surveillance cultures and colonization status, before the patient develops an infection, could be considered to enrich the patient population [Citation52].
Although mortality has traditionally been used in certain infection site-specific trials among severely ill patients with infections in the critical care setting, it is often related to underlying illnesses and disease severity [Citation53,Citation54]. In addition, the detection of inter-treatment differences in mortality rates requires larger patient numbers [Citation53], which may not be achievable in pathogen-focused studies [Citation26,Citation37]. Consideration should instead be given to composite endpoints (i.e. desirability of outcome ranking [DOOR]) [Citation55], nested trial designs, and safety endpoints, along with improved statistical analysis approaches [Citation52]. Endpoints more proximal to the treatment intervention, and ‘soft’ but still clinically meaningful endpoints, such as ICU-free or ventilator-free days, may be useful alternatives [Citation54,Citation56]. While there is increased interest in examining composite DOOR endpoints, they should be carefully discussed for their suitability prior to incorporation into the design of PF-RCTs, which are heterogeneous in terms of infection sites and pathogens. Once a heterogeneous patient population is enrolled with various infection types, one composite endpoint may not be suitable. The uniform, most objective endpoint across antibiotic trials is all-cause mortality with dichotomous results and its combination with another endpoint prerequisites that the patient is alive. Depending on the comparator arm, a safety endpoint, such as acute kidney injury, may be combined with mortality or a clinical outcome [Citation53]. However, it is known that composite endpoints are challenging to interpret if clinically more important effects (e.g. mortality) are hidden by less important components [Citation53]. Better delineations of regulatory pathways to investigate certain CR pathogens, and standardization of BAT, infection sites, and definitions of carbapenem resistance and treatment response are also required to facilitate cross-study comparisons.
We need to identify ways to enhance enrollment of sufficient patient numbers to these types of studies, which may require broader collaborations such as ECRAID or COMBACTE, innovative study designs, and more suitable study endpoints [Citation57,Citation58]. Until such innovative clinical trials become available, real-world evidence from post-marketing prospective or retrospective observational studies will provide additional information [Citation59]. For example, adaptive and hybrid clinical trial designs may enable prompt enrollment as soon as CR Gram-negative infection is confirmed [Citation27,Citation60]. In addition, rapid diagnostic testing can facilitate patients being enrolled into studies earlier, reducing the confounding effects of prior antibiotic treatment. Finally, the inclusion of pharmacokinetic/ pharmacodynamic data may enable refinement of exposure–response analyses.
Both types of studies, such as those for regulatory approval of new agents and those investigating comparative effectiveness, will improve our understanding in the treatment of multidrug-resistant and/or CR Gram-negative infections. Their success relies on the combination of inter-related factors such as global epidemiology, local antibiotic resistance, evolving mechanism of resistance, understanding of risk factors, patient factors, physicians’ experience with and availability of control agents, and regulatory requirements, study design, sample size, selected outcomes, and robustness of analysis. Nevertheless, they are equally important to combat antimicrobial resistance.
Article highlights
Carbapenem-resistant (CR) Gram-negative pathogens carry a high risk of morbidity and mortality and are on the World Health Organization’s global critical priority list of antibiotic-resistant bacteria.
Patients with infections due to these difficult-to-treat CR pathogens are often excluded from clinical trials of new antibiotics, even when the product is intended to be used to treat antibiotic-resistant infections. Regulatory agency guidance for the development of new antimicrobial agents has provided the possibility of using pathogen-focused trial approvals as an alternative to the traditional infection site-specific approach.
We conducted a literature search and subsequent narrative analysis of pathogen-focused, randomized, controlled clinical trials (PF-RCTs) of new and older generic antibiotics that were conducted in seriously ill, hospitalized patients with CR Gram-negative infections.
Eight PF-RCTs were identified: three involved only CR Enterobacterales ([CRE]; CARE, TANGO II, Ji et al. study), two involved only CR Acinetobacter baumannii (Durante-Mangoni and Sirijatuphat studies), three studies included different CR Gram-negative infections: CREs, carbapenem-resistant Pseudomonas aeruginosa (CRPA), and CR Acinetobacter spp. in AIDA and CREDIBLE-CR studies, and CREs and CR P. aeruginosa in RESTORE-IMI 1 study.
