Abstract
Objective
To assess the effect of cochlear implantation on the function of the semicircular canals (SCC) and on experienced vestibular symptoms. Second, to determine the relation between vestibular test results.
Design
Retrospective cohort study assessing absolute and categorised results of caloric irrigation test, video Head Impulse Test (vHIT) and Dizziness Handicap Inventory (DHI) before and after cochlear implantation.
Study sample: 192 patients, aged ≥7 years old, without preoperative areflexia.
Results
Mean maximum slow phase velocity decreased with 3.1°/s and 4.7°/s for warm and cold caloric irrigation respectively. About 37.4% of the patients deteriorated one or more categories on caloric testing. Complete caloric postoperative areflexia was found in 6.2%. Mean vHIT gain decreased with 0.06, 0.04 and 0.05 for anterior, lateral and posterior SCC, respectively. Seven patients (7.7%) acquired an abnormal gain value for the anterior SCC. Only mean score on DHI’s physical subdomain rose significantly (1.4 points). Overall, 9.0% of the patients deteriorated one or two categories on DHI. Only few weak correlations were found between caloric test, vHIT and DHI shifts.
Conclusions
Although mean objective and subjective-physical vestibular deteriorations were significant, its clinical impact seems limited. However, 9% of patients experience vestibular deterioration, thus, advocate assessment. Vestibular test results show no or merely weak mutual correlations.
Introduction
After Djourno and Eyriès’s auditory implantation in 1957 (Djourno and Eyries Citation1957), methods and implantable hearing devices for deaf patients have been improved and nowadays many adults and children benefit from cochlear implantation. Initially, vestibular function was not a factor of interest and cochlear implantation was generally considered safe. However, in a recent systematic review, a reduction of vestibular function, assessed with caloric testing, was reported in 37% of the implanted patients (Kuang, Haversat, and Michaelides Citation2015). Yet, many studies do not take into account the experienced impact on daily life. The Dizziness Handicap Inventory (DHI) is a widely used questionnaire that assesses subjective functional, physical and emotional handicaps, induced by possible balance problems (Jacobson and Newman Citation1990). However, the correlation between subjective vestibular symptoms and objective clinical data seems to vary (Mutlu and Serbetcioglu Citation2013). In 2017, a systematic review and meta-analysis were performed to determine which vestibular assessment should be performed to evaluate vestibular function after cochlear implantation (Abouzayd et al. Citation2017). Only a few studies used a standardised questionnaire (e.g. DHI) and the methods of vestibular testing often differed. Although the caloric irrigation test is a relatively easy and widely used clinical test to assess vestibular function, it also has its limitations. This test only measures the function of the lateral semicircular canal (SCC). Besides, between-subject differences in maximum slow phase velocity (MSPV) of the nystagmus in healthy individuals show a wide range, probably because of variations in anatomy or test execution (Curthoys Citation2012). Uniform reference values and classifications for vestibular deterioration are lacking. Furthermore, the caloric irrigation test mainly encompasses very low frequency stimulation of the human vestibular system (Shepard and Jacobson Citation2016). In contrast, the Head Impulse Test (HIT) examines all SCCs and tests the sensitivity of the vestibular system for higher frequencies (Alhabib and Saliba Citation2017). It measures the vestibulo-ocular-reflex (VOR) by observing eye movements in response to short head accelerations performed in such a way that only one specific SCC is activated. Since the introduction of the video HIT (vHIT), a more accurate and quantified observation of the VOR can be obtained and even covert saccades can easily be recognised (Ulmer and Chays Citation2005; Weber et al. Citation2009; Blodow et al. Citation2013). Recent studies have investigated the effectiveness of this VOR test in patients with different vestibular pathologies and results are promising (Alhabib and Saliba Citation2017). However, data on the value of this test for the assessment of vestibular function in patients who received a cochlear implant (CI) are limited.
The aim of this study is to evaluate the impact of cochlear implantation on vestibular function, by comparing pre- and postoperative objective data consisting of conventional low-frequency caloric irrigation, high-frequency vHIT and subjective data obtained by using the DHI questionnaire. Furthermore, this study aims to investigate the relation between these different tests.
