Abstract
Objective
There is evidence of ototoxicity from antiretrovirals (ARVs), and ARV therapy in pregnant/nursing mothers can expose offspring to these compounds. The current work modelled whether exposure to ARVs in utero and during nursing altered the functioning of the auditory system in offspring mice.
Design
The females of seven breeding pairs of C57BL6/J mice were given daily doses of ARVs lamivudine and tenofovir disoproxil fumarate by oral gavage during gestation and nursing. Three breeder females were given equivalent volumes of water as controls. At wean age (3 weeks after birth), the offspring mice were tested with auditory brainstem responses (ABRs). At the conclusion of the experiment, the offspring mice’s cochleae were examined for hair cell counts.
Study sample
Ten breeder female C57BL6/J mice and 69 offspring mice.
Results
The offspring mice exposed to ARVs during development showed higher ABR thresholds than the control offspring. No differences were found in supra-threshold ABRs. There was no evidence of missing hair cells.
Conclusions
Hearing impairment may be a possible consequence of exposure to ARVs during gestation and development. Because the threshold differences were not large, if they are occurring in humans, it is unlikely they would be identified in any hearing screening tests.
Introduction
In an effort to curb the spread of human immunodeficiency virus (HIV) and acquired immunodeficiency syndrome (AIDS), the World Health Organisation (WHO) works with local governments and researchers to find new ways to implement HIV prevention and transmission control measures. A specific focus of recent prevention efforts has been vertical transmission, where the virus is passed from mother to child during pregnancy and childbirth. The WHO recommends that all people with HIV initiate and maintain lifelong antiretroviral therapy and specifically highlights pregnant and breastfeeding women as a target population. To minimise the risk of transmission, the WHO recommends treating HIV-positive mothers with highly-active antiretroviral therapy (HAART) during pregnancy and breastfeeding (Beyrer Citation2016). Initial HAART treatment regimens usually include two nucleoside reverse transcriptase inhibitors and one or more additional drugs. The goal is stopping viral replication, reducing the viral load, and increasing the number of CD4 cells. This helps to prevent the transmission of HIV and often reduces the severity of complications, ultimately increasing survival rate (Poorolajal et al. Citation2016; Sebitloane and Moodley 2017).
The use of HAART during pregnancy has been successful in preventing vertical HIV transmission, but it is unclear what, if any, negative long-term health consequences result from HAART for the children. Children who are born HIV-negative often leave the healthcare system, and so subsequent effects of HAART exposures, such as neurological developmental abnormalities that could affect the developing auditory system may not be documented (Coelho et al. Citation2017). Previous studies have linked in utero exposure to HAART to haematologic and liver abnormalities, myopathy, central nervous system disorders, cardiac conditions, neurologic malformations, and an increased risk of language delay (Blanche et al. Citation1999; Brogly et al. Citation2007; Gerschenson and Brinkman Citation2004; Rice et al. Citation2013).
To date, studies of the auditory effects of antiretrovirals (ARVs) have focussed on the effects in patients or animal models actively being treated with the ARV compounds. Those studies found abnormalities in the auditory brainstem response (ABR) (Fokouo et al. Citation2015; Matas et al. Citation2014; Citation2015) and increased susceptibility to noise-induced hearing loss (Bektas et al. Citation2008). Other studies have found no significant effects of ARVs on hearing (Luque et al. Citation2014). It is possible that the variability in these findings comes from the range of drugs used in the treatment and management of HIV/AIDS or from the duration of treatment with a single compound. The effects of exposure to HAART during gestation and nursing and its effects on the peripheral auditory system and auditory brainstem have not been well studied. Exposure to ARVs during development may only induce sub-clinical molecular or genetic changes in the auditory structures, but these changes may pose additional risks later in life. A previous study by Bektas et al. (Citation2008) showed that exposure to ARVs can increase hearing loss from concurrent noise exposure (Bektas et al. Citation2008). There is literature indicating that those in resource-constrained countries are exposed both to damaging levels of occupational noise and elevated non-occupational noise when compared to more industrialised nations (Abubakar et al. Citation2016; Nyarubeli et al. Citation2018; Sieber et al. Citation2017; Strauss et al. Citation2014). This indicates that some workers in these countries are already at significant risk for hearing loss and should be aware of any factors that put them at greater risk or predispose them to additional hearing loss.
Given the many confounding variables and privacy concerns inherent to a study of the auditory effects of HAART in human patients, the use of an animal model to study the effects of these drugs during gestation and nursing gives the opportunity to study a controlled dosing schedule of a fixed combination of drugs over a known window. This controlled environment will allow for the study of any effects of these drugs in relative isolation to understand their safety as they are implemented in new contexts. Furthermore, if those exposed to HAART during gestation and nursing are at greater risk from later damaging exposures, this may provide not only a need for modified counselling for the mothers of these children, but also a need for follow-up audiologic monitoring and care for these children. The current study sought to explore the auditory developmental risks associated with exposure to the ARVs lamivudine and tenofovir disoproxil fumarate during gestation and development. The authors hypothesised that exposure to these drugs during gestation and nursing would cause ototoxic hearing loss due to sensory hair cell loss.
