Abstract
While the immediate effect of explosives in armed conflicts is frequently in the public eye, until recently, the insidious, longer-term corollaries of these toxic compounds in the environment have gone largely unnoticed. Now, increased public awareness and concern are factors behind calls for more effective remediation solutions to these global pollutants. Scientists have been working on bioremediation projects in this area for several decades, characterizing genes, biochemical detoxification pathways, and field-applicable plant species. This review covers the progress made in understanding the fundamental biochemistry behind the detoxification of explosives, including new shock-insensitive explosive compounds; how field-relevant plant species have been characterized and genetically engineered; and the major roles that endophytic and rhizospheric microorganisms play in the detoxification of organic pollutants such as explosives.
Keywords:
Introduction
The main explosives used by the military are 2,4,6-trinitrotoluene (TNT) and hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX), with newer compounds 2,4-dinitroanisole (DNAN) and 3-nitro-1,2,4-triazole-5-one (NTO) now being tested. However, these compounds are toxic; TNT and RDX are classified by the US Environmental Protection Agency (EPA) as Group C (possible human) carcinogen and toxic to all organisms tested and are recalcitrant to degradation in the environment. Thus the potential for progressive accumulation of these materials in soil and groundwater is a significant concern at military sites. It is estimated that in the U.S. alone, 10 million hectares of military land is contaminated with munitions components (Defense Science Board Task Force Citation1998; United States General Accounting Citation2004). An even greater number of explosives-contaminated sites exist in Europe (Kalderis et al. Citation2011); a toxic legacy from World Wars I and II. In Germany, there are an estimated 3200 contaminated sites requiring remediation, including the former ammunition site Werk Tanne in Clausthal-Zellerfeld (Eisentraeger et al. Citation2007). Much of the pollution is still present today, demonstrating the persistence of these compounds, and their metabolites, in the environment. For example, pollution from munitions disposed in the 1920s at Verdun, in France is still threatening human health (Gorecki et al. Citation2017). Explosive-contaminated sites in the US and inherent environmental problems are further described by Albright (Citation2012).
Terrestrial contamination is heterogeneous, with hotspots of grams of explosives per kilogram of soil within large areas of relatively low contamination. The nature of the explosive compounds present can also vary depending on the type of military range (artillery, anti-tank), and this topic is comprehensively reviewed by Pichtel (Citation2012). To add to this, sites can also contain phytotoxic levels of inorganic pollutants such as lead and antimony. Sites can also contain unexploded ordnance restricting ground-access. The sheer scale, inaccessibility and, on active ranges, the need to contain and perpetually remediate, are further, significant hurdles to the successful phytoremediation of military sites.
The US Department of Defense (DoD) estimated that the clean-up of unexploded ordnance, discarded military munitions and munition constituents on its active ranges would cost between $16 billion and $165 billion (United States General Accounting, Citation2004). Despite the adverse effects of explosive-derived contaminants on the environment and human health, the global market for explosives is predicted to grow from $23.8 billion in 2017 to $31.2 billion by 2022 (BCC Research LLC Citation2018), and cost-effective, clean-up methods are urgently needed.
Current remediation methods, such as landfill, incineration or advanced oxidation, are more suited to small-scale, highly polluted areas, where clean-up time is short (<3 years), and overall costs relatively low. Phytoremediation is most likely to excel as an in situ method on larger-scale sites and/or when a longer-term clean-up time (3+ years) is acceptable. Effective plant-based bioremediation requires a thorough understanding of the uptake and in planta partitioning of the xenobiotic; and subsequent biochemical detoxification pathways in plants, associated endophytes, and rhizospheric communities. Earlier reviews by experts in this scientific field have comprehensively described genetic and biochemical studies, including the development and testing of genetically-modified model plant systems for the remediation of explosives (Rylott and Bruce Citation2009; Van Aken Citation2009; Maestri and Marmiroli Citation2011; Panz and Miksch Citation2012). This review focuses on articles published more recently, summarizing the current biochemical knowledge on the phytoremediation of explosives, and how knowledge from microbial detoxification pathways might help. Alongside this, progress on establishing detoxification routes for newer, insensitive munitions; the increasingly important contribution of plant-associated bacteria; and the use of genetically modified (GM) technologies to develop plant species suitable for in situ remediations will be discussed.
Classes of explosives
The nitroaromatic and nitramine classes of explosives contain the most widely-used, and environmentally polluting compounds, 2,4,6-trinitrotoluene (TNT) and hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX), along with the newer, less shock-sensitive compounds 2,4-dinitroanisole (DNAN) and 3-nitro-1,2,4-triazol-5-one (NTO). Further details on the chemical properties of these compounds are presented in . These explosives are used in munitions as mixes, the most common of which are Composition B (RDX and TNT), IMX-101 (Insensitive Munition eXplosive; DNAN, NTO, and 1-nitroguanidine) and IMX-104 (NTO, RDX, and DNAN).
Table 1. Chemical properties of TNT, DNAN, RDX, and NTO.
Nitroaromatics
Nitroaromatic explosives contain multiple nitro groups bonded to carbon atoms on the aromatic ring. The most well-known example, and prolifically used worldwide, is TNT. Classified by the EPA as a Group C (possible human) carcinogen and toxic to all organisms tested (Rylott, Lorenz, et al. Citation2011), the EPA has established a lifetime health advisory guidance limit of 2 µg/L for TNT in drinking water. Dinitrotoluenes (2,4-DNT and 2,6-DNT), which are used in propellants and are also byproducts associated with the manufacture of TNT, are also found as contaminants in military training ranges. Both TNT, and its DNT isomers, are also listed as priority pollutants by the EPA. The TNT molecule contains three nitro groups which withdraw electrons from the aromatic ring making an electrophilic attack of the ring by microbial oxygenases difficult (Qasim et al. Citation2009). Thus TNT is notoriously recalcitrant to ring cleavage and mineralization in the environment. Stable isotope data suggest half-lives of up to 9–17 years for 2,4-DNT biodegradation and 18–34 years for the co-metabolic transformation of TNT and 2,6-DNT in soils (Wijker et al. Citation2013).