Three new antibiotics (meropenem/vaborbactam, cefiderocol, and plazomicin) were investigated against CRE infections, while colistin with or without meropenem and cefepime with amoxicillin/clavulanic acid were also investigated against CRE infections. Against CR Acinetobacter spp. infections, the older generic antibiotics studied were colistin monotherapy or the combination of colistin plus meropenem or rifampicin or fosfomycin, whereas cefiderocol was investigated mainly as monotherapy. Against CRPA, studies investigated cefiderocol and imipenem/relebactam as new antibiotics, and colistin with or without a carbapenem as older generic antibiotics.
Day 28 all-cause mortality (ACM) rates in CR Acinetobacter spp. Infections were generally higher than in CRE or CRPA infections, and were consistent with rates in previous prospective or retrospective observational studies of CR Acinetobacter spp. There was no evidence for benefit of colistin combination therapy over colistin monotherapy. Limited information is available against CRPA from PF-RCTs regarding Day 28 ACM. Day 28 ACM rates in the CRE studies broadly matched those in observational studies and mortality appeared numerically lower with newer agents.
These pathogen-focused studies provide clinically relevant information to guide clinical decisions on antibiotic treatment in target populations with CR Gram-negative infections.
Author contributions
All authors had substantially contributed to the conception and design of the review article, interpretation of the relevant literature, drafting the review article and/or revising it for intellectual content.
Declaration of interest
Thomas P Lodise has received research grants from Merck and Motif Bio, and received consultancy fees from DoseME, Melinta, Merck, Motif Bio, Nabriva, Paratek, Shionogi, Spero, and Sunovion. Outside the submitted work, Matteo Bassetti has participated in advisory boards and/or received speaker honoraria and/or study grants from Angelini, Astellas, Bayer, BioMérieux, Cidara, Gilead, Menarini, MSD, Pfizer, Roche, and Shionogi. Ricard Ferrer has received consultancy fees for advisory boards or lectures from Shionogi, MSD, Pfizer, and Gilead. Thierry Naas has received honoraria from Pfizer and Shionogi. Yoshihito Niki has no conflict of interest. David L Paterson has received research grants from Shionogi and Merck, and speaker honoraria from Accelerate, BioMérieux, Merck, and Pfizer, and has participated in advisory boards for Entasis, Merck, and VenatoRx. Markus Zeitlinger has received consultancy fees for advisory boards or lectures from Shionogi. Roger Echols is a consultant for and has received consultancy fees from Shionogi.
Geolocation information
The reviewed studies enrolled patients globally.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Data availability
Details of best available therapy and unpublished mortality in the CREDIBLE-CR study included in the current manuscript were provided by Shionogi & Co., Ltd., Osaka, Japan, on request from the authors. All other data included in the manuscript were available in the full-text articles of the studies.
Supplemental Material
Download MS Word (210.1 KB)Acknowledgments
Editorial assistance was provided by Highfield Communication, funded by Shionogi. The data included in the current analysis were partly presented in an abstract for the 30th European Congress of Clinical Microbiology & Infectious Diseases in 2020: Lodise T, Bassetti M, Ferrer R, Naas T, Niki Y, Paterson D, Zeitlinger M, Tillotson G, Echols R. Comparison of 28-day mortality rates of recently completed prospective, randomised treatment studies of adult patients with carbapenem-resistant Gram-negative bacterial infections. Abstract 2497. Available at: https://www.escmid.org/escmid_publications/eccmid_abstract_book/. Accessed: 20 October 2021.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- WHO. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. 2017 [cited 2020 Mar 2]. Available from: https://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf
- Lemos EV, de La Hoz FP, Einarson TR, et al. Carbapenem resistance and mortality in patients with Acinetobacter baumannii infection: systematic review and meta-analysis. Clin Microbiol Infect. 2014;20(5):416–423.
- Falagas ME, Lourida P, Poulikakos P, et al. Antibiotic treatment of infections due to carbapenem-resistant Enterobacteriaceae: systematic evaluation of the available evidence. Antimicrob Agents Chemother. 2014;58(2):654–663.
- Cai B, Echols R, Magee G, et al. Prevalence of carbapenem-resistant Gram-negative infections in the United States predominated by Acinetobacter baumannii and Pseudomonas aeruginosa. Open Forum Infect Dis. 2017;4(3):ofx176.
- Maraolo AE, Corcione S, Grossi A, et al. The impact of carbapenem resistance on mortality in patients with Klebsiella pneumoniae bloodstream infection: an individual patient data meta-analysis of 1952 patients. Infect Dis Ther. 2021;10(1):541–558.
- Infectious Diseases Society of America. The 10 × ’20 initiative: pursuing a global commitment to develop 10 new antibacterial drugs by 2020. Clin Infect Dis. 2010;50:1081–1083.