Materials and methods
Animals
A total of 89 C57BL6/J mice were used during the course of this study. Ten male and ten female mice were obtained from Jackson Laboratory (Bar Harbour, ME). These mice were separated into breeding pairs, and the 69 additional mice were offspring of these 20. Offspring were weaned and separated into new cages 21 days after birth. All mice were housed in a vivarium maintained by University Laboratory Animal Resources at The Ohio State University. The noise level in the room did not exceed 45 dB Leq for any 1-h period. All housing and experimental procedures were reviewed by The Ohio State University Institutional Animal Care and Use Committee.
Auditory brainstem response (ABR)
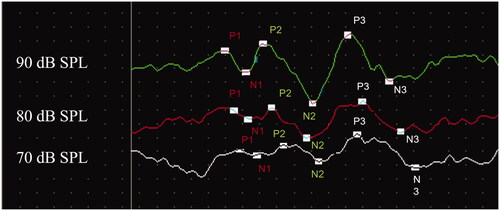
ABRs were used to assess and monitor auditory function for all time points in the study. The ABR has been shown to be a sensitive measure of hearing for more than 80 mice strains (Zheng, Johnson, and Erway Citation1999). For all ABRs, mice were anaesthetised with inhalant isoflurane (2.5% for induction and 1.2% for maintenance of anaesthesia with a 1 L/min oxygen flow rate). Subcutaneous platinum electrodes (Grass Technologies, West Warwick, RI) were inserted at the vertex (non-inverting), behind the right pinna (inverting), and near the left hind leg (ground). ABR tone burst stimuli were generated using Tucker Davis Technologies (TDT, Gainesville, FL) SigGen software (TDT). Each burst was 1 msec in duration with a .5 msec rise and fall time and no plateau. Tone bursts were presented at 21 bursts/second, and auditory thresholds were assessed at 4, 8, 12, 16, 24, and 32 kHz from 90 to 20 dB in decreasing 5-dB steps. Signals were routed from the TDT RZ6 signal processor to a speaker (TDT MF1) positioned at 90 degrees azimuth, approximately 3 cm from the right pinna. Responses were amplified 50,000× by a headstage (TDT RA4LI) connected to a preamplifier (TDT RA4PA) and bandpass filtered from 100 to 3.0 kHz. Three hundred sweeps were recorded for each presentation and threshold was defined as the lowest level at which a repeatable response could be visually identified. In order to assess supra-threshold responses and the retrocochlear pathway beyond the cochlea, P1, P2, and P3 wave amplitudes and latencies for the 90-, 80-, and 70-dB SPL stimuli at 16 kHz were evaluated in the offspring mice at wean (3 weeks after birth). Cursors were placed at each positive peak, as well as the negative troughs that followed (see for an example). Latencies were measured from the positive peaks, and amplitudes were measured as peak-to-peak amplitudes from the positive peaks to the negative troughs.
Antiretroviral exposures
Breeding pairs were assigned to one of two groups: antiretrovirals (ARV) (n = 7 pairs) or Control (CON) (n = 3 pairs). Each female breeding mouse in the ARV group was given a once daily dose of two WHO-recommended first-line ARV drugs (2 mg/kg of lamivudine and 20 mg/kg of tenofovir disoproxil fumarate) via oral gavage; both solutions were mixed at 1 mg/mL. These doses were chosen based on tolerability of these drugs observed during pilot testing. Each female breeding mouse in the CON group was given 22 mL/kg of distilled water via oral gavage once daily (typically 0.3–0.4 mL per day). Daily doses were administered beginning after baseline testing and continued until offspring used for the study were weaned. Therefore, the females were treated during the mating period, during pregnancy, and during weaning of all offspring. No female generated more than five offspring litters before removal from the study. Note, the males were not treated in any way during the experiment. 53 offspring were produced by breeding pairs in the ARV group and 16 offspring were produced by breeding pairs in the CON group.