Materials and methods
Patients and inclusion criteria
All patients who underwent (routine) vestibular testing before and after receiving a CI at our hospital between 2010 and 2017 were selected. Patients younger than 7 years of age and patients with definite preoperative areflexia in the operated ear were excluded. The former because VOR gain is highly dependent on age below 6 years of age and intra-individual VOR gain variability appears to be relatively high below 7 years of age (Wiener-Vacher and Wiener Citation2017). In patients undergoing (sequential) bilateral implantation, only data regarding the first implantation were considered. All data were retrospectively collected from patients’ medical records. The procedures in this investigation were in accordance with legislation (the Medical Research Involving Human Subjects Act) and ethical standards on human experimentation in the Netherlands.
Vestibular assessment
According to standard procedures, all included patients underwent oculomotor tests (i.e. smooth pursuit, saccades, optokinetic nystagmus, spontaneous nystagmus and gaze testing), to exclude central lesions, followed by specific vestibular assessment, i.e. vHIT, rotatory chair test (“velocity step”), bithermal caloric irrigation tests, and the DHI questionnaire. Except for vHIT, all eye movements were recorded using electronystagmography. Data of the caloric irrigation and rotatory chair tests were obtained and analysed using BalanceLab 2.0.0 (Ekida GmbH, Buggingen, Germany). Velocity step test was performed as previously described (Oonk et al. Citation2015). Caloric irrigational testing was performed using Variotherm (Atmos Medizin Technik GmbH, Lenzkirch, Germany) by applying cold water of 30 °C and warm water of 44 °C in each external auditory canal for 30 s. The MSPV of the nystagmus was subsequently determined for each condition and analysed. Caloric irrigations were performed in a fixed order: warm followed by cold irrigation and when applicable started with the subjective worst side. vHIT was performed using the vHIT Ulmer Evolution (Synapsys, Marseille, France). This device consists of a motorised camera on a monopod placed at 0.9 m in front of the patient’s eyes, capturing eye movements in relation to head movement with a video sampling rate of 100 Hz. To focus on calibrated points, three small red dots were presented on the wall, at central gaze and at 20° left and right. Performing the vHIT yielded the (mean) gains of the VOR for the six SCCs (i.e. right anterior, left anterior, right lateral, left lateral, right posterior and left posterior), which were calculated by dividing the velocity of the eyes (degrees/second) by the velocity of the head (degrees/second). A validated translation of the DHI was used to assess vestibular function subjectively (Vereeck et al. Citation2007). This yielded scores on the emotional, functional and physical domain and an accumulated total score. A higher DHI score indicates more (severe) symptoms.
Analysis
Data was analysed using IBM SPSS Statistics for Windows Version 22 (IBM Corp., Armonk, NY). The level of significance was defined at 5%. The last preoperative and first postoperative vestibular tests were included in the analyses. A paired Student’s t-test was used to analyse differences in absolute pre-operative and post-operative outcome of the vestibular tests. Individual vestibular function was defined according to different categories for each assessment, as shown in . DHI scores were categorised according to Whitney et al. (Citation2004) and vHIT categories were based on manufacturer’s normative data. Vestibular preponderance (unilateral weakness) on the caloric test was determined using Jongkees’ formula (Jongkees and Philipszoon Citation1964), with an absolute value of more than 20% considered as abnormal. The sign test was used to determine the significance of categorical shifts.
For caloric irrigation testing, best responses (either warm or cold) were used for categorical allocation, pre- and postoperatively per side. Since the normal range for caloric testing is known to be very wide, an additional analysis was performed to avoid underestimation of individual caloric deterioration, by dividing the normal caloric category into two subcategories (warm irrigation: MSPV 10–30°/s normal-lower-half and MSPV 31–52°/s normal-upper-half; cold irrigation: MSPV 7–18°/s normal-lower-half and MSPV 19–31°/s normal-upper-half). Additionally, contralateral deterioration of more than one caloric category was calculated, in order to account for individual non-CI-related vestibular deterioration in interpreting the results.
Definite areflexia was defined as MSPV of caloric responses ≤ 4°/s for warm caloric irrigation and/or ≤ 3°/s for cold caloric testing in combination with short time constants, i.e. tau ≤ 3 s obtained with rotary velocity step chair testing. The latter prerequisite was added to minimise the chance that low/absent responses on caloric testing were only the result of poor irrigation.