- Infectious Diseases Society of America (IDSA). White Paper: recommendations on the conduct of superiority and organism-specific clinical trials of antibacterial agents for the treatment of infections caused by drug-resistant bacterial pathogens. Clin Infect Dis. 2012;55(8):1031–1046.
- Talbot GH, Jezek A, Murray BE, et al. The Infectious Diseases Society of America’s 10 × ‘20 Initiative (10 new systemic antibacterial agents US food and drug administration approved by 2020): is 20 × ‘20 a possibility?. Clin Infect Dis. 2019;69(1):1–11.
- Kirby T. Europe to boost development of new antimicrobial drugs. Lancet. 2012;379(9833):2229–2230.
- Gajdács M. The concept of an ideal antibiotic: implications for drug design. Molecules. 2019;24(5):892.
- Echols R, Ariyasu M, Nagata TD. Pathogen-focused clinical development to address unmet medical need: cefiderocol targeting carbapenem resistance. Clin Infect Dis. 2019;69(Suppl 7):S559–564.
- Doi Y. Treatment options for carbapenem-resistant Gram-negative bacterial infections. Clin Infect Dis. 2019;69(Suppl 7):S565–575.
- Connolly LE, Riddle V, Cebrik D, et al. A multicenter, randomized, double-blind, phase 2 study of the efficacy and safety of plazomicin compared with levofloxacin in the treatment of complicated urinary tract infection and acute pyelonephritis. Antimicrob Agents Chemother. 2018;62(4):e01989–17.
- Wagenlehner FME, Cloutier DJ, Komirenko AS, et al. Once-daily plazomicin for complicated urinary tract infections. N Engl J Med. 2019;380(8):729–740.
- Kaye KS, Bhowmick T, Metallidis S, et al. Effect of meropenem-vaborbactam vs piperacillin-tazobactam on clinical cure or improvement and microbial eradication in complicated urinary tract infection: the TANGO I randomized clinical trial. JAMA. 2018;319(8):788–799.
- Sims M, Mariyanovski V, McLeroth P, et al. Prospective, randomized, double-blind, Phase 2 dose-ranging study comparing efficacy and safety of imipenem/cilastatin plus relebactam with imipenem/cilastatin alone in patients with complicated urinary tract infections. J Antimicrob Chemother. 2017;72(9):2616–2626.
- Lucasti C, Vasile L, Sandesc D, et al. Phase 2, dose-ranging study of relebactam with imipenem-cilastatin in subjects with complicated intra-abdominal Infection. Antimicrob Agents Chemother. 2016;60(10):6234–6243.
- Titov I, Wunderink RG, Roquilly A, et al. A randomized, double-blind, multicenter trial comparing efficacy and safety of imipenem/cilastatin/relebactam versus piperacillin/tazobactam in adults with hospital-acquired or ventilator-associated bacterial pneumonia (RESTORE-IMI 2 study). Clin Infect Dis. 2021;73(11):e4539–e4548. DOI:https://doi.org/10.1093/cid/ciaa803.
- Portsmouth S, van Veenhuyzen D, Echols R, et al. Cefiderocol versus imipenem-cilastatin for the treatment of complicated urinary tract infections caused by Gram-negative uropathogens: a phase 2, randomised, double-blind, non-inferiority trial. Lancet Infect Dis. 2018;18(12):1319–1328.
- Wunderink RG, Matsunaga Y, Ariyasu M, et al. Cefiderocol versus high-dose, extended-infusion meropenem for the treatment of Gram-negative nosocomial pneumonia (APEKS-NP): a randomised, double-blind, phase 3, non-inferiority trial. Lancet Infect Dis. 2021;21(2):213–225.
- Tabak YP, Sung AH, Ye G, et al. Attributable clinical and economic burden of carbapenem-non-susceptible Gram-negative infections in patients hospitalized with complicated urinary tract infections. J Hosp Infect. 2019;102(1):37–44.
- Bassetti M, Welte T, Wunderink RG. Treatment of Gram-negative pneumonia in the critical care setting: is the beta-lactam antibiotic backbone broken beyond repair?. Crit Care. 2016;20:19.
- Skrupky LP, Tellor BR, Mazuski JE. Current strategies for the treatment of complicated intraabdominal infections. Expert Opin Pharmacother. 2013;14(14):1933–1947.