Hair cell counts
A subset of the animals from group ARV was sacrificed after their wean-age ABR tests in order to count the outer (OHCs) and inner hair cells (IHCs) in the cochlea. The mice were rapidly decapitated following inhalation of CO2. Both auditory bullae were removed from each animal. The stapes was removed from each cochlea, the oval window was punctured, and a small hole was made in the apex. The cochlea was perfused with a fixation solution of 10% buffered formalin. The cochleae were dissected in phosphate buffered saline (PBS) to expose the organ of Corti and then were stained with DAPI (4′,6-diamidino-20-phenylindole dihydrochloride, ThermoFisher Scientific, D1306, Waltham, MA, USA) at room temperature for 20 min. To validate the results, a few cochleae were doubly stained with DAPI and Alexa Fluor 488-phalloidin (ThermoFisher Scientific, A12379, Waltham, MA, USA). Specifically, the tissues were transferred into the staining solution containing DAPI (1:100 in 1 mM PBS) and phalloidin (1:50 in 1 mM PBS) for 20 min. After staining, tissues were rinsed three times with PBS and were observed and photographed using a fluorescence microscope (Leica Z6 APO Manual MacroFluo, 10x objective) equipped with a Leica DFC digital camera. The collected images were processed to illustrate hair cell nuclei using Adobe Photoshop CS6 (RRID: SCR_014199) and ImageJ (NIH). Sections of individual images were aligned and stitched to generate a merged view of the tissue. Sensory cells were counted at 150 µm intervals from the apex to the base of the cochlear spiral.
Statistical analysis
To assess the effect of gestation/nursing exposure to ARVs on ABR thresholds at weaning, a two-factor (frequency × group) analysis of variance (ANOVA) was performed comparing the CON and ARV groups. For supra-threshold 16 kHz ABR latencies and amplitudes at wean, a two-factor ANOVA (group × stimulus level) was used. The latency and amplitude data of each of the three waves (P1, P2, P3) were analysed with separate ANOVAs. In the event of a two-way interaction, Welch’s t-testing was used for all comparisons, due to the unequal sample sizes. A p value of < .05 was considered significant for all interactions. All statistics were analysed using IBM SPSS version 25 (IBM, Armonk, NY).
Results
Breeder female pre-exposure thresholds
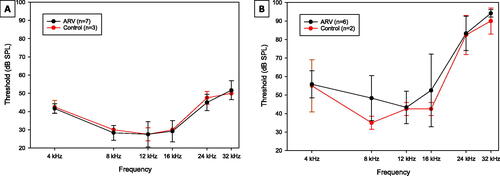
For breeding females in both the CON and ARV groups, baseline thresholds were recorded for all subjects prior to exposure to lamivudine and tenofovir disoproxil fumarate. Pre-exposure thresholds are presented in . The females were also tested at the conclusion of the experiment, which was approximately four months after the onset of the drug dosing and breeding (). A three-factor ANOVA (frequency × group × time) analysing these thresholds across both time points indicated no significant interactions or main effects involving group. Thus, there was no evidence that the female breeders had any underlying differences in susceptibility to hearing loss that would be expected to cause any differences in the hearing of their offspring at wean. There was a significant two-way interaction of frequency × test time (F(5,30) = 7.992, p < 0.001). Paired samples t-tests comparing pre-exposure thresholds to post-exposure thresholds at each frequency revealed significant threshold changes at each frequency, ranging from mean changes in the range of 13–21 dB at 4–16 kHz, and 37–42 dB at 24 and 32 kHz. These changes are consistent with the accelerated age-related hearing loss exhibited by adult C57BL6/J mice (Li and Borg Citation1991).
Figure 2. Breeder female ABR thresholds. Mean ABR thresholds from female breeder mice from Groups exposed to ARVs (ARV) and controls (CON). Error bars are ± 1 s.d. (A) Pre-exposure thresholds at the beginning of the experiment, prior to any breeding or dosing with ARV compounds. (B) Thresholds at the conclusion of the experiment. The ARV (black lines) and CON (red lines) groups were not significantly different from each other at any frequency or time point.
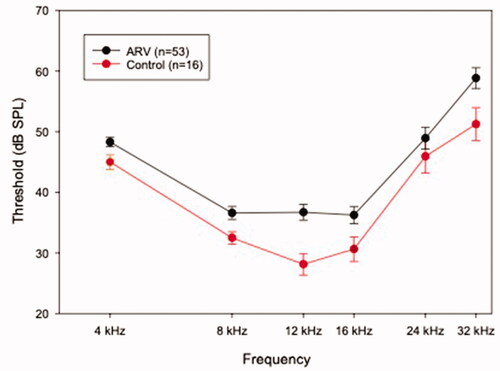
Wean thresholds
Wean thresholds for all offspring in the ARV and CON groups are presented in . Offspring in the ARV group had mean thresholds 3–9 dB higher at all frequencies than offspring in the CON group. A two-factor ANOVA (frequency × group) analysing these thresholds indicated a significant main effect of group (F(1,66)= 6.385, p = 0.014). Welch’s t-tests to compare the groups were run at each frequency. Offspring of the ARV group had higher thresholds than the offspring of the CON group at 4 kHz (p = 0.029), 8 kHz (p = 0.008), 12 kHz (p < 0.0005), 16 kHz (p = 0.030), and 32 kHz (p = 0.025).