The newer, shock-insensitive munition compound, DNAN has been developed to replace TNT. Assessment by Dodard et al. (Citation2013) found DNAN to be 100 times less toxic than TNT to bacteria, 3–8 times less toxic for algae and 1–3 times less toxic for earthworms (Eisenia fetida). The exact reason for the interspecies differences in nitroaromatic ecotoxicity is not known, and there is no consensus on the mode of DNAN toxicity. Currently, DNAN is not listed by the EPA as a contaminant of concern, although the fate of DNAN in the environment is still being established.
Both TNT and DNAN have relatively low aqueous solubility and high octanol/water partition coefficient (log Kow) values () and thus migrate slowly through soil columns, binding tightly to organic matter (Arthur et al. Citation2018). For DNAN, successive replacement of the nitro groups with amines reduces hydrophobicity, increasing its potential to migrate through soils (Boddu et al. Citation2008; Hawari et al. Citation2015).
Nitramines
Nitramines contain nitro groups bonded to nitrogen present within an alicyclic ring, the most commonly used nitramine explosive, RDX, is listed by the EPA as toxic and a Group C (possible human) carcinogen. Octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine (Her Majesty’s Explosive, High Melting point Explosive, HMX also known as octogen) occurs as an impurity in military grade RDX, but is also used as a component in anti-tank weapons. The newer, non-nitramine, insensitive munition, NTO, is being tested as a replacement for RDX. Compared to nitroaromatics, RDX, HMX, NTO, and their transformation products are very water soluble, with low log Kow values () and are highly mobile in soils (Becker Citation1995; Badgujar et al. Citation2008; Toghiani et al. Citation2008; Bhatnagar et al. Citation2013). This property has resulted in the buildup of RDX in soil and groundwater at military sites (Clausen et al. Citation2004; Yamamoto et al. Citation2004) which has leached into surrounding geographical areas. As is the case for TNT, the EPA has established a lifetime health advisory guidance limit for RDX in drinking water of 2 µg/L. Cases where RDX contamination has threatened, or affected, human health such as contamination of a sole source aquifer at Cape Cod, and the exposure of individuals to RDX in the Bickford Case in Utah (Albright Citation2012), underline the seriousness and urgency with which effective containment and decontamination methodologies are needed.
A log Kow value greater than 5 is a predictive indicator for bioaccumulation. Unlike famously bioconcentrating organic pollutants such as DDT (dichlorodiphenyltrichloroethane) and polychlorinated biphenyls (PCBs), which have log Kow values of 4.89 to 6.91; and upwards of 6.47, respectively, values for TNT, DNAN, RDX, and NTO are low, and these compounds, fortunately, have a correspondingly low tendency to bioconcentrate (Lotufo et al. Citation2015).
A comparatively recent addition to the nitramine class, is the cyclic nitroamine compound 2,4,6,8,10,12-hexanitro-2,4,6,8,10,12-hexaazaisowurtzitane (CL-20; China Lake compound #20). This compound is being tested as a propellant because it has higher energy per mass and energy density levels than the currently used HMX. Also, unlike the bright flame and dense smoke trails produced by aluminium-based propellants, CL-20-based propellants are described as smokeless. Testing from 2000 to 2010 (reviewed by Crocker et al. Citation2006; Rylott and Bruce Citation2009; Rylott, Lorenz, et al. Citation2011) found acute and chronic toxicities for CL-20 in both invertebrates and vertebrates, with CL-20 now described as a neurotoxin (Gong et al. Citation2012). In plants and microorganisms, CL-20 causes little toxicity, and although biodegradation rates in the soil are low, mineralization by fungi and bacteria has been demonstrated. Costs remain prohibitively high for wide-scale production of CL-20 (Chaturvedi and Dave Citation2015), and there has been little scientific research in this area in recent years.
Nitrate esters
An additional class of explosives, nitrate esters, contain NO2 groups bonded to an oxygen atom attached to an aliphatic carbon and include pentaerythritol tetranitrate (PETN), glyceryl trinitrate (GTN; nitroglycerin), and ethylene glycol dinitrates (EGDN). The GTN is used by the military in munition propellants, and more widely by construction, demolition, and mining industries, EGDN is used to reduce the freezing temperature of the nitroglycerin component of dynamite. PETN is used in plastic explosives such as Semtex. PETN is toxic to plants and animals (Hawari Citation2014), and the medical use of GTN as a vasodilator in humans demonstrates it has biological activity. These nitrate esters also persist in the environment, and often as co-pollutants with explosives such as TNT (Arbeli et al. Citation2016). Although nitrate ester detoxification pathways have been described for bacteria, fungi and plants, along with some remediation studies (Rylott, Lorenz, et al. Citation2011), there has been little progress in bioremediation strategies in the last decade.
Transformation and degradation of explosives
Phytodetoxification of TNT
Analyses of monocot grass species, dicot annuals and trees, and gymnosperm trees, have all found TNT and metabolites to be localized predominantly in root tissues (Schoenmuth and Pestemer Citation2004; Rylott and Bruce Citation2009; Rylott, Johnston, et al. Citation2015). Detoxification in plants follows classic reductive transformation pathways, outlined in , to form hydroxyl-amino-DNTs (HADNT), then amino-DNTs (ADNT). In Arabidopsis thaliana (Arabidopsis), a model plant species for molecular and biochemical studies, these steps can be catalyzed by oxophytodienoate reductases (OPRs), which are members of the Old Yellow Enzyme (OYE) family (Beynon et al. Citation2009). The OPRs have also been shown to catalyze the reductive attack of the aromatic ring, releasing nitrite and producing hydride (H–TNT) and dihydride (2H–TNT) Meisenheimer TNT adducts. Gene expression studies on plants treated with TNT implicate cytochrome P450s, classic xenobiotic detoxification enzymes, and intriguingly, members of this family are upregulated in all gene expression studies on TNT-dosed plants (Ekman et al. Citation2003; Tanaka et al. Citation2007; Gandia-Herrero et al. Citation2008; Ali et al. Citation2014), although their involvement with TNT detoxification is, as yet, still unconfirmed.