- FDA. Antibacterial therapies for patients with an unmet medical need for the treatment of serious bacterial diseases. Guidance for Industry. 2017. [cited 2021 Apr 14]. Available from: https://www.fda.gov/files/drugs/published/Antibacterial-Therapies-for-Patients-With-an-Unmet-Medical-Need-for-the-Treatment-of-Serious-Bacterial-Diseases.pdf
- EMA. Guideline on the evaluation of medicinal products indicated for treatment of bacterial infections. Rev. 3. 2018 [cited 2021 Nov 12]. Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/draft-guideline-evaluation-medicinal-products-indicated-treatment-bacterial-infections-revision-3_en.pdf
- Bassetti M, Ariyasu M, Binkowitz B, et al. Designing a pathogen-focused study to address the high unmet medical need represented by carbapenem-resistant Gram-negative pathogens – the international, multicentre, randomized, open-label, Phase 3 CREDIBLE-CR study. Infect Drug Resist. 2019;12:3607–3623.
- Paul M, Scudeller L. Clinical research designs to study treatment effects for multidrug-resistant bacteria. Clin Microbiol Infect. 2019;25(8):929–931.
- Jorgensen SCJ, Rybak MJ. Pathogen-specific clinical trials: a new paradigm in clinical trials for multidrug-resistant organisms. Infect Dis Ther. 2018;7(4):401–405.
- McKinnell JA, Dwyer JP, Talbot GH, et al. Plazomicin for infections caused by carbapenem-resistant Enterobacteriaceae. N Engl J Med. 2019;380(8):791–793.
- Wunderink RG, Giamarellos-Bourboulis EJ, Rahav G, et al. Effect and safety of meropenem-vaborbactam versus best-available therapy in patients with carbapenem-resistant Enterobacteriaceae infections: the TANGO II randomized clinical trial. Infect Dis Ther. 2018;7(4):439–455.
- Bassetti M, Echols R, Matsunaga Y, et al. Efficacy and safety of cefiderocol or best available therapy for the treatment of serious infections caused by carbapenem-resistant Gram-negative bacteria (CREDIBLE-CR): a andomized, open-label, multicentre, pathogen-focused, descriptive, phase 3 trial. Lancet Infect Dis. 2021;21(2):226–240.
- Paul M, Daikos GL, Durante-Mangoni E, et al. Colistin alone versus colistin plus meropenem for treatment of severe infections caused by carbapenem-resistant Gram-negative bacteria: an open-label, randomised controlled trial. Lancet Infect Dis. 2018;18(4):391–400.
- Durante-Mangoni E, Signoriello G, Andini R, et al. Colistin and rifampicin compared with colistin alone for the treatment of serious infections due to extensively drug-resistant Acinetobacter baumannii: a multicentre, randomized clinical trial. Clin Infect Dis. 2013;57(3):349–358.
- Sirijatuphat R, Thamlikitkul V. Preliminary study of colistin versus colistin plus fosfomycin for treatment of carbapenem-resistant Acinetobacter baumannii infections. Antimicrob Agents Chemother. 2014;58(9):5598–5601.
- Ji S, Lv F, Du X, et al. Cefepime combined with amoxicillin/clavulanic acid: a new choice for the KPC-producing K. pneumoniae infection. Int J Infect Dis. 2015;38:108–114.
- Motsch J, Murta de Oliveira C, Stus V, et al. RESTORE-IMI 1: a multicenter, randomized, double-blind trial comparing efficacy and safety of imipenem/relebactam vs colistin plus imipenem in patients with imipenem-nonsusceptible bacterial infections. Clin Infect Dis. 2020;70(9):1799–1808.
- Yahav D, Tau N, Shepshelovich D. Assessment of data supporting the efficacy of new antibiotics for treating infections caused by multidrug-resistant bacteria. Clin Infect Dis. 2021;72(11):1968–1974.
- Savoldi A, Carrara E, Piddock LJV, et al. The role of combination therapy in the treatment of severe infections caused by carbapenem resistant gram-negatives: a systematic review of clinical studies. BMC Infect Dis. 2021;21(1):545.
- Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281.
- Schmid A, Wolfensberger A, Nemeth J, et al. Monotherapy versus combination therapy for multidrug-resistant Gram-negative infections: systematic review and meta-analysis. Sci Rep. 2019;9(1):15290.
- Matsunaga Y, Ariyasu M, Takemura M, et al. Cefiderocol treatment for serious infections caused by carbapenem-resistant bacteria: post-hoc analysis of outcomes by pathogen in the CREDIBLE-CR study. Abstract 165. Open Forum Infect Dis. 2020;7(Suppl 1):S212.