Figure 3. Wean-age ABR thresholds in the ARV and Control offspring. Wean age was three weeks for the offspring mice. Frequencies denoted with an asterisk are those at which the ARV group (black line) had significantly (p < 0.05) higher thresholds than the CON group (red line). Error bars are ± 1 s.d.
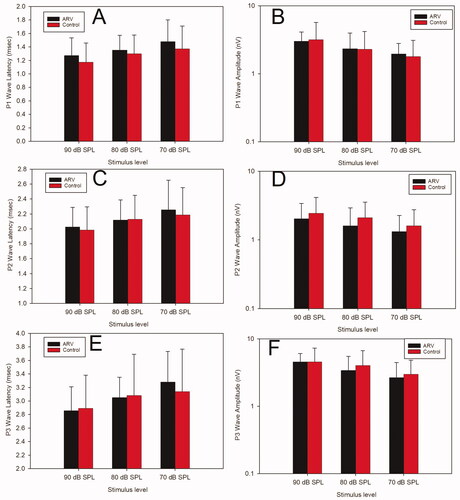
Supra-threshold ABR amplitudes and latencies
Peak latencies and P1-N1 peak-to-peak amplitudes at 16 kHz for 90-, 80-, and 70-dB SPL stimuli were analysed to gain insight into the IHC-auditory nerve-auditory brainstem pathway. Mean data are presented in . Two-way ANOVAs (group × stimulus level) for each latency and each amplitude of the P1-N1, P2-N2, and P3-N3 waves revealed no significant differences between the ARV and CON offspring groups for any measure or any wave.
Figure 4. Supra-threshold ABR wave amplitudes and latencies in the ARV and Control offspring. Panels A and B display latency and amplitude, respectively, of the P1 wave. Panels C and D display latency and amplitude, respectively, of the P2 wave. Panels E and F display latency and amplitude, respectively, of the P3 wave. The ARV group (black bars) and the CON group (red bars) were not significantly different from each other at any stimulus level for any of the measures. Error bars are +1 s.d.
Hair cell losses
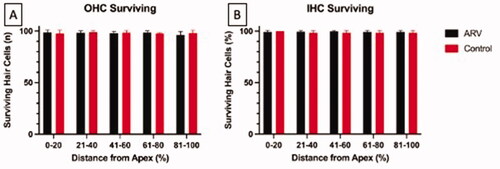
In order to assess a possible underlying pathology to which the higher ABR thresholds at wean in the ARV mice could be attributed, OHCs and IHCs were counted across the turns of the cochlea in a subset of the ARV group. A representative image of the apex (A), middle (B), and basal (C) turns of the cochlea in an ARV mouse is presented in . The image includes sporadic missing OHCs (denoted with white arrows on the figure). The percentages of missing OHCs were low in the animals evaluated (n = 16), with the range of intact OHCs at 95–99% in the ARV mice, and the IHCs 96–99% in the portions of the cochlea that map to the 4–32 kHz frequency range, as can been seen in . These findings of intact hair cells in these sections of the cochlea indicate that hair cell absence does not account for the elevated ABR thresholds detected in the ARV mice compared to the CON offspring.
Figure 5. Representative image of a cochlea from a mouse in the ARV group. Samples are from the apical (A), middle (B), and basal (C) turns of the cochlea. Hair cells are stained with DAPI (blue fluorescence). Points of missing OHCs in Panels A and B are denoted with white arrows.
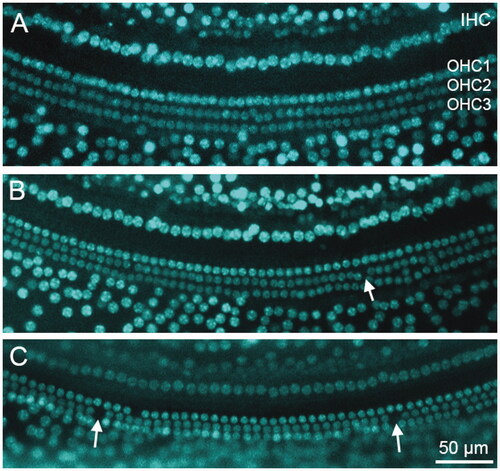
Figure 6. Panel A represents the number of intact OHCs surviving in each cochlear segment (denoted by their distance from the cochlear apex). Panel B indicates the percent of surviving IHCs for each cochlear segment. No significant differences exist across groups for any segment. For both panels, offspring from the ARV group are represented by black bars and the CON group by red bars. Error bars are + 1 s.d.