Figure 1. Summary of the predominant known routes for TNT detoxification in bacteria, fungi and plants. Stars indicate degradative routes. TNT: 2,4,6-trinitrotoluene, HADNT: hydroxyl-amino-dinitrotoluene, ADNT: amino-dinitrotoluene, TAT: triaminotoluene, DNT: dinitrotoluene, H-TNT: hydride-, and 2H-TNT: dihydride-Meisenheimer complexes, GSH: glutathione, Glu: glucose, TCA: Tricarboxylic Acid Cycle.
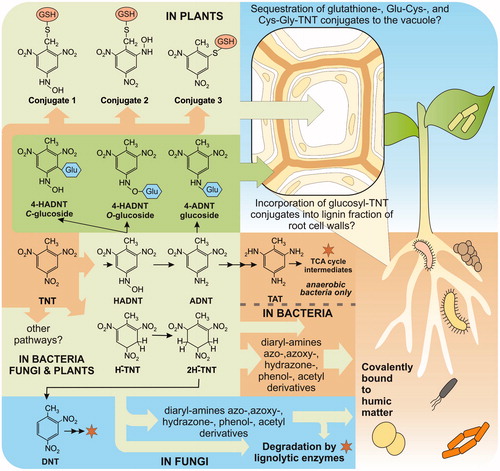
Microbial TNT transformation follows similar pathways to those for plants, with additional, sequential reduction of up to all three nitro groups to ADNT, diamino-nitrotoluene (DANT) and triamino-toluene (TAT). Additional reactions between HADNTs and Meisenheimer adducts product diaryl-amines, azo, azoxy-, hydrazone-, phenol-, acetyl derivatives; compounds which are highly recalcitrant to further degradation (Wittich et al. Citation2008, Citation2009) (). However, a fundamental difference between plant and microbial xenobiotic metabolism is that plants conjugate transformed intermediates to a range of hydrophilic molecules including sugars and glutathione. This step usually, but not always, decreases the toxicity of the compound, enabling it to then be incorporated into plant tissues. Gandia-Herrero et al. (Citation2008) demonstrated that six uridine 5′-diphospho-glucuronosyltransferases (UGTs), five of which are in the same phylogenetic group N, were able to conjugate HADNT or ADNT, at either the 2- or 4- isomer positions forming either C or O-glucosidic bonds, with UGT73B4 exhibiting the highest activity (Gandia-Herrero et al. Citation2008). Bhattacherjee et al. (Citation2014) have used homology modeling and molecular docking of the modeled structure, to show π-cation interaction between a conserved tryptophan in UGT73B4 and 2-HADNT. This amino acid is located within the active site of the five TNT-conjugating UGTs, but absent from the active site in eight, non-TNT-conjugating, group N, UGTs.
Studies have now confirmed the involvement of glutathione transferases (GSTs) in TNT detoxification, with the unexpected finding that TNT is conjugated directly to glutathione (γGlu-Cys-Gly; GSH) without an initial transformation step (Gunning et al. Citation2014; Rylott, Gunning, et al. Citation2015). The GSTs are specifically upregulated in response to TNT exposure, and when over-expressed in Arabidopsis GSTU24 and GST-U25, conferred significantly enhanced ability to withstand and detoxify TNT. The plants were also able to remove more TNT from contaminated soil; showing a corresponding reduction in GSH levels when compared to unmodified plants. Purified U24 and U25 GSTs were shown to form three TNT-glutathionyl products (). While GSTU24 produces almost exclusively conjugate 2, GSTU25 produces conjugates 1, 2; and 3 (2-glutathionyl-4,6-dinitrotoluene), dependent on pH. To produce conjugate 3, GSTU25 catalyzes the denitration of TNT. Given the stability conferred by the electron-withdrawing properties of the three nitro groups of TNT, the substitution of a nitro group for sulfur in conjugate 3 could render the aromatic ring more susceptible to subsequent degradation; DNT mineralization has been shown in both bacteria and fungi; alternatively, endogenous degradative pathways for conjugate 3 may already exist. To identify target amino acids within GSTU25 that might be involved in the formation of conjugate 3, the structure for GSTU25 has recently been determined, in complex with oxidized glutathione. Findings from the structure, in combination with site-directed mutagenesis studies, identified five key amino acids involved in the production of conjugate 3 (Tzafestas et al. Citation2018).
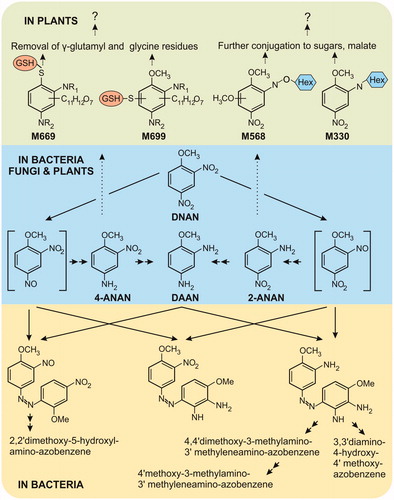
Figure 2. Summary of biotransformation routes for 2,4-dinitroanisole (DNAN) in bacteria, fungi and plants based on (Olivares et al. Citation2016; Schroer, Langenfeld, et al. Citation2017; Schroer, Li, et al. Citation2017). DAAN; 2,4-diaminoanisole; ANAN; methoxy-5-nitroaniline. Dotted arrows indicate putative pathways.
To enhance the production of conjugate 3 in planta a Drosophila melanogaster GST (dGST), that produces almost exclusively conjugate 3, was expressed in Arabidopsis. Although enhanced resistance to TNT was observed in the dGST plants, at higher TNT concentrations, detoxification was limited by GSH availability (Tzafestas et al. Citation2017). Thus, increasing TNT phytodetoxification in plants might require correspondingly increasing GSH availability. In more remediation-suitable species, two poplar (Populus trichocarpa) Tau class GSTs are upregulated in response to TNT with recombinantly expressed, purified enzymes also found to have activity towards TNT (Musdal and Mannervik Citation2015).
