Abstract
The role of sewage sludge as an immobilising agent in the phytostabilization of metal-contaminated soil was evaluated using five grass species viz., Dactylis glomerata L., Festuca arundinacea Schreb., F. rubra L., Lolium perenne L., L. westerwoldicum L. The function of metal immobilization was investigated by monitoring pH, Eh and Cd, Pb, and Zn levels in column experiment over a period of 5-months. Grasses grown on sewage sludge-amendments produced high biomass in comparison to controls. A significant reduction in metal uptake by plants was also observed as a result of sewage sludge application, which was attributed to decreased bioavailability through soil stabilisation. We have observed that the sludge amendment decreased metal bioavailability and concentrations in soil at a depth of 25 cm, in contrast to untreated columns, where metal concentrations in the soil solution were very high.
INTRODUCTION
“Soil is the soul of life”. Therefore, soil must be clean. Soil contamination with toxic metals is of serious health concern. Factors for soil pollution differ from country to country, but industrial activities are responsible for over 60% of Europe's soil pollution (the oil sector accounts for 14% of this total). Generally, severe soil contamination by non-point sources of industrial waste is rather diffused, with the exception of acidified soils. (Evans et al. Citation2012). Local (or point source) contamination is mainly associated with mining, industrial facilities and waste landfills (municipal and industrial). In the Silesian region of Poland, metal contamination is localised in specific areas, such as the “Black Triangle” as well as around cities and other industrial areas (Szewrański et al. Citation2003).
Conventional soil cleanup techniques for metal contamination include bioleaching with bacteria, solidification, vitrification, electrokinetics, chemical oxidation or reduction, excavation and off-site treatment (Padmavathiamma and Li, Citation2009). However, these operations are cost prohibitive (from 0.27 to 1.6 million USD per hectare) and can disturb soil structure and function (Kidd et al. Citation2009). Hence, these approaches are limited to relatively small areas. Recently, intensive and eco-friendly soil remediation techniques have been developed using different plants, known as phytoremediation.
Phytoremediation includes several processes in which “phytostabilization” has convincing success. It aims to establish a vegetation cover with metal-tolerant plants and thus reduce leaching of metals. Organic/inorganic amendments to soil also reduce the metal mobility and bio/phytoavailability. These measures change the chemical state of metals against potential migration pathways and, at the same time, increase soil fertility and promote plant growth (Vangronsveld et al., Citation1996, Yu et al., Citation2008). For these purposes, the following characteristics of plants are beneficial: (i) ability to grow in nutrient-poor soil; (ii) a deep root system; (iii) a rapid growth rate; and (iv) metal resistance and low levels of metal translocation from roots to above ground tissues to avoid further flow through the food chain (Wong Citation2003, Mendez and Maier Citation2008, Santibáñez et al., Citation2008). Hence, the choice of suitable species is crucial for success (Rizzi et.al. 2004, Arienzo et. al Citation2004, Prasad Citation2006, Zhang et al. Citation2010). Therefore phytostabilization may be a viable alternative for large areas with high and multi-elemental contamination (Mench et al., Citation2003, Kidd at al., 2009).
High concentrations of Zn, Pb, Cd and As are found near metal smelters and mining areas. Acidification can be accelerated by anthropogenic deposition of SO2, NH3 and NOx by the metallurgical and mining industry. In sandy soils, with little buffering capacity, this decrease in pH may be fast (Degryse et al. Citation2007). Agrochemicals contribute to soil acidification which subsequently leads to reductions in macronutrient concentrations. Metal-tolerant grasses are reported by many authors as being suitable for phytostabilization (Vangronsveld et al. Citation1996, Rizzi et al. Citation2004, Prasad Citation2006, Sagyndyk et al. Citation2007,). The most studied species of grasses are the following: Lolium perenne (Alvarenga et al. Citation2008, Santibáñez et al. Citation2008); L. perenne, Festuca rubra, and Poa pratensis (Padmavathiamma and Li Citation2009); Agrostis capillaris and F. rubra (Vangronsveld et al. Citation1996); Agrostis tenuis (Dahmani-Muller et al. Citation2000); Lolium italicum and Festuca arundinacea (Rizzi et al. Citation2004). Often plants growing on metalliferous substrates also face nutrient and water limitation. Therefore, as an improved management practice use of soil additives and ameliorants (e.g., cyclonic ashes, lime, compost, sewage sludge, phosphate, diatomaceous earth and agrowaste) including biofertilizers and rhizobial cultures are administered (e.g., the introduction of mycorrhizal fungi, such as Hebeloma crustuliniforme) (Vangorsveld et al. Citation1996, Rizzi et al. Citation2004, Ruttens et al. Citation2006, Alvarenga et al. Citation2009a, Padmavathiamma and Li Citation2009, Kacprzak et al. Citation2010). It is envisaged that enhanced technical and engineering applications of sewage sludge would contribute efficiently for phytostabilization.
Therefore, the objective of this study was to investigate the combined effect of sewage sludge application and growth of grass species on the pH, Eh and Cd, Pb, and Zn levels in soil solution during a 5- month column experiment.
MATERIALS AND METHODS
Characterisation of Soil and Biosolids
Contaminated soil was collected from the area surrounding a zinc smelter in Miasteczko Slaskie, in the Silesia region of Poland. This zinc smelter has been in operation since 1969 and is one of the main producers of Zn, Pb and Cd in Poland. According to the Central Statistical Office, annual Zn production is 85,000 Mg, which constitutes approximately 40% of the total domestic production and approximately 50% of the total Pb production. Forest cover up to a radius of about 3 km degraded due to the emissions of the zinc smelter. Soil in the study area has been contaminated with metals, especially Cd, Pb and Zn. Moreover, soils of the study area are also acidified ().