- Chen Z, Chen Y, Fang Y, et al. Meta-analysis of colistin for the treatment of Acinetobacter baumannii infection. Sci Rep. 2015;5:17091.
- Lyu C, Zhang Y, Liu X, et al. Clinical efficacy and safety of polymyxins based versus non-polymyxins based therapies in the infections caused by carbapenem-resistant Acinetobacter baumannii: a systematic review and meta-analysis. BMC Infect Dis. 2020;20(1):296.
- Papst L, Beović B, Pulcini C, et al. Antibiotic treatment of infections caused by carbapenem-resistant Gram-negative bacilli: an international ESCMID cross-sectional survey among infectious diseases specialists practicing in large hospitals. Clin Microbiol Infect. 2018;24(10):1070–1076.
- Paterson DL, Isler B, Stewart A. New treatment options for multiresistant Gram negatives. Curr Opin Infect Dis. 2020;33(2):214–223.
- Gniadek TJ, Carroll KC, Simner PJ. Carbapenem-resistant non-glucose-fermenting Gram-negative bacilli: the missing piece to the puzzle. J Clin Microbiol. 2016;54(7):1700–1710.
- Hsueh SC, Chao CM, Wang CY, et al. Clinical efficacy and safety of cefiderocol in the treatment of acute bacterial infections: a systematic review and meta-analysis of randomised controlled trials. J Glob Antimicrob Resist. 2021;24:376–382.
- Theuretzbacher U, Outterson K, Engel A, et al. The global preclinical antibacterial pipeline. Nat Rev Microbiol. 2020;18(5):275–285.
- Pew Charitable Trust. Tracking the global pipeline of antibiotics in development. March 2021 [cited 2021 Jul 14]. Available from: https://www.pewtrusts.org/en/research-and-analysis/issue-briefs/2021/03/tracking-the-global-pipeline-of-antibiotics-in-development
- Clinicaltrials.gov. Study to evaluate the efficacy and safety of intravenous sulbactam-ETX2514 in the treatment of patients with infections caused by Acinetobacter baumannii-calcoaceticus complex (ATTACK). [cited 2021 Jul 13]. Available from: https://clinicaltrials.gov/ct2/show/NCT03894046
- Clinicaltrials.gov. Efficacy, safety, and tolerability of ATM-AVI in the treatment of serious infection due to MBL-producing Gram-negative bacteria. [cited 2021 Nov 5]. Available from: https://clinicaltrials.gov/ct2/show/NCT03580044
- Rex JH, Talbot GH, Goldberger MJ, et al. Progress in the fight against multidrug-resistant bacteria 2005–2016: modern noninferiority trial designs enable antibiotic development in advance of epidemic bacterial resistance. Clin Infect Dis. 2017;65(1):141–146.
- Timsit JF, de Kraker MEA, Sommer H, et al. Appropriate endpoints for evaluation of new antibiotic therapies for severe infections: a perspective from COMBACTE’s STAT-Net. Intensive Care Med. 2017;43(7):1002–1012.
- Farkas J. PulmCrit – chasing mortality endpoints is a fool’s errand. 2018; March 12. [cited 2021 Apr 14]. Available from: https://emcrit.org/pulmcrit/mortality
- Evans SR, Rubin D, Follmann D, et al. Desirability of outcome ranking (DOOR) and response adjusted for duration of antibiotic risk (RADAR). Clin Infect Dis. 2015;61(5):800–806.
- Weiss E, Zahar J-R, Alder J, et al. Elaboration of consensus clinical endpoints to evaluate antimicrobial treatment efficacy in future hospital-acquired/ventilator-associated bacterial pneumonia clinical trials. Clin Infect Dis. 2019;69(11):1912–1918.
- ECRAID. European clinical research alliance on infectious diseases. [cited 2021 Jul 14]. Available from: https://www.ecraid.eu
- COMBACTE. Combatting bacterial resistance in Europe. [cited 2021 Jul 14]. Available from: https://www.combacte.com
- Groft LM, Claeys KC, Heil EL. An evaluation of meropenem/vaborbactam for the treatment of nosocomial pneumonia. Expert Opin Pharmacother. 2021;22(3):265–271.
- Lanini S, Ioannidis JPA, Vairo F, et al. Non-inferiority versus superiority trial design for new antibiotics in an era of high antimicrobial resistance: the case for post-marketing, adaptive randomised controlled trials. Lancet Infect Dis. 2019;19(12):e444–e451.