The fate of UGT and GST-catalyzed TNT conjugates is still to be determined. In Arabidopsis, glutathionylated xenobiotics can be compartmentalized to the vacuole by MRP2, an ATP-binding cassette transporter belonging to the multidrug resistance-associated protein (MRP) family. MRP2 is upregulated in TNT-fed Arabidopsis (Gandia-Herrero et al. Citation2008), but compartmentation and the ultimate fate of TNT conjugates are still to be elucidated. Towards this, subcellular fractionation studies on bean (Phaseolus vulgaris) fed 14C TNT found over half of the label in the lignin fraction (Sens et al. Citation1998, Citation1999). Tobacco (Nicotiana tabacum) cell suspension cultures fed TNT, accumulate more mono and diglycoside conjugates of 2‐ and 4‐HADNT than are found in whole‐plant cultures (Vila et al. Citation2005), possibly because of the lack of a significant lignin sink for incorporation of these conjugates. In agreement with this finding, a gene expression study in Arabidopsis roots treated with TNT identified upregulation of lignin biosynthesis genes (including phenyl ammonium lyase, cinnamate 4‐hydroxylase, 4‐coumarate CoA ligase, hydroxycinnamoyltransferase, cinnamoyl‐CoA reductase, caffeic acid O‐methyltransferase and cinnamyl alcohol dehydrogenase; Ekman et al. Citation2003). Thus is it plausible that TNT conjugates are incorporated, through non-enzymatic free radical polymerization and self-assembly, into root cell wall lignin? Subsequent metabolism is likely to occur during natural composting of dead plant material, with mineralization of any re-released TNT-intermediates by fungal lignolytic activities, as described above (Rylott and Bruce Citation2009; Rylott, Lorenz, et al. Citation2011).
There are many reports on the phytotoxicity of TNT, and the mode of action has now been established. Arabidopsis plants carrying a mutation in monodehydroascorbate reductase 6 (mdhar6) are resistant to TNT. MDHAR6 is duel-localized to the mitochondria and plastids where is thought to contribute to the recycling of the antioxidant compound ascorbate; however, MDHAR6 also catalyzes the one electron reduction of TNT to a nitro radical that spontaneously regenerates, with the production of superoxide. In vivo, toxicity occurs through the futile cycling of TNT by MDHAR6, which depletes NADH and produces damaging superoxide within sensitive organelles (Johnston et al. Citation2015). This finding presents an opportunity whereby inactivating MDHAR6 in remediation suitable species could potentially be used to enhance tolerance and thus increase the remediation of TNT.
Microbial mineralization of TNT
Although mineralization has not been demonstrated in plants, TNT breakdown, albeit limited, has been demonstrated some time ago in some bacteria and fungi (, Spain et al. Citation2000); findings that could be key to developing degradation pathways using plant-based systems.
Obligate anaerobic bacteria, such as Clostridium sp. are able to oxidize the amino groups of TAT, to form 2,4,6-trihydroxytoluene, which is then degraded to intermediates in the Tricarboxylic Acid (TCA) cycle (; Esteve-Nunez et al. Citation2001). Compared to bacterial rates, more substantial mineralization of TNT has been reported for a number of lignolytic fungi including Phanerochaete chrysosporium (Hawari et al. Citation1999; Esteve-Nunez et al. Citation2001). Following reductive transformation of one or more nitro groups, mineralization is catalyzed nonspecifically by the indirect activity of lignin-degrading enzymes including laccases and manganese peroxidases. Mineralization of DNTs has also been shown in P. chrysosporium (Valli et al. Citation1992), with bacterial pathways elucidated for both 2,4-DNT (Nishino et al Citation2000) and 2,6-DNT (Johnson and Spain Citation2003).
A recent proteomic study to identify the enzymes behind fungal TNT metabolism in Yarrowia lipolytica found the expected enzymes of the TNT-transformation, and stress response protein, (Khilyas et al. Citation2017) but enzyme-based mechanisms to catalyze the denitration of TNT-dihydride-Meisenheimer complexes to potentially mineralize DNT remain elusive. Studies on bacterial consortia highlight the synergistic effect of microbial communities on TNT degradation, with significant mineralization observed in TNT-contaminated soils (Robertson and Jjemba Citation2005).
Phytodetoxification of DNAN
Compared to TNT, relatively little is known about the phytotoxicity of DNAN and detoxification pathways in plants. Soil studies with DNAN and IMX-101 showed that DNAN is toxic to grasses (Dodard et al. Citation2013; Richard and Weidhaas Citation2014b). Plant studies using perennial ryegrass (Lolium perenne L.) found DNAN to be more phytotoxic than TNT (Dodard et al. Citation2013). IMX-101 inhibited growth in big bluestem grass (Andropogon gerardii), Nash Indiangrass (Sorghastrum nutans), and switchgrass (Panicum virgatum) (Richard and Weidhaas Citation2014b).
A new study on liquid culture grown Arabidopsis fed radio-labeled DNAN identified compounds containing both sugar and glutathione conjugates, as shown in , but no detectable mineralization of DNAN (Schroer, Li, et al. Citation2017). Initial transformation involves reduction of the nitro groups through nitroso-intermediates to 2- and 4- isomers of aminonitroanisiole (ANAN), 2- and -4-ANAN isomers. Glycosylated conjugates were identified including a 2-ANAN-derived compound N-glycosylated to a hexose sugar (M330), with a malonylated form of this compound appearing to undergo abiotic decarboxylation to an acetyl glycoside; and an O-glycosidic derivative of 2-ANAN (M568). Two glutathionylated products, one by a sulfhydryl substitution of the methoxy group (M669), and the other directly conjugated to the aromatic ring at a previously unsubstituted position (M699) were described. Alongside this, two cysteinyl conjugates were also identified (Schroer, Li, et al. Citation2017) indicating that both the γ-glutamyl and carboxy-terminal glycine residues of the tripeptide, glutathione, can be removed. These activities allow the reuse of nitrogen, a limiting resource on range soils. The detoxification route parallels that for TNT, and gene expression studies could be used to identify the plant GSTs, with members of the 28-strong tau-class sub-clade likely candidates, along with the GST-conjugate processing phytochelatin synthases and transpeptidases (Rylott, Gunning, et al. Citation2015). Schroer, Li, et al. (Citation2017) also reported significant, light-dependent photolysis (measured by nitrite release) in leaf tissues, but soil-based studies are still needed to determine if there are significant uptake and partitioning of DNAN into aerial tissues for photolysis.