Table 1 Selected chemical and physical characteristics of the contaminated soil (S), sewage sludge (SS) and mixed material (S+SS)
Table 2 Multivariate analysis of variance for the effects of time (t), sewage sludge (SS), grass species (GS) and depth (d) on the Eh, pH and metals (Cd, Zn, Pb) concentration.
The soil used in the experiment is classified as a sandy soil with 2.2% gravel, 96% sand, and 1.8% clay, is highly acidic, and is low in N, P, K, organic matter and CEC. Selected physical and chemical characteristics of this soil are shown in . The biosolid used was anaerobically digested sewage sludge (SS), collected from the industrial waste water treatment plant and juices manufacturer (food industry) ().
The Column Device Experiment
Top soil from the contaminated site was collected in the spring. One-meter-high (15-cm diameter) columns were filled with soil to ensure unlimited root growth. The structure of the soil profile was preserved in each column. The columns were filled with soil down to the bedrock layer (1 m), consisting of gravel, clay, and stones, and the upper part of the column was capped and the column is then inverted. A column experiment was performed via application of SS. Soil samples were obtained from the top of each column (30 cm deep), reweighed and mixed with sewage sludge (SS accounted for 2% of dry weight; according to the Polish Minister of Environmental Regulation, SS application for land reclamation cannot exceed 45 Mg d.m./ha/3 years). The columns were subsequently refilled with soil mixed with SS. Control columns without any amendment were also prepared (the 30 cm deep soil sample was mixed without sewage sludge). The columns were prepared with three replicates for each grass species. After a two-week incubation period under growth chamber conditions (columns were irrigated twice in order to maintain constant moisture and prevent dryness), the five grass species were seeded according to their remediation potential: Festuca arundinacea Schreb. (Fa); Lolium perenne L. (Lp); Lolium westerwoldicum L. (Lw); Dactylis glomerata L. (Dg); and Festuca rubra L. (Fr). The columns with and without amendment were sown with 4 g of grass seeds per column. The seeds were planted at approximately 0.5 cm deep and watered daily, using a spray bottle, until germination. Unvegetated columns with and without the application of SS were used as controls. A plant growth experiment was conducted in a growth chamber for 5 months. The plants were grown under artificial light (350 μmol m−2 s−1). The temperature was set to 20°C and 14°C (day and night, respectively), and the relative humidity was maintained at 70%. During the growing season, fertiliser was not applied, and irrigation with deionised water was applied as needed (15 mL every two days for every column). To collect soil solution, micro-samplers (underpressure MicroRhizon micro-samplers, consisting of a porous length of 15 mm with an outer diameter of 1 mm, PEEK tubing with a connector fit to a syringe and a microfiltration Teflon membrane with a pore size of 0.15- 0.20 μm) were installed in the columns at three depths: 25 cm, 50 cm and 90 cm. During the experiment, monthly soil solution samples were collected separately from each depth (20 mL of soil solution collected for 48 hours). Stones and the gravel bedrock layer at the bottom of the columns became a drainage layer (lixiviation from the base of the columns was allowed by equipping columns at a depth of 1 m with a connector for the discharge of leachate, but no significant leachate was collected during the experiment). The plants were harvested after 5 months. The roots were gently separated from the soil and rinsed under running water, followed by a final rinse in deionised water. Root samples were dried at 70°C for 48 h. Soil samples were obtained from each column from the plant rhizosphere (depth <30 cm). The root system, together with adhering soil was carefully removed from the column. Rhizosphere soil sample adhered to the roots was carefully collected by gentle shaking of the root system.
Chemical Analysis of Soil, Soil Solution and Plants
Before and after the plant growth experiment, soil subsamples were air-dried and passed through a 2 mm mesh screen. The following parameters were determined for the subsamples: pH in H2O and KCl deionised water suspension (soil to water ratio of 1: 2.5) (PN-ISO 10390:1997); CEC (Cation Exchange Capacity) according to Kappen's method (Karczewska and Kabała, Citation2008); humid acids (Stevenson Citation1994); total Kjeldahl N (PN-ISO 11261:2002); total organic carbon using a Multi N/C H1300 Analitykjena (PN-ISO 10694:2002); available P using the Enger-Riehm method (Karczewska and Kabała, 2008); total P (PN-ISO 11263:2002); and moisture (PN-ISO 11465:1999). Soil solution samples were filtered with a Whatman 0.45 μm filter prior to pH and redox analysis. Redox potential (Eh) was measured using a platinum (Pt) electrode, and the reference electrode was calomel. We made the a correction for the reference electrode by adding 244 mV to the measured value. Plant material, shoots and roots separately (0.3 g), sewage sludge (0.3 g) and soil (0.5 g) material were digested using hot aqua regia (PN-ISO 11047:2001) (1995). The digested experimental material and pore soil solution were analysed for the presence of Cd, Pb and Zn using inductively coupled plasma atomic emission spectrometry (ICP-AES; Optima 200 apparatus). All measurements were obtained in triplicate. As an environmental matrix, the following reference materials were used (LGC standards): plant material (ERM-CD281); soil material (LGCQC3004); and sewage sludge material (BCR-146R).