Discussion
The current study sought to analyse the effects of exposure during gestation and nursing to lamivudine and tenofovir disoproxil fumarate on the health and functionality of the auditory system in mice. The key result was elevated thresholds at 4–16 kHz and at 32 kHz in offspring mice that were exposed to the ARVs during gestation and nursing. As the exposure volumes and number of exposures were matched between the ARV and distilled water control group, these threshold differences are not the result of stress caused by the gavaging/handling processes or other factors outside of the content of the exposure. The elevated thresholds were the only significant difference between the ARV and control offspring. Differences were not found in supra-threshold ABR wave amplitudes or latencies. This indicates that the IHC-spiral ganglion neuron pathway is intact, as supra P1 wave amplitude decreases have been associated with IHC-spiral ganglion synapse injury in animal models of noise injury (Kujawa and Liberman Citation2009; Lin et al. Citation2011) and age-related hearing loss (Sergeyenko et al. Citation2013). Further, P1 latency shifts have been identified as an early consequence of cisplatin ototoxicity associated with injury to the auditory nerve neurons’ mitochondria and myelin (Chen et al. Citation2021). The absence of any supra-threshold ABR physiology differences between the groups signals that the afferent pathway from the IHCs is intact through the brainstem level. It is unknown if disruptions to that afferent pathway could develop in the ARV-exposed mice at a later age.
The final measurement explored in the study was counting of OHCs and IHCs in a subset of ARV mice in order to determine underlying pathology associated with the threshold shift. Very few missing OHCs and IHCs were detected in the ARV mice. Therefore, it was concluded that OHC/IHC death was not the underlying pathology to explain the elevated thresholds. That said, there are several pathologies that can cause dysfunction of the hair cells, even if the cell itself is physically present. Stereocilia injury can prevent K+ ions from flowing into the hair cells, thus impairing their ability to undergo depolarisation and elevating auditory thresholds (Patuzzi Citation2002; Saunders and Flock Citation1986). OHCs without prestin are physically intact, but unable to provide cochlear amplification through electromotility (Liberman et al. Citation2002). Further, the depolarisation process of the hair cells is driven by the strong endocochlear potential in the endolymph, in which the approximately +80 mV positive charge creates a significant electrical gradient with the negative charge inside the hair cells. The endocochlear potential is generated by the active ion pumping from the marginal cells of the stria vascularis, and reduction of the endocochlear potential leads to elevated auditory thresholds (Mills and Schmiedt Citation2004). None of the aforementioned pathologies were visible with the assays employed in the current study. Therefore, none can be ruled out as possible contributors to the elevated ABR thresholds detected in the ARV mouse offspring group.
While there were significant group differences at a number of frequencies in the offspring of females exposed to tenofovir disoproxil fumarate and lamivudine, compared to the offspring of females exposed to distilled water, there was significant variability in the observed thresholds across the ARV offspring. Levene’s test of homogeneity of variances found that the assumption of equal variance was not violated, but survey of the results, especially in the mid frequencies, shows thresholds with a range of 40–60 dB between subjects at a single frequency. It is reasonable to conclude that ARV exposure during foetal development and nursing can lead to offspring with higher auditory thresholds than would normally be expected. The large range of post-wean thresholds could be the result of varied delivery of these compounds to each foetus (Tomi, Nishimura, and Nakashima Citation2011; Van den Hof et al. Citation2018) or could be indicative of individual susceptibility to ARV-induced ototoxicity. Previous literature on sensorineural hearing loss has shown individual differences in susceptibility to cisplatin (Lanvers-Kaminsky and Ciarimboli Citation2017), kanamycin (Wu et al. Citation2001), and noise (Ohlemiller and Gagnon Citation2007).
It is important to acknowledge that the primary timepoint of interest for this study was wean age (21 days after birth). This is a typically-used timepoint in the study of murine hearing, as mice are altricial, meaning that their auditory and other systems continue to develop in the period following birth. This is unlike humans who are precocial, meaning that they are born with more fully developed systems. One example of this development that could be relevant to future research in this area is the maturation of cochlear hair cells. OHCs are not fully developed in the murine cochlea until approximately seven days after birth (Marcotti and Kros Citation1999), with IHCs not developing until approximately twelve days after birth (Kros, Ruppersberg, and Rüsch Citation1998). Because of the delays in maturation of the mouse cochlea, the sense of hearing does not develop until approximately twelve days after the mouse has been born. This differs from humans, who are born with fully mature cochlear cells. In order to control for some of the potential differences that may exist due to this difference, female breeding mice were exposed to ARVs not only during gestation but also during the 21 days of nursing following birth. This was intended to capture any potential exposure to ARVs delivered during nursing following in utero exposure. It is important to acknowledge this difference, however, as it is possible that these results would not be seen in precocial species such as humans. Preliminary investigation of human children exposed to ARVs during gestation and nursing did not show significant differences based on trimester of exposure (Torre et al. Citation2017), despite the most significant cochlear damage typically resulting from toxic exposures during the first and second trimesters. Additional investigation of these effects in both mice and humans is required to determine if the timing of exposures or differences in auditory development affect the translatability of these findings. Furthermore, it bears acknowledging that the current study looked only at a single mouse strain, and so future work in this area should evaluate the consistency of these findings in other strains as previous studies have observed differences in the way that commonly-used mouse models respond to the same ototoxic exposures (DeBacker, Harrison, and Bielefeld Citation2020).