Spearman’s rank correlation coefficients (rs) were calculated to determine the correlation between the pre- and postoperative changes of the different tests (caloric test, vHIT and DHI). This test was also used to determine whether gender, side of implantation, age of implantation and/or timing of postoperative assessment were correlated with these changes. The correlation of age of implantation was also separately tested for patients younger than 18 years of age and for patients of 18 years and older. Significant correlations were divided into weak (rs ≤ 0.3), moderate (0.3 < rs ≤ 0.7) and strong (rs > 0.7) correlations.
Results
One hundred ninety-two patients with pre- and postoperative vestibular test results were included in this study (six other patients were excluded because of pre-existing definite areflexia). This comprised 83 males (43.2%) and 109 females (56.8%). One hundred right ears (52.1%) and 92 left ears (47.9%) were implanted. The mean age at implantation was 53.4 years (range 7.1–84.3 years). Patients had postoperative vestibular testing at a mean follow-up interval of 7.9 months (range 1.2–45.1 months; 79% of the patients was tested within the first postoperative year). Mean test results are presented in .
Objective and subjective vestibular deterioration
Caloric irrigation
Paired data of warm and cold caloric tests were available for 172 and 152 patients respectively, comprising a total of 179/192 patients. Mean MSPV of warm and cold irrigation testing decreased with 3.1°/s and 4.7°/s, respectively (). This is reflected by the regression lines in . A majority of patients showed a postoperative deterioration in MSPV. Contralateral ears showed no significant mean change for either warm (p = 0.99) or cold (p = 0.13) caloric irrigation testing. Three patients deteriorated two categories (5-category scale) in the contralateral ear: one deteriorated to areflexia and the other two patients deteriorated to the lower half of the normal category. No patients deteriorated more than two categories in this ear. Ipsilaterally, 35 patients (19.6%) showed a decrease of one or more categories postoperatively, when the best value (warm or cold) of the four caloric categories of was applied (). However, by using the 5-category scale, the number of deteriorating patients is almost twice as high (i.e. 67 patients, 37.4%; ). The deterioration within the normal group was significant (Sign test, p < 0.001). 11 patients (6.2%) acquired caloric areflexia postoperatively and 24 patients (14.3%) dropped from normal or hyperreflexia to hypo- or areflexia. Differences between the pre- and postoperative caloric diagnoses were significant (Sign test, p < 0.001). Taking into account velocity step test results, eventually four patients (2.2%) developed definite areflexia after implantation. Furthermore, 26.1% of the patients acquired vestibular asymmetry.
Figure 1. Individual changes on the caloric test (A) and video Head Impulse Test (B–D) before and after implantation. (A) Change in MSPV for warm (light grey dots with striped regression line) and cold (dark grey squares with continuous regression line) caloric irrigation. Horizontal and vertical dashed lines indicate lower normative MPSV values for warm (10°/s) and cold (7°/s) irrigation. (B–D) Change in gain for anterior (B), lateral (C) and posterior (D) SCC, with corresponding regression lines. Shaded areas show subjects with absolute deterioration. MSPV: maximum slow phase velocity. SCC: semicircular canal.
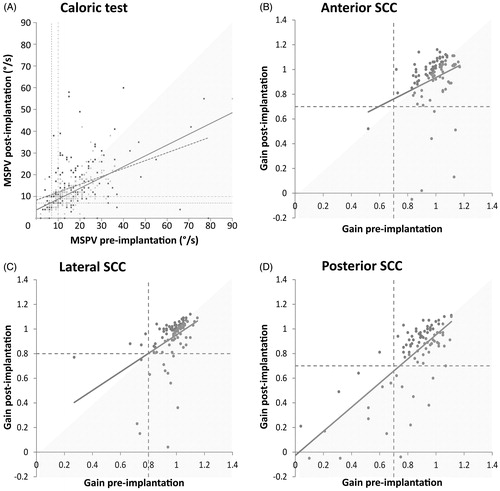
Table 1. Normative ranges of vestibular outcomes and category definitions.
Table 2. Mean changes in vestibular test results after cochlear implantation.
Table 3. Categorical comparison of caloric test results pre- and post-implantation.
vHIT
The vHIT was performed in 104 patients pre- and postoperatively. For 13 patients, only VOR gains of the lateral SCC were available. shows the categorical pre- versus postoperative VOR gain. For both anterior and posterior SCC, seven patients (7.7%) acquired abnormal VOR gain, eight patients (7.7%) moved to abnormal for the lateral SCC. Only the pre- versus postoperative difference for the anterior SCC was significant (Sign test, p = 0.016). vHIT diagnoses of 15 patients (14.4%) showed a deterioration of one or more SCCs.