Statistical Treatment of Data
Multivariate analysis of variance (MANOVA) was used to determine significant effects of time (t), sewage sludge (SS), grass species (gs) and depth (d) on the Eh, pH and metals (Cd, Zn, Pb) concentration. Significant effects were identified at the 0.05 probability level and the mean comparisons were based on Fisher's protected LSD.
RESULTS AND DISCUSSION
Influence of Sewage Sludge on Plant Growth and Cd, Pb and Zn Concentrations
Plants growing on amended soil were devoid of any macroscopic symptoms of metal toxicity or nutrient deficiency, in contrast to plants grown on non-amended soil, where growth was inhibited and some phytotoxic effects were observed. Additionally, a very dense root system was achieved in columns with a sewage sludge application, especially for F. arundinacea, L. westerwoldicum and L. perenne. The presence of 2% d.m. of SS (45 Mg ha−1) did not negatively influence plant growth, whereas Alvarenga et al. (Citation2009b) observed some phytotoxic effects using compost from the organic fraction of unsorted municipal solid waste at this same ratio. This difference is likely due to a 3-fold higher concentration of metals in biosolids compared to those used in this study, as well as much higher metal contamination of the experimental soil. The C:N ratio directly affects the properties of the SS and was similar (20) in both studies. This similarity suggests that the toxic symptoms in plants observed by Alvarenga resulted from the use of sludge with a high metal content. In our study, there was little or no plant biomass production in soil without SS despite favourable water and temperature conditions. For amended soil, plant growth was most likely influenced by improved soil conditions due to nutrient-rich SS and metals immobilisation by organic matter. Moreover, an improvement in pH also occurred, which also had a positive effect on plant growth. Nitrogen and phosphorus (bio)fertilisers facilitated remediation of degraded areas according to Tordoff et al. (Citation2000).
The addition of organic material is reported to decrease the bioavailability of metals (Alvarega et al., 2009b, Juwarkar et al., Citation2008). This is supported in our study. Cadmium concentrations in grass shoots grown in SS treatments ranged from 0.25–1.5 mg kg−1 d.m., whereas the control soil without SS had 5-fold higher Cd concentrations (). This value exceeds the maximum tolerable level (0.5 mg kg−1 d.m.) according to the National Research Council (2002) and is within the critical concentration for plants (5–30 mg kg−1 d.m.) (Kabata-Pendias, Citation2010). The Cd concentration in shoots was significantly lower in treatments that received SS amendment for all grass species. For L. perenne L., Alvarenga et al. (Citation2009a) noted no significantly different concentrations of Cu, Pb or Zn in shoots after SS application, but this result was due to high metal concentrations in the sludge, whereas the metal contamination of SS was lower in the present study (within the range of standards for agricultural purposes). Metal concentrations in plant tissues are influenced by many factors that affect metal dynamics in the soil (Park et al., Citation2011). The addition of organic material has been reported to decrease the bioavailability of metals (Alvarega et al., 2009b, Juwarkar et al., Citation2008). The Pb concentration in the aboveground biomass was low compared to the total Pb concentration in soil. In all plants derived from SS-amended soil, the Pb concentration ranged from 3–9 mg kg−1 d.m., and the Pb concentration in the plants derived from control soil was higher (25 mg kg−1 d.m.) (). Alvarenga et al. (Citation2009b) found that the Pb concentration in L. perenne L. shoots was 140 mg kg−1 d.m., whereas the total Pb concentration in soil was 5500 mg kg−1 d.m. Despite high differences in Pb concentration in soil and shoots in both experiments, 2.5% of the total Pb concentration in soil accumulated in aboveground plant tissues. In the present study, the Pb concentration in the shoots was quite low compared to the concentrations in the soil and roots. Zn concentrations in shoots did not exceed the critical concentration for plants (100–400 mg kg−1 d.m.) (Kabata-Pendias, Citation2010). Zn concentrations ranged from 25–30 mg kg−1 and 35 mg kg−1 for treated and untreated columns, respectively.
Figure 1 Metals concentrations in the roots and shoots of grass species, Fa- Festuca arundinacea Schreb., Lw- Lolium westervoldicum L., Lp- Lolium perenne L., Fr- Festuca rubra L., Dg- Dactylis glomerata L., S+SS-soil amended with sewage sludge, S- soil without amendment; error bars represent ± SE.
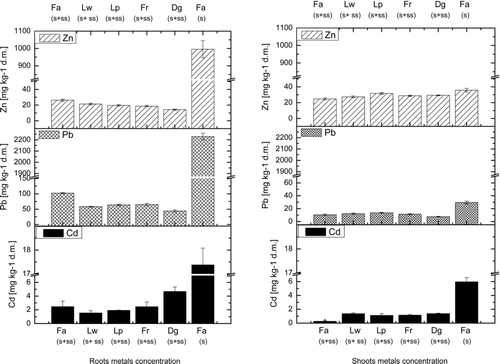
The amounts of Zn taken into the roots and sorbed onto the root surface () were similar for grass grown on sludged soil, or slightly higher for species in which the biomass was highest (i.e., F. arundinacea Schreb., L. westerwoldicum L. and L. perenne L.), and ranged from 15 to 25 mg kg−1 d.m. (). For F. arundinacea Schreb. grown in soil without any amendment, this value reached almost 1000 mg kg−1 d.m. and was 40-fold higher compared to sewage sludge-amended soil. The Pb concentration in roots of F. arundinacea Schreb. grown in unamended soil was very high (2250 mg kg−1 d.m.) compared to sludged soil (100 mg kg−1 d.m.). Lower concentrations of lead (50–75 mg kg-1 d.m.) were observed for L. westerwoldicum L., L. perenne L., F. rubra L. and D. glomerata L., similar to the Zn accumulation in roots. Among the grasses tested, F. arundinacea Schreb. had the highest Pb and Zn concentrations in the root tissues and the lowest concentrations of these metals in the shoot tissues. The Cd concentration in F. arundinacea Schreb. grown in unamended soil was 17.4 mg kg−1 d.m. compared to 1.7–2.3 mg kg−1 d. m. in amended soil. All metals accumulated mainly in root tissues for the SS application. A significant reduction in metal uptake into the roots (and sorbed onto the root surface) was achieved after sewage sludge application, which can be attributed to decreased bioavailability and the stabilisation of metals in soil.