As the results of this study suggest an increased risk of hearing loss for those exposed to the ARV drugs lamivudine and tenofovir disoproxil fumarate during gestation and nursing, future studies should also continue to investigate this risk using other ARV compounds recommended by the WHO. Lamivudine and tenofovir disoproxil fumarate are both recommended as a part of HAART alongside one or more other compounds. Future studies should use the WHO guidelines for HAART in pregnant and nursing mothers to explore the ototoxic risk associated with these recommendations. The WHO has previously modified the recommendations for use of HAART in pregnant and nursing mothers due to risk to the foetus associated with use of dolutegravir (Beyrer Citation2016) and so it is possible that these recommendations could be further altered upon evidence of additional risk posed by one of these compounds.
Conclusion
The findings of elevated thresholds for the offspring of the mice exposed to lamivudine and tenofovir disoproxil fumarate during pregnancy and when nursing suggest that these drugs can cause auditory damage when the developing offspring is exposed to them during development. If these findings are representative of human pregnant and breastfeeding mothers with HIV/AIDS, then the current study suggests that the children of these mothers are at risk for ARV-induced hearing loss. The hearing losses may be slight and could fall below clinical detection. As previous studies have indicated that in utero exposure to ARVs can lead to mitochondrial damage (Brogly et al. Citation2007; Coelho et al. Citation2017) and mitochondrial damage has been linked to hearing loss (Estivill et al. Citation1998), it is possible that this in utero exposure is causing mitochondrial damage which leads to the hearing loss observed in this study. Further study in other animal models and human clinical patients is indicated based on these findings.
Acknowledgements
The authors would like to thank Jordan Belanger for assistance with cochlear dissections, Drs. Christina Roup, Eric Healy, and Gail Whitelaw for editorial feedback, and Dr. Cara Hollander for introducing us to this area of inquiry.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Abubakar, T., A. Labaran, G. Mohammed, A. Kirfi, and O. B. Nwaorgu. 2016. “Hearing Threshold of Sawmillers in Kaduna, Nigeria.” Indian Journal of Otology 22 (3): 152. doi:10.4103/0971-7749.187974.
- Bektas, D., G. K. Martin, B. B. Stagner, and B. L. Lonsbury-Martin. 2008. “Noise-Induced Hearing Loss in Mice Treated with Antiretroviral Drugs.” Hearing Research 239 (1–2): 69–78. doi:10.1016/j.heares.2008.01.016.
- Beyrer, C. 2016. “Consolidated Guidelines on HIV Prevention, Diagnosis, Treatment and Care for Key Populations World Health Organization.” Available from: http://proxy.library.carleton.ca/loginurl=https://www.deslibris.ca/ID/10063272.
- Blanche, Stéphane, Marc Tardieu, Pierre Rustin, Abdelhamid Slama, Béatrice Barret, Ghislaine Firtion, Nicole Ciraru-Vigneron, et al. 1999. “Persistent Mitochondrial Dysfunction and Perinatal Exposure to Antiretroviral Nucleoside Analogues.” The Lancet 354 (9184): 1084–1089. doi:10.1016/S0140-6736(99)07219-0.
- Brogly, Susan B., Nathalie Ylitalo, Lynne M. Mofenson, James Oleske, Russell Van Dyke, Marilyn J. Crain, Mark J. Abzug, et al. 2007. “In Utero Nucleoside Reverse Transcriptase Inhibitor Exposure and Signs of Possible Mitochondrial Dysfunction in HIV-Uninfected Children.” AIDS 21 (8): 929–938. doi:10.1097/QAD.0b013e3280d5a786.
- Chen, Y., E. C. Bielefeld, J. G. Mellott, W. Wang, A. M. Mafi, E. N. Yamoah, and J. Bao. 2021. “Early Physiological and Cellular Indicators of Cisplatin-Induced Ototoxicity.” Journal of the Association for Research in Otolaryngology 22 (2): 107–126. doi:10.1007/s10162-020-00782-z.
- Coelho, A., P. Tricarico, F. Celsi, and S. Crovella. 2017. “Antiretroviral Treatment in HIV-1-Positive Mothers: Neurological Implications in Virus-Free Children.” International Journal of Molecular Sciences 18 (2): 423. doi:10.3390/ijms18020423.