Table 4. Categorical comparison of video Head Impulse Test results pre- and post-implantation.
Individual vHIT data are shown in . Mean gain dropped minimally for all three SCCs (). This minimal decrease is reflected in the regression lines for each SCC.
DHI
Paired DHI data were available for 144 patients. Total DHI score, as well as the score on the functional or emotional domain did not change significantly after cochlear implantation. In contrast, the DHI score on the physical domain revealed a statistically significant increase of 1.4 points (). Categorical comparison of the (total) DHI score is shown in . A significant number of patients (n = 13, 9.0%) showed an increase of symptoms of dizziness, resulting in a change of one (n = 12) or two (n = 1) categories, while 4 patients (2.8%) revealed a categorical decrease of symptoms (Sign test, p = 0.049).
Table 5. Categorical comparison of DHI results.
Correlating factors
Changes in vestibular tests were not correlated with gender, side of implantation and generally not correlated with age of implantation and timing of postoperative assessment (p > 0.05). Age of implantation was only weakly correlated with the shift in vHIT VOR gain for the anterior (rs = 0.21, p = 0.049) and lateral (rs = 0.27, p = 0.005) SCC and shift in DHI physical subdomain score (rs = 0.16, p = 0.049), while for the length of follow-up interval only a weak (negative) correlation was found with the anterior SCC VOR gain (rs = −0.22, p = 0.038). When the 18 patients younger than 18 years of age were excluded from the correlation analysis regarding age of implantation, still weak to moderate correlations were found; with the shift in vHIT VOR gain for the lateral (rs=0.35, p = 0.001) and posterior (rs = 0.28, p = 0.013) SCC and with shift in DHI physical subdomain score (rs=0.20, p = 0.021).
Correlation between vestibular assessments
Correlation analysis between pre- and postoperative differences of the caloric test (warm and cold MSPV), vHIT (gain for anterior, lateral and posterior SCC) and DHI (functional, emotional, physical and total score) results showed only few significant correlations. Merely weak correlations were found between the warm irrigational caloric test and either the vHIT gain on the lateral (rs=0.30, p = 0.003) or the posterior SCC (rs=0.25, p = 0.024) and also between the cold irrigational caloric test and the physical subdomain of DHI (rs=0.19, p = 0.038). Moderate correlations were found between gains of the several SCCs (anterior, lateral and posterior) mutually (rs=0.37–0.44, p ≤ 0.001) and also between the DHI domains mutually (rs=0.47–0.65, p < 0.001). Other correlations were not statistically significant (Table S1, Supplemental Material).
Discussion
This study investigated the effect of cochlear implantation on vestibular function by retrospectively comparing pre- and postoperative results of the caloric irrigational test, vHIT and DHI. For each assessment, categories were defined to compare the pre- versus postoperative vestibular function. Although mean changes for the whole group were minimal, the present findings show evidence of vestibular deterioration in a substantial group. In 6.2% of the patients, cochlear implantation led to caloric areflexia and 26.1% of the patients acquired vestibular asymmetry. Furthermore, in 8% of the patients the anterior SCC deteriorated on vHIT (while the other SCCs showed a similar trend). Mean DHI score on the physical subdomain increased statistically significant, albeit merely 1.4 points. Different studies have reported a range from 3 to 18 points difference as clinically significant (Jacobson and Newman Citation1990; Friscia et al. Citation2014). Therefore, we considered these small mean changes on DHI as not clinically relevant. However, still a small but significant number of patients (13/145) deteriorated one or more categories, as their subjective responses showed a change from mild to moderate or severe handicap. Caloric irrigation, vHIT and DHI tests seem not or only weakly correlated to each other in detecting postoperative changes due to cochlear implantation.