The Role of Amendments on Soil Chemical Characteristics
The results show that SS amendment improved the soil parameters (). Stańczyk-Mazanek and Sobik- Szołtysek (2010) also found SS to be effective in the improvement of soil fertility. In the present experiments, all chemical and physical parameters improved after SS application, including higher pH, organic matter, N, C, P and sorption capacity, similarly to previous research (Ociepa et al., Citation2010, Kacprzak and Grobelak, Citation2011). Nevertheless, the by-products used do not appear to be effective at increasing the soil pH of acidic soil (Maddocks et al., Citation2004), contrary to the results obtained in this study (). The soil pH from the contaminated site was 5.2, whereas the pH of amended soil was 6.8. Acid conditions of soil usually indicate deficiencies in the availability of cations and, therefore, limit plant growth (Whitehead, Citation2000). In the present study, increased soil pH was the result of the SS amendment. The pH buffering of a soil arises from cation exchange reactions and from dissolution and precipitation reactions. Organic matter is a main contributor to pH buffering in these soils. In the pH range 4–6, cation exchange is an important buffeting mechanism (Degryse et all, 2007). Moreover, the soil CEC was low (3.62 cmol (+) kg−1 soil, which could be explained by the high sand proportion in the soil of this experimental site (Brady and Weil, Citation2008). The pH and CEC (6.1 cmol (+) kg−1) were improved by the SS treatment. Moreover in the research described by Lo′pez-Dı′az et al. (2007), a positive effect of sewage sludge application on soil pH was found, when the initial soil pH was very low (4–5). Moreover the addition of biosolids increases the surface charge of the amended soils, which is attributed to the higher pH and surface charge of the biosolid compost. Organic amendments supply micronutrients particularly biosolids and MSW, and often possess moderate to high pH buffering capacity (Park et al., 2011). In this study, the soil fertility was enhanced by SS treatment and grass growth was promoted.
The immobilisation of metals occurs through adsorption reactions (increase in surface charge and the presence of metal-binding compounds) (Park et al., Citation2011). The presence of phosphates in SS is mainly believed to be responsible for increased lead sorption. In contrast, organic material amendments have been shown to increase metal leaching losses (Jackson et al., Citation1999, McBride, Citation2003).
Soil Solution Analysis
Metal solubility
The Cd concentrations in the soil solution of control- and sludge–amended columns are shown in . At a 25 cm depth, very high Cd concentrations were observed in soil solution from unamended columns, for both control soil and planted soil, compared to columns with SS amendments. At 25 cm depth in all unamended soils, Cd concentrations were highest during the first few months after lysimeter deployment. At the fourth and fifth week, Cd concentrations in amended soil increased slightly. Similar results were achieved by Ruttens et al. (Citation2006). The mobility of Cd at the end of the experiment may have been enhanced by complexation with colloidal organic molecules of low stability (McBride, Citation2003). At 50 cm depth, the Cd concentration decreased slowly during the experiment. Slightly higher concentrations were also observed for unamended compared to amended soils. At a 80 cm depth, there was a slight decrease in Cd concentration at the beginning of the experiment, but was not clear. Furthermore, no differences were observed between amended and unamended columns at this depth. In summary, the lowest soil solution Cd concentrations were found in the sludge and planted columns. No significant differences between grass species were observed for Cd percolation.
Figure 2 The Cd concentrations of the soil solution; Fa- Festuca arundinacea Schreb., Dg- Dactylis glomerata L., S+SS-soil amended with sewage sludge, S- soil without amendment; error bars represent ± SE.
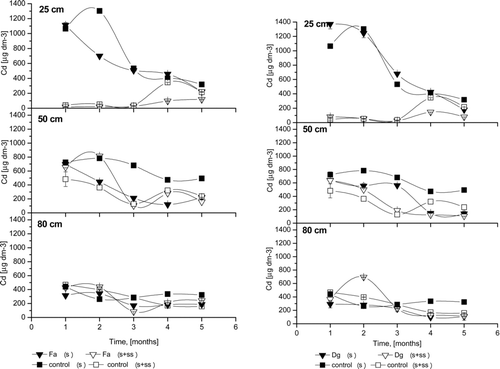
Pb concentrations in the percolate at three depths are presented in . Compared to Cd () and Zn (), the maximum amount of lead in soil solution was obtained in the third and fourth months of the experiment. However, this pattern was observed only for untreated soils. Delayed percolation of Pb is the result of its lower availability compared to other metals. Tenfold decreases of Pb in the soil solution from 25 cm depth were observed in SS-treated columns, whereas Pb concentrations did not change during the experimental period. High amounts of phosphate from the SS may have complexed Pb into less available forms (Park et al., Citation2011).