- DeBacker, J. R., R. T. Harrison, and E. C. Bielefeld. 2020. “Cisplatin-Induced Threshold Shift in the CBA/CaJ, C57BL/6J, BALB/cJ Mouse Models of Hearing Loss.” Hearing Research 387: 107878. doi:10.1016/j.heares.2019.107878.
- Estivill, Xavier, Nancy Govea, Anna Barceló, Enric Perelló, Cèlia Badenas, Enrique Romero, Luis Moral, et al. 1998. “Familial Progressive Sensorineural Deafness is Mainly Due to the mtDNA A1555G Mutation and Is Enhanced by Treatment with Aminoglycosides.” The American Journal of Human Genetics 62 (1): 27–35. doi:10.1086/301676.
- Fokouo, Jean Valentin F., Jean Espoir E. Vokwely, Jean Jacques N. Noubiap, Brice Enid Nouthe, Joseline Zafack, Esthelle Stéphanie Minka Ngom, Asmaou Bouba Dalil, et al. 2015. “Effect of HIV Infection and Highly Active Antiretroviral Therapy on Hearing Function: A Prospective Case-Control Study from Cameroon.” JAMA Otolaryngology- Head & Neck Surgery 141 (5): 436–441. doi:10.1001/jamaoto.2015.125.
- Gerschenson, M., and K. Brinkman. 2004. “Mitochondrial Dysfunction in AIDS and Its Treatment.” Mitochondrion 4 (5–6): 763–777. doi:10.1016/j.mito.2004.07.025.
- Kros, C. J., J. P. Ruppersberg, and A. Rüsch. 1998. “Expression of a Potassium Current in Inner Hair Cells during Development of Hearing in Mice.” Nature 394 (6690): 281–284. doi:10.1038/28401.
- Kujawa, S. G., and M. C. Liberman. 2009. “Adding Insult to Injury: Cochlear Nerve Degeneration after “"temporary" noise-induced hearing loss.” The Journal of Neuroscience 29 (45): 14077–14085. doi:10.1523/JNEUROSCI.2845-09.2009.
- Lanvers-Kaminsky, C., and G. Ciarimboli. 2017. “Pharmacogenetics of Drug-Induced Ototoxicity Caused by Aminoglycosides and Cisplatin.” Pharmacogenomics 18 (18): 1683–1695. doi:10.2217/pgs-2017-0125.
- Li, H. S., and E. Borg. 1991. “Age-Related Loss of Auditory Sensitivity in Two Mouse Genotypes.” Acta Oto-Laryngologica 111 (5): 827–834. doi:10.3109/00016489109138418.
- Liberman, M. C., J. Gao, D. Z. He, X. Wu, S. Jia, and J. Zuo. 2002. “Prestin Is Required for Electromotility of the Outer Hair Cell and for the Cochlear Amplifier.” Nature 419 (6904): 300–304. doi:10.1038/nature01059.
- Lin, H. W., A. C. Furman, S. G. Kujawa, and M. C. Liberman. 2011. “Primary Neural Degeneration in the Guinea Pig Cochlea after Reversible Noise-Induced Threshold Shift.” Journal of the Association for Research in Otolaryngology 12 (5): 605–616. doi:10.1007/s10162-011-0277-0.
- Luque, A. E., M. S. Orlando, U. C. Leong, P. D. Allen, J. J. Guido, H. Yang, and H. Wu. 2014. “Hearing Function in Patients Living with HIV/AIDS.” Ear and Hearing 35 (6): e282–e290. doi:10.1097/AUD.0000000000000064.
- Marcotti, W., and C. J. Kros. 1999. “Developmental Expression of the Potassium Current IK,n Contributes to Maturation of Mouse Outer Hair Cells.” Journal of Physiology.520 (3): 653–660. doi:10.1111/j.1469-7793.1999.00653.x.
- Matas, C., R. Angrisani, F. Magliaro, and A. Segurado. 2014. “Audiological Manifestations in HIV-Positive Adults.” Clinics 69 (7): 469–475. doi:10.6061/clinics/2014(07)05.
- Matas, C. G., A. G. Samelli, R. G. Angrisani, F. C. L. Magliaro, and A. C. Segurado. 2015. “Brainstem Auditory Evoked Potential in HIV-Positive Adults.” Medical Science Monitor 21: 3172–3178. doi:10.12659/msm.894958.
- Mills, D. M., and R. A. Schmiedt. 2004. “Metabolic Presbycusis: Differential Changes in Auditory Brainstem and Otoacoustic Emission Responses with Chronic Furosemide Application in the Gerbil.” Journal of the Association for Research in Otolaryngology 5 (1): 1–10. doi:10.1007/s10162-003-4004-3.