A deterioration of mean caloric response after cochlear implantation was also found in a recent meta-analysis across 19 heterogeneous studies (Ibrahim et al. Citation2017). Although, in the present study, these changes were very slight. This emphasises the assessment of individual changes. Since the normal range of caloric testing is very broad, the caloric test results in the present study were also evaluated by splitting the broad MSPV range of the normal category into two categories, in order to obtain a more realistic view of possible postoperative changes on the individual level. A systematic review by Kuang, Haversat, and Michaelides (Citation2015), reported that 37% of the implanted patients exhibit reduced MSPV values postoperatively and 34% of the patients acquire asymmetry after implantation. Unfortunately, absolute MSPV values were often not reported by the individual studies, thus hindering interpretation of caloric deterioration between research groups. Nevertheless, the present analysis shows similar deterioration, i.e. 37.4% dropped one or more categories and 26.3% acquired vestibular asymmetry.
Until now, vHIT results in adult patients undergoing cochlear implantation have only been reported in a few studies. Batuecas-Caletrio et al. (Citation2015) found that six patients (20%) acquired abnormal lateral SCC gain values after cochlear implantation, using the same cut-off value (0.8) as used in the present study. This percentage is higher than found in the present study (7.7%), but they tested patients on the second day after surgery, which may mainly reflect a very acute change after surgery. In contrast, Korsager et al. (Citation2018) showed no difference of mean lateral SCC gain values up to one month postoperatively. Regarding mean lateral SCC VOR gain changes, the meta-analysis of Ibrahim et al. that pooled five studies (n = 122 patients) with several methods of HIT execution, including search coils, did not find a mean change after cochlear implantation (Ibrahim et al. Citation2017). The very small mean VOR gain deteriorations found in the present study were not considered clinically relevant in any SCC.
This meta-analysis also reported a non-significant effect of cochlear implantation on DHI total score (Ibrahim et al. Citation2017), which is in line with our findings. Interestingly, Buchman et al. (Citation2004) found no significant worsening in the DHI for all subdomains, but a significant handicap improvement was found on the emotional subdomain. In contrast, we did not find any change on this subdomain, while the physical subdomain decreased a little. Nine per cent of the patients experienced deterioration of DHI category. Even much higher proportions of subjective vestibular deterioration have been reported by other studies, but often assessed without using standardised questionnaires. Reported deterioration of DHI category varies from 0 to 28% of the patients (Migliaccio et al. Citation2005; Basta et al. Citation2008; Wagner et al. Citation2010; Le Nobel et al. Citation2016). An explanation for this variation might be the relatively small number of patients in other studies.
The lack of good correlation of caloric test and vHIT with DHI in patients who underwent cochlear implantation has been described previously in the systematic review and meta-analysis of Abouzayd et al. (Citation2017). Since many patients have dizziness complaints preoperatively and the relation of the changes was deemed important, we used the pre- versus postoperative difference to assess correlations. Our analyses support the conclusion that a single test would not suffice for vestibular assessment and that vestibular tests are rather complementary (Abouzayd et al. Citation2017; Alhabib and Saliba Citation2017). This relates to the relative specificity of the tests for a certain vestibular sensor, in a specific frequency range.
Interestingly, our data also show that a few patients exhibit improvement after implantation on caloric testing, vHIT and/or DHI. Some authors have suggested that CI stimulation can influence vestibular test results. Bance et al. (Citation1998) showed cross-stimulation of vestibular afferents of the implanted ear in one patient and Filipo et al. (Citation2006) described improvement of results of caloric test and/or rotational chair testing in some patients after CI activation. Some other studies described significant improvements of the vestibulo-spinal reflex measured by postural stability tests (Buchman et al. Citation2004; Filipo et al. Citation2006). However, we assume that vestibular improvement in our study is inherent to the relative test variability.