DNAN-detoxifying microbial systems
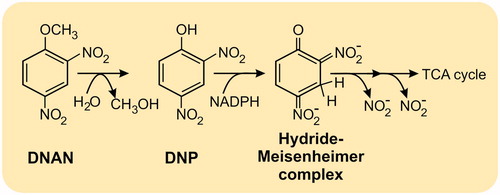
Compared to the studies performed on plants, relatively more is known on the microbial detoxification of DNAN. Biotransformation pathways for DNAN have been elucidated from a range of mixed consortia and pure cultures in both aerobic and anaerobic conditions (Platten et al. Citation2010; Perreault et al. Citation2012; Liang et al. Citation2013; Fida et al. Citation2014; Hawari et al. Citation2015; Schroer et al. Citation2015). The route for DNAN biotransformation () commences, as for plants, with the production of 2- and 4-ANAN isomers, with reduction of the ortho nitro group, preferred. Coupling between nitroso-intermediates and aromatic amines then leads to the formation of azo dimers. Additional transformations of the functional groups then follow including demethylations, demethoxylation, and dehydroxylations (Perreault et al. Citation2012; Liang et al. Citation2013; Olivares et al. Citation2013; al. 2016). The denitrated azo dimer, 2,2’-dimethoxy-5-hydroxyl-amino-azobenzene, has been identified, and nitrite release, through hydride-Meisenheimer complex formation, has been reported for DNAN-derived transformation products (Fida et al. Citation2014). The longer-term fate of DNAN-derived, azo dimers is not known, but they are tightly bound to the humic fraction of soils (Olivares et al. Citation2017). Similar intermediate metabolites have also been reported for the fungus Penicillium sp. KH1 (Schroer, Langenfeld, et al. Citation2017), and an endophytic DNAN-transforming bacterium isolated from willow (Schroer et al. Citation2015). Although DNAN is seldom mineralized by indigenous soil bacteria, encouragingly for bioremediation, Nocardioides sp. strain JS1661 has been isolated from activated sludge based on its ability to grow on DNAN as the sole source of carbon and energy. JS1661 is able to degrade DNAN in non-sterile soil, aqueous media and in a fluidized bed bioreactor (Fida et al. Citation2014; Karthikeyan and Spain Citation2016). To achieve this, an existing hydrolase has evolved the ability to catalyze the hydrolytic release of methanol from DNAN to form 2,4-dinitrophenol, as shown in . Degradation then occurs through an established pathway involving hydride-Meisenheimer complex formation, denitration then metabolism by the TCA cycle (Fida et al. Citation2014).
Figure 3. Degradation route for 2,4-dinitroanisole (DNAN) in Nocardioides sp. strain JS1661 (Fida et al. Citation2014), TCA: Tricarboxylic Acid Cycle.
Toward the identification of microbial genes responsible for DNAN detoxification, transcriptome analysis on microbial populations fed DNAN and NTO reported the upregulation of nitroreductase, hydroxylaminophenol mutase, 4-hydroxylphenyl acetate-associated genes, and quinone oxidoreductases (Weidhaas et al. Citation2018).
RDX in plants
The phytotoxicity of RDX, which is less toxic than TNT, has been well-studied, and is reviewed in Rylott and Bruce (Citation2009), Crocker et al. (Citation2006), and Van Aken (Citation2009). A recent report tested 18 herbaceous dicotyledonous plants adapted to eastern and central North American conditions and found growth inhibition in 16 of the 18 species tested at RDX levels upwards of 100 mg/kg (Hagan et al. Citation2016). Following uptake, RDX is predominantly localized to the aerial tissues of angiosperm species, whereas coniferous trees retain RDX mainly in the roots (Schoenmuth et al. Citation2014). In planta, RDX is reduced to MNX and DNX, with further, light-dependent mineralization of the heterocyclic ring yielding formaldehyde, methanol and carbon dioxide (Van Aken et al. Citation2004). The initial reductive steps are light independent, suggesting the involvement of plant biological activities. Expression studies in Arabidopsis (Ekman et al. Citation2003; Mezzari et al. Citation2005) and poplar (Tanaka et al. Citation2007) identified genes involved in the classic xenobiotic detoxification pathway, including cytochrome P450s, reductases, peroxidases and GSTs (Rylott and Bruce Citation2009; Maestri and Marmiroli Citation2011), but plant enzymes potentially able to transform RDX have not been isolated. Although RDX is readily taken up by plants, and mineralization observed, plants have inherently low endogenous abilities to degrade RDX. Arabidopsis plants grown in soil containing 100 mg/kg RDX accumulated c. 5 µg/g dry weight in their aerial tissues. This stored RDX becomes biologically available through the food chain through herbivory or is returned, as RDX, back to the soil when the plant dies (Rylott, Budarina, et al. Citation2011).
RDX-degrading microbial systems
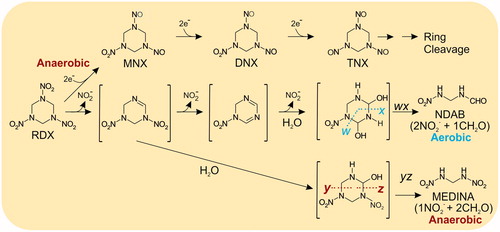
Given that RDX is a relatively recent xenobiotic in our environment, it is perhaps not surprising that plants have not evolved degradative pathways. However, several microbial RDX-metabolizing enzyme systems have been well-characterized and established routes for RDX biodegradation in soils are shown in . Four main enzymatic systems have been characterized: The flavin mononucleotide-containing oxidoreductases XenA and XenB, from Pseudomonas putida and P. fluorescens I-C, respectively (Fuller et al. Citation2009). The XplAB system, which comprises a cytochrome P450 XplA and associated reductase XplB, (together termed XplAB), initially characterized from Rhodococcus rhodochrous 11Y (Kloepper et al. Citation1980; Rylott et al. Citation2006; Jackson et al. Citation2007; Rylott, Jackson, et al. Citation2011). A diaphorase (DiaA) from Clostridium kluyveri (Bhushan et al. Citation2002b) and a nitrate reductase (NR) from Aspergillus niger (Bhushan et al. Citation2002a). The XenA, XenB, DiaA, and NR degrade RDX under anaerobic conditions, through the transformation intermediates hexahydro-1-nitroso-3,5-dinitro-1,3, 5-triazine (MNX), hexahydro-1,3-dinitroso-5-nitro-1,3,5-triazine (DNX) and hexahydro-1,3,5-trinitroso-1,3, 5-triazine (TNX), or by denitration to methylenedinitramine (MEDINA) (Bhushan et al. Citation2002a, Citation2002b; Fuller et al. Citation2009). The XplAB system catalyzes the reductive denitration of RDX to form, under aerobic conditions, 4-nitro-2,4-diazbutanal (NDAB), and anaerobically MEDINA. Aerobic rates of RDX degradation by XplAB are significantly faster than anaerobic systems, but, while MEDINA is converted to formaldehyde and nitrous oxide, products that are mineralizable in soils, the aerobic product NDAB has been detected in groundwater from RDX-contaminated soils (Paquet et al. Citation2011). Despite numerous stable isotope-based studies, additional modes of RDX metabolism have not been found.