- Nyarubeli, I. P., A. M. Tungu, M. Bråtveit, E. Sunde, A. V. Kayumba, and B. E. Moen. 2018. “Variability and Determinants of Occupational Noise Exposure among Iron and Steel Factory Workers in Tanzania.” Annals of Work Exposures and Health 62 (9): 1109–1122. doi:10.1093/annweh/wxy071.
- Ohlemiller, K. K., and P. M. Gagnon. 2007. “Genetic Dependence of Cochlear Cells and Structures Injured by Noise.” Hearing Research 224 (1–2): 34–50. doi:10.1016/j.heares.2006.11.005.
- Patuzzi, R. 2002. “Non-Linear Aspects of Outer Hair Cell Transduction and the Temporary Threshold Shifts after Acoustic Trauma.” Audiology & Neuro-Otology 7 (1): 17–20. doi:10.1159/000046857.
- Poorolajal, J., E. Hooshmand, H. Mahjub, N. Esmailnasab, and E. Jenabi. 2016. “Survival Rate of AIDS Disease and Mortality in HIV-Infected Patients: A Meta-Analysis.” Public Health 139: 3–12. doi:10.1016/j.puhe.2016.05.004.
- Rice, Mabel L., Bret Zeldow, George K. Siberry, Murli Purswani, Kathleen Malee, Howard J. Hoffman, Toni Frederick, et al. 2013. “Evaluation of Risk for Late Language Emergence after in Utero Antiretroviral Drug Exposure in HIV-Exposed Uninfected Infants.” The Pediatric Infectious Disease Journal 32 (10): e406–e413. doi:10.1097/INF.0b013e31829b80ee.
- Saunders, J. C., and A. Flock. 1986. “Recovery of Threshold Shift in Hair-Cell Stereocilia Following Exposure to Intense Stimulation.” Hearing Research 23 (3): 233–243. doi:10.1016/0378-5955(86)90112-7.
- Sebitloane, H. M., and D. Moodley. 2017. “The Impact of Highly Active Antiretroviral Therapy on Obstetric Conditions: A Review.” European Journal of Obstetrics, Gynecology, and Reproductive Biology 210: 126–131.
- Sergeyenko, Y., K. Lall, M. C. Liberman, and S. G. Kujawa. 2013. “Age-Related Cochlear Synaptopathy: An Early-Onset Contributor to Auditory Functional Decline.” The Journal of Neuroscience 33 (34): 13686–13694.
- Sieber, Chloé, Martina S. Ragettli, Mark Brink, Olaniyan Toyib, Roslyn Baatjies, Apolline Saucy, Nicole Probst-Hensch, et al. 2017. “Land Use Regression Modeling of Outdoor Noise Exposure in Informal Settlements in Western Cape, South Africa.” International Journal of Environmental Research and Public Health 14 (10): 1262. doi:10.3390/ijerph14101262.
- Strauss, S., D. W. Swanepoel, P. Becker, Z. Eloff, and J. W. Hall. 2014. “Noise and Age-Related Hearing Loss: A Study of 40 123 Gold Miners in South Africa.” International Journal of Audiology 53 (sup2): S66–S75. doi:10.3109/14992027.2013.865846.
- Tomi, M., T. Nishimura, and E. Nakashima. 2011. “Mother-to-Fetus Transfer of Antiviral Drugs and the Involvement of Transporters at the Placental Barrier.” Journal of Pharmaceutical Sciences.100: 37.
- Torre, P., B. Zeldow, T. Yao, H. Hoffman, G. Siberry, M. Purswani, T. Frederick, S. Spector, and P. Williams. 2017. “Newborn Hearing Screenings in Human Immunodeficiency Virus-Exposed Uninfected Infants.” J AIDS Immune Res 1: 3708–3718.
- Van den Hof, Malon, Charlotte. Blokhuis, Sophie Cohen, Henriette J. Scherpbier, Ferdinand W. N. M. Wit, M. C. M. Pistorius, Neeltje A. Kootstra, et al. 2018. “CNS Penetration of ART in HIV-Infected Children.” The Journal of Antimicrobial Chemotherapy 73 (2): 484–489. doi:10.1093/jac/dkx396.
- Wu, W.-J., S.-H. Sha, J. D. McLaren, K. Kawamoto, Y. Raphael, and J. Schacht. 2001. “Aminoglycoside Ototoxicity in Adult CBA, C57BL and BALB Mice and the Sprague-Dawley Rat.” Hearing Research 158 (1-2): 165–178. doi:10.1016/s0378-5955(01)00303-3.
- Zheng, Q. Y., K. R. Johnson, and L. C. Erway. 1999. “Assessment of Hearing in 80 Inbred Strains of Mice by ABR Threshold Analyses.” Hearing Research 130 (1–2): 94–107. doi:10.1016/s0378-5955(99)00003-9.