The postoperative evaluation in the present study was performed after a mean follow up interval of approximately eight months. This timing of postoperative assessment varies between studies and could influence test results (Abouzayd et al. Citation2017), although the present study showed that in general the length of the postoperative follow up interval was not correlated with any vestibular outcome. The evolution of vestibular symptoms over time may indicate a central compensatory effect (Kubo et al. Citation2001; Buchman et al. Citation2004; Filipo et al. Citation2006; Krause et al. Citation2009). The present findings of a clinically non-significant effect on DHI score might therefore possibly be influenced by the time of assessment. Immediate postoperative assessment might lead to worse DHI results. It is not clear whether a delayed postoperative assessment also influences caloric or vHIT results, due to vestibular recovery (Abouzayd et al. Citation2017), although a few studies reported VOR recovery already one month after implantation (Katsiari et al. Citation2013; Korsager et al. Citation2017). In our study, the moment of the postoperative assessment was just weakly correlated, with only the anterior SCC gain. It is interesting for future studies to perform vestibular examination at different follow-up intervals, in order to observe VOR evolution after implantation and the role of central compensation. Furthermore, our patients were implanted at various ages. Some studies suggest that older patients are more prone to develop dizziness symptoms and worsening of the VOR after cochlear implantation (Fina et al. Citation2003; Enticott et al. Citation2006). In the present study, weak correlations between age and vHIT VOR gain changes and between age and changes in the DHI physical subdomain score were found, although no significant correlation between age of implantation and any other change of variable was found. In this study, the objective vestibular tests were only focussed on the function of the semicircular canals, so possible saccule or utricle deteriorations were not evaluated. Vestibular evoked myogenic potentials may reveal additional information about the effect of cochlear implantation on vestibular function (Ibrahim et al. Citation2017).
Conclusions
This study evaluated the effect of cochlear implantation on the vestibular system in a large sample of patients who underwent vestibular tests that cover all semicircular canals and completed a validated subjective questionnaire. Mean changes in test results were slight; however, for each assessment (caloric irrigation, vHIT and DHI), a substantial group of patients showed deterioration after cochlear implantation. An increase of vestibular symptoms (DHI) was present in 9% of the patients, showing that for some individuals postoperative impact can be very serious. In 37% of the patients, a deterioration of one or more categories (5-category scale) was found for caloric testing and 14% of the patients developed a vHIT VOR gain reduction of one or more semicircular canals. No good correlations were found between the objective and subjective changes. These results emphasise the necessity of thorough vestibular assessment and adequate patient counselling. The preoperative findings can also aid in choosing the most optimal ear of implantation in cases of unilateral cochlear implantation. Moreover, postoperative vestibular tests may facilitate a future diagnosis when late onset balance problems in cochlear implant recipients arise, by defining a new reference point of vestibular function after a cochlear implant surgery.
Geolocation information
This study was conducted with data from patients who received a cochlear implant at the affiliated Dutch tertiary referral centre.
TIJA-2020-02-0071-File005.pdf
Download PDF (159.4 KB)Acknowledgements
The authors thank Marloes Graauwmans, Karin Krommenhoek and Jacquelien Jilissen for their help in collecting the data.
Disclosure statement
The authors declare no conflicts of interest nor external financial support for this study.
References
- Abouzayd, M., P. F. Smith, S. Moreau, and M. Hitier. 2017. “What Vestibular Tests to Choose in Symptomatic Patients after a Cochlear Implant? A Systematic Review and Meta-Analysis.” European Archives of Oto-Rhino-Laryngology: Official Journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS): Affiliated with the German Society for Oto-Rhino-Laryngology – Head and Neck Surgery 274 (1): 53–63. doi:10.1007/s00405-016-4007-4.
- Alhabib, S. F., and I. Saliba. 2017. “Video Head Impulse Test: A Review of the Literature.” European Archives of Oto-Rhino-Laryngology: Official Journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS): Affiliated with the German Society for Oto-Rhino-Laryngology – Head and Neck Surgery 274 (3): 1215–1222. doi:10.1007/s00405-016-4157-4.
- Bance, M. L., M. O'Driscoll, E. Giles, and R. T. Ramsden. 1998. “Vestibular Stimulation by Multichannel Cochlear Implants.” The Laryngoscope 108 (2): 291–294. doi:10.1097/00005537-199802000-00025.
- Basta, D., I. Todt, F. Goepel, and A. Ernst. 2008. “Loss of Saccular Function after Cochlear Implantation: The Diagnostic Impact of Intracochlear Electrically Elicited Vestibular Evoked Myogenic Potentials.” Audiology & Neuro-Otology 13 (3): 187–192. doi:10.1159/000113509.
- Batuecas-Caletrio, A., M. Klumpp, S. Santacruz-Ruiz, F. Benito Gonzalez, E. Gonzalez Sánchez, and M. Arriaga. 2015. “Vestibular Function in Cochlear Implantation: Correlating Objectiveness and Subjectiveness.” The Laryngoscope 125 (10): 2371–2375. doi:10.1002/lary.25299.