Figure 3 The Pb concentrations of the soil solution; Fa- Festuca arundinacea Schreb., Dg- Dactylis glomerata L., S+SS-soil amended with sewage sludge, S- soil without amendment; error bars represent ± SE.
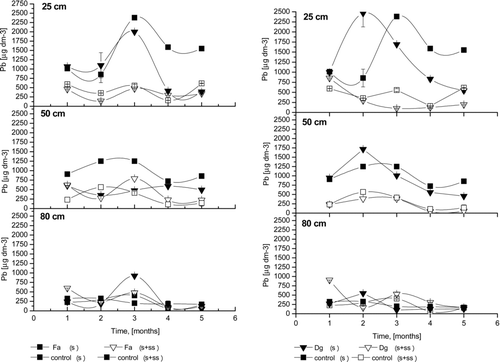
Figure 4 The Zn concentrations of the soil solution; Fa- Festuca arundinacea Schreb., Dg- Dactylis glomerata L., S+SS-soil amended with sewage sludge, S- soil without amendment; error bars represent ± SE.
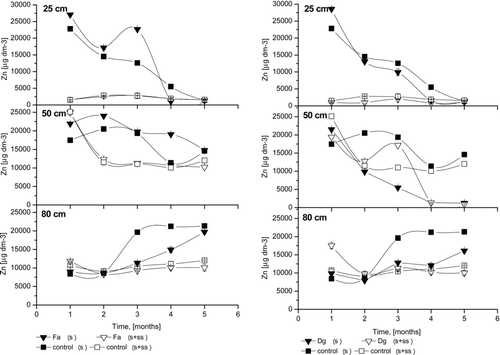
At a 25 cm depth in all untreated soils, Zn concentrations () were highest during the first and third months. Similar results were reported by Ruttens et al. (Citation2006). In the sludged columns, Zn release to the soil solution was very low compared to untreated columns. No differences were observed between planted or non-planted columns, as well as between grass species, at 25 cm depth in sludged columns. The high mobility of Zn was confirmed at 50 cm depth, where intense changes were noted. In this layer, irregular changes in zinc concentrations were observed, except for the first month, during which the value was steady. For F. arundinacea, the only grass species with roots passing through the sludge layer and achieving 50 cm depth, Zn concentration remained stable throughout the experiment. For other grass species, the differences were not significant. Surprising results were observed for 80 cm depth, where metal concentrations were less stable. From the third sampling, Zn concentrations increased significantly for untreated columns. Zn was the only metal for which changes were observed at all depths, confirming the mobility of this element for these soil conditions. The main factor affecting Zn percolation was likely the low pH of the soil. High initial metal concentrations in the soil solution were also likely the result of soil loosening during the filling of the columns, despite soil profile preservation. Soil manipulations tend to increase metal mobility (Vangronsveld et al. 1995). Due to similar results (metals, pH, Eh) between grass species, only two of five figures are shown.
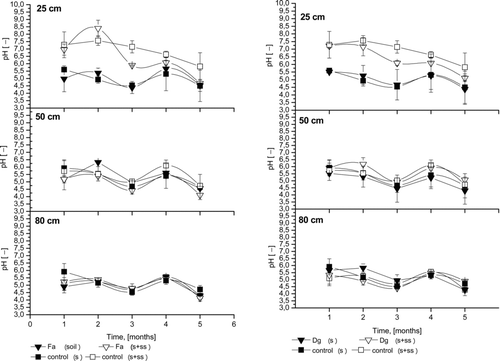
pH of the Soil Solution
The pH values () in the soil solution from control and sludged columns were also determined. At 25 cm depth, relatively high pH values were observed consistently throughout the percolation, and these values were higher than the pH values noted for control soils. Contrary results were reported by Ashworth and Alloway (Citation2004), with slightly higher values than those of the sludged soil. The pH values in the soil solution from untreated control soils at 25 cm depth ranged from 4.5–5.5 during all experimental points, with no significant differences between planted and non-planted columns. The pH of soil solution from sludged columns was higher and ranged from 5.5–8.0. After the first month, the pH was 5 for control and 7 for sludged columns, which was the result of SS application (pH 8) to the topsoil. For amended control columns (control S+SS), as well as for poorly rooted plants (e.g., F. rubra and D. glomerata), similar pH was found, with mild decreases during the experiment. The relationship between metal adsorption and soil pH depends on the initial concentration of the metal. At low concentrations, initial metal adsorption (Pb, Cd, Cu, Ni, and Zn) is independent of the soil pH. However, at high concentrations, the initial adsorption of these metals increases with increasing soil pH (Basta and Sloan, Citation1999). Intensive Cd and Zn desorption was observed at pH 5–5.5 in untreated columns. The addition of SS caused an increase in pH (7–7.5), above the threshold for zinc desorption (5.8; Huang et al., Citation1997), and most likely also for other metals. In sludged columns with well-rooted plants, increasing pH was observed in the second month, with a subsequent decrease. Increasing pH in the second month is most likely the result of intensive absorption of anions, especially phosphate and nitrate (from SS), in the rhizosphere of rapidly growing plants. However, the long-term application of biosolids leads to decreasing pH due to organic matter mineralisation (Park et al., Citation2011, Neczaj et al., Citation2011). At 50 cm depth, pH ranged from 4.5–6.5, and a small decrease in pH was observed at the end of the experiment, with high variability. No influence of sludge and plants was observed at this depth. Similar results were observed at 80 cm depth. No impact of sewage sludge application was observed on the pH in the soil solution during the experiment in the deepest soil layer, despite organic matter (dissolved) mineralisation processes. Adsorption of compounds as they move through 1 m of soil is the likely explanation, as observed by Ashworth and Alloway (Citation2004). In summary, the most significant influence of SS on pH in soil solution was observed at 25 cm depth, with no clear patterns at other depths.