Figure 4. Bacterial degradation of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) in bacteria (adapted from Jackson et al. Citation2007). MNX: Hexahydro-1-nitroso-3,5-dinitro-1,3, 5-triazine, DNX: hexahydro-1,3-dinitroso-5-nitro-1,3,5-triazine, TNX: hexahydro-1,3,5-trinitroso-1,3,5-triazine. Dotted lines indicate position of ring cleavage, compounds in brackets are hypothetical.
Fate of NTO in plants
Compared to TNT, RDX, and DNAN, relatively little is known about the detoxification of NTO in plants. A study using IMX-101 indicated that NTO is toxic to grasses (Richard and Weidhaas Citation2014b) but no other plant-based studies have been reported to date, and detoxification pathways are still to be elucidated.
NTO-degrading microbial systems
Under anaerobic conditions, Bacillus licheniformis, present in aqueous industrial wastes, transforms NTO to 3-amino-1,2,4,-triazol-5-one (ATO). Subsequent ring cleavage then occurs yielding N-hydroxylcarbamic acid and urea which both become mineralized (Le Campion et al. Citation1999). In agreement, bioreactors using soil as inoculum, were found to produce ATO, but subsequent biodegradation, to ammonium and nitrate, only occurred under aerobic conditions, and urea was not found (Madeira et al. Citation2017). Enrichment cultures dosed with IMX-101, identified an additional intermediate: 1,2-dihydro-4H-1,2,4-triazol-3-one, and found slow transformation rates (Richard and Weidhaas Citation2014a), plausibly due to the microbial toxicity of DNAN. To profile the communities involved in NTO biodegradation, a metagenomic approach used enrichment cultures supplemented with molasses, methanol, or thiosulfate and identified predominately Pseudomonadaceae, Clostridiaceae and Sporolactobacillaceae (Eberly et al. Citation2016). The subsequent, whole community, transcriptome profiling on batch reactors dosed with NTO and DNAN revealed the upregulation of many genes implicated in the classic detoxification of xenobiotics (Weidhaas et al. Citation2018). Among these was N-ethylmaleimide reductase, a member of the OYE family; other OYE homologs are able to transform nitrate ester and nitroaromatic explosives (Williams et al. Citation2004).
At present, no system exists for the aerobic biodegradation, rather than transformation, of NTO from wastewaters. A study has implicated cytochromes P450 in the detoxification of NTO in the mammalian liver (Le Campion et al. Citation1997). It is possible that, as seen for RDX, microbial systems will evolve to degrade NTO under the aerobic conditions found in surface run-off waters, subsurface soil and the rhizosphere.
Enhancing phytodetoxification through genetic modification
The previous decade saw a flurry of publications demonstrating the promise of GM technologies to remediate explosives (reviewed in Van Aken Citation2008, Citation2009; Rylott and Bruce Citation2009; Maestri and Marmiroli Citation2011; Rylott, Lorenz, et al. Citation2011). These studies, in model plant systems, expressed bacterial genes to introduce, or enhance, detoxification pathways in Arabidopsis and tobacco. For TNT and PETN, bacterial NAD(P)H nitroreductases such as nfsI, and OYE family member onr, both from Enterobacter cloacae, conferred significant resistance and detoxification abilities, whereas the capability of plants to degrade RDX was conferred by the introduction of xplA/B. Such plants are able to grow in soils containing levels of explosives inhibitory to unmodified plants and, in the case of xplA/B-expressing plants, contain dramatically reduced levels of RDX in their tissues, with potentially beneficial impacts on the food chain (Rylott, Budarina, et al. Citation2011). TNT and RDX are commonly found together, and TNT detoxifying nitroreductases have now been combined with xplA/B to generate Arabidopsis plants able to grow and remediate soils contaminated with these co-pollutants (Rylott, Budarina, et al. Citation2011). Towards developing TNT-mineralizing systems in plants, TNT-denitrating GSTs and dioxygenase activities show promise. Plant-expressed fungal laccases also have potential; although not commonly found in plants, these xylem-localized enzymes were first characterized from the Japanese lacquer tree, Rhus vernicifera.
GM technology is expanding into phytoremediation-applicable species, with the first step being the successful generation of hybrid aspen (Populus tremula x tremuloides var. Etroploe) expressing a bacterial TNT-detoxifying nitroreductase (van Dillewijn et al. Citation2008). Further progress has since been made with RDX-degrading switchgrass (Panicum virgatum), wheatgrass (Pascopyrum smithii) and creeping bentgrass (Agrostis stolonifera) transformed with xplA, xplB and nfsI (Zhang, Rylott, et al. Citation2017; Zhang et al. Citation2018, 2016). The next steps now require the development of transformation protocols for a broader range of plant species such as vetiver and miscanthus. Panja et al. (Citation2018). For transgene expression in grass species, identification and testing of gene promoters from monocots, or ideally the genome of the target plant own genome are prerequisites for reliable expression. Given the differences in the spatial distribution of explosives in plants, for example, TNT is predominantly localized in the root, RDX in aerial tissues, there is also the opportunity to use tissue-specific promoters to target detoxification activity and reduce the metabolic load associated with constitutive transgene expression. However, despite promising lab-based results, gaining regulatory approval for much-needed testing of GM plants in field trials is both financially prohibitive and time-consuming (Rylott, Johnston, et al. Citation2015). To perhaps address perceived concern over the spread of GM pollen, a study has created transplastomic TNT-detoxifying tobacco expressing a bacterial nitroreductase. Restricting transgenes to the plastid genome prevents pollen-based transgene dispersal routes (Zhang, Routsong, et al. Citation2017). Reports enhancing the ability of endogenous detoxification pathways in plants (Gunning et al. Citation2014; Johnston et al. Citation2015) could pave the way for selective breeding screens.