- Blodow, A., S. Pannasch, and L. E. Walther. 2013. “Detection of Isolated Covert Saccades with the Video Head Impulse Test in Peripheral Vestibular Disorders.” Auris, Nasus, Larynx 40 (4): 348–351. doi:10.1016/j.anl.2012.11.002.
- Buchman, C. A., J. Joy, A. Hodges, F. F. Telischi, and T. J. Balkany. 2004. “Vestibular Effects of Cochlear Implantation.” The Laryngoscope 114 (S103): 1–22. doi:10.1097/00005537-200410001-00001.
- Curthoys, I. S. 2012. “The Interpretation of Clinical Tests of Peripheral Vestibular Function.” The Laryngoscope 122 (6): 1342–1352. doi:10.1002/lary.23258.
- Djourno, A., and C. Eyries. 1957. “Auditory Prosthesis by means of a Distant Electrical Stimulation of the Sensory Nerve with the Use of an Indwelt Coiling.” La Presse Medicale 65 (63): 1417.
- Enticott, J. C., S. Tari, S. M. Koh, R. C. Dowell, and S. J. O’Leary. 2006. “Cochlear Implant and Vestibular Function.” Otology & Neurotology: Official Publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology 27 (6): 824–830. doi:10.1097/01.mao.0000227903.47483.a6.
- Filipo, R., M. Patrizi, R. La Gamma, C. D'Elia, G. La Rosa, and M. Barbara. 2006. “Vestibular Impairment and Cochlear Implantation.” Acta Oto-Laryngologica 126 (12): 1266–1274. doi:10.1080/00016480600678789.
- Fina, M., M. Skinner, J. A. Goebel, J. F. Piccirillo, J. G. Neely, and O. Black. 2003. “Vestibular Dysfunction after Cochlear Implantation.” Otology & Neurotology: Official Publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology 24 (2): 234–242. discussion 242. doi:10.1097/00129492-200303000-00018.
- Friscia, L. A., M. T. Morgan, P. J. Sparto, J. M. Furman, and S. L. Whitney. 2014. “Responsiveness of Self-Report Measures in Individuals with Vertigo, Dizziness, and Unsteadiness.” Otology & Neurotology: Official Publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology 35 (5): 884–888. doi:10.1097/MAO.0000000000000421.
- Ibrahim, I., S. D. da Silva, B. Segal, and A. Zeitouni. 2017. “Effect of Cochlear Implant Surgery on Vestibular Function: Meta-Analysis Study.” Journal of Otolaryngology – Head & Neck Surgery = Le Journal D'oto-Rhino-Laryngologie et de Chirurgie Cervico-Faciale 46 (1): 44. doi:10.1186/s40463-017-0224-0.
- Jacobson, G. P., and C. W. Newman. 1990. “The Development of the Dizziness Handicap Inventory.” Archives of Otolaryngology – Head & Neck Surgery 116 (4): 424–427. doi:10.1001/archotol.1990.01870040046011.
- Jongkees, L. B., and A. J. Philipszoon. 1964. “Electronystagmography.” Acta Oto-Laryngologica 189(0): 1.
- Katsiari, E., D. G. Balatsouras, J. Sengas, M. Riga, G. S. Korres, and J. Xenelis. 2013. “Influence of Cochlear Implantation on the Vestibular Function.” European Archives of Oto-Rhino-Laryngology: Official Journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS): Affiliated with the German Society for Oto-Rhino-Laryngology – Head and Neck Surgery 270 (2): 489–495. doi:10.1007/s00405-012-1950-6.
- Korsager, L. E, J. H. Schmidt, C. Faber, and J. H. Wanscher. 2017. “Temporary Loss of Vestibular Function after Cochlear Implant Surgery.” Ugeskr Laeger 179 (4): V07160503.
- Korsager, L. E. H., J. H. Schmidt, C. Faber, and J. H. Wanscher. 2018. “Vestibular Outcome after Cochlear Implantation is Not Related to Surgical Technique: A Double Blinded, Randomized Clinical Trial of Round Window Approach versus Cochleostomy.” Otology & Neurotology: Official Publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology 39 (3): 306–312. doi:10.1097/MAO.0000000000001695.
- Krause, Eike, J. Wechtenbruch, T. Rader, A. Berghaus, and R. Gürkov. 2009. “Impaired Fixation Suppression is a Risk Factor for Vertigo after Cochlear Implantation.” The Journal of Laryngology and Otology 123 (8): 845–850. doi:10.1017/S0022215109004812.