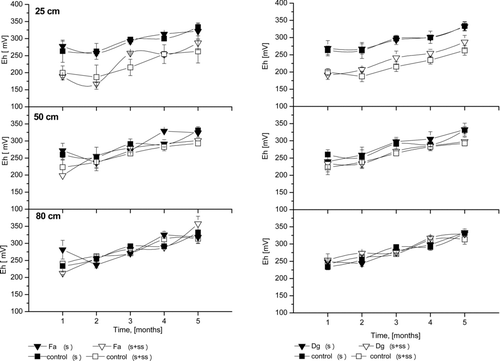
Eh of the Soil Solution
Redox values () measured for soil solution are consistent with moderately reduced soils (+100 to +350 mV). Similarly, as in the case for pH, SS addition affected the Eh of soil solution at the 25 cm depth. For the soil solution from soil treated with sewage sludge, Eh was much lower and ranged from 180 mV (in the first month) to 270 mV (in the fifth month). However, for columns without sludge addition, Eh ranged from 270 to 350 mV. Similar to results reported by Ugwuegbu et al. (Citation2001), the Eh in all control columns for the other two depths generally did not vary. The Eh trend was similar at all depths. In both studies, values for the treatment columns were lower than for control columns at the first depth. The addition of organic carbon accelerated oxygen depletion and established a more reduced state, which was pronounced in the top layer where SS was applied. Generally, most of the metals have higher mobility in acidifying and oxidising conditions, while reducing and alkaline conditions are conducive to reduction in metal mobility (Adriano, Citation2001). The results confirmed that food industry SS effectively retained Zn, Pb and Cd at 25 cm depth throughout the experiment (5 months), likely due to the Eh decrease. In the deeper layers, there was no difference in Eh between columns with and without sewage sludge. As with pH, there were no differences in Eh of soil solution between grass species. A lower Eh in treatment columns suggest the importance of Eh in the design and monitoring of SS soil amendment. Soil redox potentials indicate that soils were aerobic (oxidised) for most of the study period. This pattern also indicates that watering was sufficient and did not produce saturated conditions.
CONCLUSIONS
Phytostabilization is expected to serve as a natural barrier to erosion and leaching of pollutants. In our experiments, a significant reduction in metal uptake by plants was achieved after sewage sludge application, which is attributed to decreased bioavailability and the stabilisation of metals in soil. A decreased Eh of the soil solution after SS amendment reduced water-soluble metallic elements and concomitantly reduced desorption processes. The results from the five-month experiment showed that SS application reduced negative impacts on the physical and chemical parameters of the soil and facilitated plant cover. Phytostabilization has been complemented by dense root systems of grass as well as biosolid amendments facilitating metal immobilisation. Most of the heavy metals were captured in the roots of test plants. The aplication of sewage sludge and the growth of grass species can stabilize metals in acidic and phytotoxic mine spoil, and by the phytostabilization process they can reduce the risk of metal toxicity to the food chain. The results indicate that changes in pH, Eh and metal content in the soil solution may be the most sensitive indicators of soil changes during improved phytostabilization procedures. The use of biosolids is sufficient to restore vegetation in contaminated areas but not enough for long-term stabilisation of soil solution pH. Knowledge of correlations between pH, Eh and ions contained in soil solution, combined with knowledge of potential bioremediation advantages, can be a tool for the practical use of these methods for large-scale areas. Large quantities of sewage sludge is produced in wastewater treatment plants. Disposal and appropriate utilization of wastewater produced sewage sludge is a challenging issue. Our experiments demonstrated that sewage sludge can assist and accelerate phytostabilization of metalliferous acidic sandy soils with the help of grass species which possess thick adventitious root mat that would further aid in phytostabilization. Long-term monitoring is needed for proper evaluation of the processes occurring in phytostabilization of metalliferous soils.
ACKNOWLEDGEMENTS
The project was supported by National Science Centre grant UMO-2011/ 03/N/ NZ9/ 02034 and University Internal Grant BS PB/401/304/11.
REFERENCES
- Adriano , D. 2001 . Trace elements in terrestrial environments: biogeochemistry, biavailability and risk of metals , New York , , USA : Springer Verlag .
- Alvarenga , P , Gonçalves , A P , Fernandes , R M , de Varennes , A , Vallini , G , Duarte , E and Cunha-Queda , A C . 2008 . Evaluation of composts and liming materials in the phytostabilization of a mine soil using perennial ryegrass . Sci Total Environ , 406 : 43 – 56 .
- Alvarenga , P , Gonçalves , A P , Fernandes , R M , de Varennes , A , Duarte , E , Cunha-Queda , A C and Vallini , G . 2009a . Reclamation of a mine contaminated soils using biologically reactive organic matrices . Waste Manage Res , 27 : 101 – 111 .