Remediation in-the-field
While experiments to identify the genetic basis behind detoxification pathways use pure compounds, detoxification in-the-field often requires the ability of the plant to withstand the effects of two, or more, explosive co-contaminants, and confoundingly, interactions between formulation ingredients can influence degradation products (Halasz et al. Citation2018). Degradation rates also vary wildly depending on soil type, and to overcome this, Katseanes et al. (Citation2017) used types multivariate analysis across a range of soil types to successfully predict environmental persistence of TNT and RDX (Katseanes et al. Citation2017). However, plant-based field-studies are not forthcoming. Field trials are critical in supplying a broad range of parameters that stakeholders need to effectively assess the viability of phytoremediation as a clean-up approach (Kennen and Kirkwood Citation2015).
Suitable plant species
Species suitable for effective remediation in-the-field are usually quick-growing phreatophytic species with relatively high biomass production rates and low maintenance. On military training ranges, species also need to be relatively low growing, fire resistant and able to withstand disruption by military equipment used on ranges. Prevalent species for temperate regions include perennial grass species such as switchgrass (Panicum virgatum); miscanthus (Miscanthus × giganteus); vetiver (Vetiveria zizaniodes); and hybrid poplar (Populus sp.) and willow (Salix sp.) trees. Recently, vetiver has been shown to effectively remove explosives from wastewater effluents from an industrial munition facility, with RDX, HMX, and DNAN transformation products detected in root and aerial tissues (Panja et al. Citation2018). Studies have also identified the cool season orchardgrass (Dactylis glomerata; Duringer et al. Citation2010), and species native to the Caribbean (Correa-Torres et al. Citation2012). Still, perhaps a contentious issue, is the use of human food crop species for in-the-field remediation, for example, some studies have used sunflower (Helianthus annuus) which would perhaps require constraints to be in place to prevent plant materials entering the human food chain.
While planting buffer zones around contaminated sites is usually achievable, on-site access is often restricted due to unexploded ordnance. This constraint can be overcome by the use of air-borne dispersal methods such as the use of helicopters to scatter seed balls (Margot Citation2009). As TNT is particularly inhibitory to germinating seedlings, the use of seed balls would also provide a localized area of uncontaminated soil for germination and early plant establishment. The presence of germination-inhibiting levels of TNT could also mean that seed-dispersed, annual species colonize less effectively than vegetatively propagated perennials species. To aid the establishment of the seedlings, seed balls could contain a robust matrix that includes nutrients, an improved water holding capacity and a mix of rhizospheric and endophytic bacteria previously isolated from the contaminated site. Genetically modifying these bacteria to contain explosive-detoxifying genes could also be considered. Phytoremediation is likely to take a number of years, with continuous remediation strategies required for explosives containment to be in place on active ranges. For these conditions, perennial species, rather than annuals appear best-suited for phytoremediation purposes (Jiamjitrpanich et al. Citation2012).
Herbivory
Phytoremediation strategies have to consider the effects of mobilizing explosive contaminants and detoxification intermediates on the ecology of the site. While studies indicate that the major nitroaromatic and nitramine explosives, TNT, RDX, DNAN, and NTO do not bioaccumulate (Lotufo et al. Citation2015), there is the potential for toxicity to herbivores. As discussed earlier, the majority of TNT is stored and detoxified in below-ground tissues, where it will be accessible to root herbivores, but; TNT transformed intermediates are detectable in aerial tissues. Radiolabelled TNT fed to sheep was found to be reduced mostly by bacteria in the gastrointestinal tract, immobilized to organic matter then excreted (Smith et al. Citation2008). Similar studies using RDX identified rumen bacteria responsible for anaerobic RDX metabolism through MNX, DNX to TNX (Eaton et al. Citation2011, Citation2013). These studies, which used relatively low doses of TNT or RDX to avoid toxicity to the animal, demonstrate that sheep ruminant bacteria have the ability to transform explosives to less toxic intermediates. But research is still needed to establish the effects of direct aerial and root herbivory on plants grown in soils contaminated with explosives.
Endophytic and rhizospheric communities
Military ranges are challenging environments for plant growth: as well as the presence of toxic explosives, the soils are often shallow, limited in nutrients and organic matter, and adverse climatic conditions. Plant endophytic and associated rhizospheric bacteria make highly significant contributions to xenobiotic detoxification, along with significant positive contributions to plant health (Finkel et al. Citation2017). Increasingly, this plant and microbial collection are being seen as a single unit or “holobiont” that responds to environmental pressures as one (Dessaux et al. Citation2016).