- Kuang, H., H. H. Haversat, and E. M. Michaelides. 2015. “Impairment of Caloric Function after Cochlear Implantation.” Journal of Speech, Language, and Hearing Research: JSLHR 58 (4): 1387–1395. doi:10.1044/2015_JSLHR-H-15-0010.
- Kubo, T., K.-i. Yamamoto, T. Iwaki, K. Doi, and M. Tamura. 2001. “Different Forms of Dizziness Occurring after Cochlear Implant.” European Archives of Oto-Rhino-Laryngology 258 (1): 9–12. doi:10.1007/PL00007519.
- Le Nobel, G. J., E. Hwang, A. Wu, S. Cushing, and V. Y. Lin. 2016. “Vestibular Function following Unilateral Cochlear Implantation for Profound Sensorineural Hearing Loss.” Journal of Otolaryngology – Head & Neck Surgery = Le Journal D'oto-Rhino-Laryngologie et de Chirurgie Cervico-Faciale 45 (1): 38. doi:10.1186/s40463-016-0150-6.
- Migliaccio, A. A, C. C. Della Santina, J. P. Carey, J. K. Niparko, and L. B. Minor. 2005. “The Vestibulo-Ocular Reflex Response to Head Impulses Rarely Decreases after Cochlear Implantation.” Otology & Neurotology 26 (4): 655–660.
- Mutlu, B., and B. Serbetcioglu. 2013. “Discussion of the Dizziness Handicap Inventory.” Journal of Vestibular Research: Equilibrium & Orientation 23 (6): 271–277. doi:10.3233/VES-130488.
- Oonk, A. M. M., A. J. Beynon, T. A. Peters, H. P. M. Kunst, R. J. C. Admiraal, H. Kremer, B. Verbist, et al. 2015. “Vestibular Function and Temporal Bone Imaging in DFNB1.” Hearing Research 327: 227–234. doi:10.1016/j.heares.2015.07.009.
- Shepard, N. T., and G. P. Jacobson. 2016. “The Caloric Irrigation Test.” Handbook of Clinical Neurology 137: 119–131. doi:10.1016/B978-0-444-63437-5.00009-1.
- Ulmer, E., and A. Chays. 2005. “Curthoys and Halmagyi Head Impulse Test: An Analytical Device.” Annales D'oto-Laryngologie et de Chirurgie Cervico Faciale: Bulletin de la Societe D'oto-Laryngologie Des Hopitaux de Paris 122 (2): 84–90. doi:10.1016/S0003-438X(05)82329-1.
- Vereeck, L., S. Truijen, F. L. Wuyts, and P. H. Van De Heyning. 2007. “Internal Consistency and Factor Analysis of the Dutch Version of the Dizziness Handicap Inventory.” Acta Oto-Laryngologica 127 (8): 788–795. doi:10.1080/00016480601075464.
- Wagner, J. H., D. Basta, F. Wagner, R. O. Seidl, A. Ernst, and I. Todt. 2010. “Vestibular and Taste Disorders after Bilateral Cochlear Implantation.” European Archives of Oto-Rhino-Laryngology: Official Journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS): Affiliated with the German Society for Oto-Rhino-Laryngology – Head and Neck Surgery 267 (12): 1849–1854. doi:10.1007/s00405-010-1320-1.
- Weber, K. P., H. G. MacDougall, G. M. Halmagyi, and I. S. Curthoys. 2009. “Impulsive Testing of Semicircular-Canal Function Using Video-Oculography.” Annals of the New York Academy of Sciences 1164: 486–491. doi:10.1111/j.1749-6632.2008.03730.x.
- Whitney, S. L., D. M. Wrisley, K. E. Brown, and J. M. Furman. 2004. “Is Perception of Handicap Related to Functional Performance in Persons with Vestibular Dysfunction?” Otology & Neurotology 25 (2): 139–143.
- Wiener-Vacher, S. R., and S. I. Wiener. 2017. “Video Head Impulse Tests with a Remote Camera System: Normative Values of Semicircular Canal Vestibulo-Ocular Reflex Gain in Infants and Children.” Frontiers in Neurology 8: 434. doi:10.3389/fneur.2017.00434.