- Alvarenga , P , Gonçalves , A P , Fernandes , R M , de Varennes , A , Vallini , G , Duarte , E and Cunha-Queda , A C . 2009b . Organic residues as immobilizing agents in aided phytostabilization: (I) Effects on soil chemical characteristics . Chemosphere , 74 : 1292 – 1300 .
- Anonymous . 1997 . Soil quality - determination of pH . PN ISO , 10390 : 1997 Polish Committee for Standardization
- Anonymous . 1999 . Soil Quality - Determination Of Dry Matter And Water Content On A Mass Basis - Gravimetric Method. PN ISO 11465:1999 moisture determination. Polish Committee for Standardization .
- Anonymous . 2001 . Soil Quality - determination of cadmium, chromium, cobalt, copper, lead, manganese, nickel and zinc in aqua regia extracts of soil - flame and electrothermal atomic absorption spectrometric methods.PN ISO 11047 . : 2001
- Anonymous . 2002 . Soil quality - determination of total nitrogen - modified Kjeldahl method. PN ISO 11261:2002. Polish Committee for Standardization .
- Anonymous . 2002 . Soil quality - determination of organic and total carbon after dry combustion (elementary Analysis).Polish Committee for Standardization. PN ISO 10694:2002 Carbon determination .
- Anonymous . 2002 . Soil quality - determination of phosphorus - spectrometric determination of phosphorus soluble in a solution of sodium bicarbonate. PN ISO 11263:2002. Polish Committee for Standardization .
- Arienzo , M and Adamo, Cozzolino , V. 2004 . The potential of Lolium perenne for revegetation of contaminated soils from a metallurgical site . Sci Total Environ , 319 : 13 – 25 .
- Ashworth , D J and Alloway , B J . 2004 . Soil mobility of sewage sludge-derived dissolved organic matter, copper,nickel and zinc . Environ Pollut , 127 : 137 – 144 .
- Basta , N T and Sloan , J J . 1999 . Bioavailability of metals in strongly acidic soils treated with exceptional quality biosolids . J. Environ. Qual. , 28 : 633 – 638 .
- Directive of Integrated Pollution Prevention and Control (96/61/WE directive of EU) .
- Brady , N C and Weill , R R . 2008 . The nature and properties of soils, 14th edn , Upper Saddle River , New Jersey : Prentice Hall .
- Dahmani–Muller , H , van Oort , F , Gélie , B and Balabane , M . 2000 . Strategies of heavy metal uptake by three plant species growing near a metal smelter . Environ Pollut , 109 : 231 – 238 .
- Degryse , F , Vlassak , V , Solders , E and Merckx , R . 2007 . Mobilization of Cd upon acidification of agricultural soils: column study and field modeling . European Journal of Soil Science , 58 : 152 – 165 .
- Evans , C D , Chadwick , T , Norris , D , Rowe , E C , Heaton , T HE , Brown , P , Richard , W and Battarbee , R. 2012 . Persistent surface water acidification in an organic soil-dominated upland region subject to high atmospheric deposition: The North York Moors, UK Ecological Indicators, In Press, Corrected Proof, Available online 28 March 201 . : 2
- Huang , J W , Chen , J , Berti , W R and Cunningham , S . 1997 . Phytoremediation of lead contaminated soils—role of synthetic chelates in lead phytoextraction . Environ. Sci. Technol. , 31 : 800 – 805 .
- Jackson , B P , Miller , W P , Schumann , A W and Sumner , M E . 1999 . Trace element solubility from land application of fly ash/organic waste mixtures . J environ Quali , 28 : 639 – 647 .
- Juwarkar , A A , Yadav , S K , Kumar , P and Singh , S K . 2008 . Effect of biosludge and biofertilizer amendment on growth of Jatropha curcas in heavy metal contaminated soils . Environ Monit assess , 145 : 7 – 15 .
- Kabata-Pendias . 2010 . A Trace Elements in Soils and Plants, Fourth Edition , 520 USA : Taylor & Francis .
- Kacprzak , M , Fijałkowski , K and Nowak , M . 2010 . The role of sewage sludge In improving of phytoremediation of heavy metal contaminated terrains . Journ Environ Stud , 2 : 73 – 77 .
- Kacprzak , M and Grobelak , A . 2011 . The influence of different sewage sludge doses on phytostabilization process (in Polish) . Inżynieria Ekologiczna , 25 : 99 – 109 .
- Karczewska , A and Kabała , C . 2008 . Metodyka analiz laboratoryjnych gleb i roślin, Instytut Gleboznawstwa i Ochrony Środowiska Rolniczego, Wrocław
- Kidd , P , Barceló , J , Bernal , M P , Navari-Izzo , F , Poschenrieder , Ch , Shilev , S , Clemente , R and Monterroso , C . 2009 . Trace element behavior at the root-soil interface: Implications in phytoremediation . Environ Exp Bot , 67 : 243 – 259 .
- Lopez O′ pez-Diaz i′ az , M L , Riguerio-Rodri′Guez , A and Mosquera- Losada , M R . 2009 . Influence of pasture botanical composition and fertilization treatments on tree growth . Forest Ecology and Management , 257 : 1363 – 1372 .
- Maddocks , G , Lin , C and McConchie , D . 2004 . Effects of Bauxsol and biosolids on soil conditions of acid-generating mine spoil for plant growth . Environ Pollut , 127 : 1327 – 1337 .
- Mench , M , Bussière , S , Boisson-Gruppen , J , Castaing , E , Vangronsveld , J Ruttens , A . 2003 . Progress in remediation and revegetation of the barren Jales gold mine spoil after in situ treatments . Plant Soil , 249 : 187 – 202 .
- Mendez , M O and Maier , R M . 2008 . Phytostabilization of mine tailings in arid and semiarid environments -an emerging remediation technology . Environ Health Perspect , 116 : 278 – 83 .
- McBride , MB. 2003 . Toxic metals in sewage sludge- amended soils: has promotion of beneficial use discounted the risk? . Adv Environ Res , 8 : 5 – 19 .
- National Research Council . 2002 . Nutrient requirements of domestic animals. 7th ed. National academy of Science , Washington, DC : National Academy Press .
- Neczaj , E , Bień , J B , Grosser , A , Worwag , M and Kacprzak , M . 2011 . Anaerobic treatment of sewage sludge and grease trap sludge in continuous co- digestion . Global Nest Journal , 14 ( 2 ) : 141 – 148 .
- Ociepa , E , Kisiel , A and Lach , J . 2010 . Effect of fertilization with sewage sludge and composts on the change of cadmium and zinc solubility in soils . Journ Environ Stud , 2 : 171 – 175 .
- Padmavathiamma , P and Li , L . 2009 . Phytostabilization—a sustainable remediation technique for zinc in soils . Water Air Pollut , 9 : 253 – 260 .
- Park , J H , Lamb , D , Paneerselvam , P , Choppala , G , Bolan , N and Chung , J W . 2011 . Role of organic amendments on enhanced bioremediation of heavy metal(loid)contaminated soils . J Hazard Mater , 185 : 549 – 574 .
- Prasad , MNV. 2006 . “ Stabilization, Remediation, and Integrated Management of Metal-Contaminated Ecosystems by Grasses (Poaceae) ” . In Trace elements in the environment. Biogeochemistry, Biotechnology and Bioremediation , Edited by: Prasad , M NV , Sajwan , K S and Naidu , R . 405 – 424 . Boca Raton , London, New York : Taylor &Francis Group .
- Rizzi , L , Petruzelli , G , Poggio , G and Vinga Guidi , G. 2004 . Soil physical changes and plant availability of Zn and Pb in a treatability test of phytostabilization . Chemosphere , 57 : 1039 – 1046 .
- Ruttens , A , Mench , M , Colpaert , J V , Carleer , R and Vangronsveld , J . 2006 . Phytostabilization of a metal contaminated sandy soil. Influence of compost and/or inorganic metal immobilizing soil amendements on phytotoxicity and plant availability of metals . Environ Pollut , 144 : 524 – 532 .
- Santibáñez , C , Verdugo , C and Ginocchio , R . 2008 . Phytostabilization of copper mine tailings with biosolids: implications for metal uptake and productivity of Lolium perenne . Sci Total Environ , 395 : 1 – 10 .
- Stańczyk-Mazanek , E and Sobik-Szołtysek , J . 2010 . Investigation of accumulation of heavy metals In soils and flotation discards fertilized with selected sewage sludge . Journ Environ Stud , 2 : 221 – 224 .
- Stevenson , FJ. 1994 . Humus Chemistry, Genesis, Composition, Reactions , New York : John Wiley&Sons .
- Sagyndyk , K S , Aidossova , S S and Prasad , M NV . 2007 . Grasses tolerant to radionuclides growing in kazakhstan nuclear test sites exhibit structural and ultrastructural changes - implications for phytoremedaition and involved risks . Terrestrial and Aquatic Ecotoxicology , 1 : 70 – 77 .
- Szewrański , Sz , Sasik , J and Żmuda , R . 2003 . Land degradation in Poland. In: the JRC enlargement action Workshop 10-B Land degradation Edited by: Jones , Robert J. A. and Montanarella , Luca . 225 – 239 .
- Tordoff , G M , Baker , A JM and Willis , A J . 2000 . Current approaches to the revegetation and reclamation of metalliferous mine wastes . Chemosphere , 41 : 219e228
- Ugwuegbu , B U , Prasher , S O , Ahmad , D and Dutilleul , P . 2001 . Bioremediation of residual fertilizer nitrate: II. Soil redox potential and soluble iron as indicators of soil health during treatment . Journal of Environmental Quality , 30 : 11 – 18 .
- Vangronsveld , J , Colpaert , J V and van Tichelen , K K . 1996 . Reclamation of a bare industrial area contaminated by non-ferrous metals: physicochemical and biological evaluation of the durability of soil treatment and revegetation . Environ Pollut , 94 ( 2 ) : 131 – 140 .
- Whitehead , D. C. 2000 . Nutrient elements in grassland. Soilplant- animal relationships , Wallingford , , UK : CAB International .
- Wong , MH. 2003 . Ecological restoration of degraded soils with emphasis on metal contaminated soils . Chemosphere , 50 : 775 – 780 .
- Yu , A , Muratova , T , Dmitrieva , V , Panchenko , L V and Turkovskaya , O V . 2008 . Phytoremediation of Oil-Sludge–Contaminated Soil . International Journal of Phytoremediation , 10/6 : 486 – 502 .
- Zhang , X , Xia , H , Li , Z , Zhuang , P and Gao , B . 2010 . Potential of four grasses in remediation of Cd and Zn contaminated soils . Bioresour Technol , 101 : 2063 – 2066 .