To utilize the potential of plant-associated bacterial communities to remediate 2,4-DNT, Thijs, Weyens, et al. (Citation2014) performed selective enrichments using 2,4-DNT as the sole nitrogen source on bacteria isolated from DNT-contaminated military soil. Strains able to transform 2,4-DNT were further screened for potential plant growth-promoting properties, then a seven-species consortium assembled. When inoculated with the consortium, Arabidopsis plants exhibited enhanced root growth in the presence of 2,4-DNT. Despite screening, the 2,4-DNT mineralizing enzyme DNT dioxygenase, and downstream pathway enzymes were not found in the Thijs, Weyens, et al. (Citation2014) study; perhaps a target for future studies. Following on from the Thijs, Weyens, et al. (Citation2014) study which focused exclusively on soil bacteria Thijs, Van Dillewijn, et al. (Citation2014) and Thijs et al. (Citation2018) used the remediation-relevant tree species Acer pseudoplatanus found growing at a historically TNT-contaminated location, to isolate rhizosphere, root endosphere, and endo-phyllosphere bacteria. The bacteria were identified using 16S ribosomal RNA sequencing, then screened for plant growth-promoting properties, and the presence of genes likely to encode TNT-transformation. These genes were identified using PCR with DNA primers designed to amplify the Xenobiotic Reductase B gene (XenB) from Pseudomonas putida KT2440 (van Dillewijn et al. Citation2008) and an internal fragment of members of the OYE family with similarity to known type II hydride transferases, with subsequent homology searches of the amplified sequences (Thijs, Van Dillewijn, et al. Citation2014). Isolates from a broader (300 isolates) population were also subsequently screened for nitroreductase enzyme activity and concurrent TNT-transformation. Inoculation of bent grass (Agrostis capillaris) with optimized consortia of beneficial strains was demonstrated to confer significantly enhanced abilities to withstand TNT-induced stress and detoxify TNT (Thijs, Van Dillewijn, et al. Citation2014; Thijs et al. Citation2018).
Towards harnessing the potential of plant-associated microbial communities, rhizosphere-colonizing Pseudomonas fluorescens engineered to contain xplA, was shown to degrade RDX in the rhizosphere (Lorenz et al. Citation2013). However, this study highlighted the need for long-term genetic stability, for example, incorporation of the genetic material into the bacterial genome. Similar results have been reported for the bioaugmentation of RDX-contaminated water with the xplAB-containing bacterial strain Gordonia sp. KTR9. Despite encouraging initial results, both bacterial numbers of Gordonia sp. KTR9 and xplA levels declined over time (Crocker et al. Citation2015; Michalsen et al. Citation2016; Fuller et al. Citation2017). Factors preventing the accumulation of sufficient numbers of xplAB-containing soil bacteria to remove RDX-contamination, preventing downstream contamination are unknown, but xplAB-engineered plant endophytic, and associated rhizospheric bacterial communities indigenous to a contaminated site would be predicted to successfully reestablish in that environment.
Soil amendments
The use of soil amendments such as biochar, compost, and molasses to ‘kick-start’ microbial degradation has been well-studied for many xenobiotics and is reviewed in Wu et al. (Citation2017). But few studies have yet investigated the effect of amendments for phytoremediation on explosives. A laboratory study (Lamichhane et al. Citation2012) showed that the application of molasses to open burn/detonation areas enabled Guinea Grass (Panicum maximum) to remediate RDX, with plant and microbial communities fully remediating soil contaminated with 1.3 mg/kg RDX within the 15-week study. These promising results show that amendments can help remediation, but experiments are needed to understand the longevity of amendment-induced effects. The application of these materials to large areas of potentially inaccessible, contaminated land is likely to be prohibitively costly, and this approach is perhaps best focused on localized areas of relatively high contamination, to mitigate contamination in groundwater run-off.
Site monitoring
A significant cost factor of site remediation is monitoring of contaminant levels, and this is particularly difficult given the large scale, and inaccessibility of military sites. Novel studies have quantified the response to explosives of plant species native to military ranges in temperate regions. By measuring multiple variables representing morphological and physiological responses, such studies identified vegetation responses that could potentially be used to determine levels of TNT and RDX contamination (Ali et al. Citation2014; Via and Zinnert Citation2016; Via et al. Citation2017, Citation2015). Hyperspectral imaging technologies are also being developed to remotely detect vegetation response (Naumann et al. Citation2010) using aerial drones as suitable carriers to record contamination levels on surface-inaccessible sites.
Future directions
It is clear that plant-associated microbial communities make an important contribution to the remediation of organic environmental pollutants. Promising studies have selected and manipulated plant microbial communities to successfully confer resistance to explosives, increased detoxification, and enhance plant growth. Efforts towards developing effective plant-based remediation tools should move towards the holobiont model to harness the inherent significant microbial contributions.
Natural attenuation rates are insufficient to detoxify current levels of TNT contamination and rely primarily on transformation activities rather than mineralization. Developing plant-based systems able to biodegrade TNT directly or support and enhance populations of TNT-degrading fungi and bacteria, would elicit a paradigm shift in the remediation of this global pollutant.
Core genes responsible for the detoxification of TNT and RDX have been well-characterized and these studies will be valuable tools to help understand the fate of newer, insensitive explosives in plants. Future research will extrapolate the genetic-based knowledge from model plant systems into species suitable for in-the-field remediation. However, despite encouraging, laboratory-based results using GM-based technologies, field trials are yet to materialize, with delays due to the prohibitive expense and time issues associated with obtaining regulatory approval (Rylott, Johnston, et al. Citation2015). In the US and Japan, these issues could perhaps be overcome by gene editing technologies enabling the development of plant lines immune to GM regulations, but recent rulings by the EU have moved to classify plants developed using these techniques as requiring GM approval (Callaway Citation2018). Either with or without GM, field trials are critical to delivering the necessary data for commercial companies and military agencies to be able to consider the viability of phytoremediation as an approach to clean-up explosives-contaminated soil.
Finally, the pioneering studies presented in this review are paving the way for similar strategies on other organic environmental pollutants. Global environmental pollution has reached unprecedented levels: according to a national soil quality survey, 16% of all soil samples in China contained contaminants exceeding recommended soil quality standards and affecting human health. Increased public awareness and concern are now pressing for more effective remediation solutions, and China’s ambitious 2016 Soil Pollution Prevention and Control action plan, estimated to cost US$90 billion (Hou and Li Citation2017), is primed to fully utilize, and further develop remediation technologies. In conclusion, the technical advancements described in this review, increasing public concern and pressure, and projected funding increases, will hopefully result in the development of effective, plant-based xenobiotic remediation strategies for use in-the-field.
Additional information
Funding
References
- Albright R. 2012. Cleanup of chemical and explsosive munitions, vol 2nd Edition. Kidlington, UK: Elsevier.
- Ali A, Zinnert JC, Muthukumar B, Peng Y, Chung SM, Stewart CN Jr. 2014. Physiological and transcriptional responses of Baccharis halimifolia to the explosive “composition B” (RDX/TNT) in amended soil. Environ Sci Pollut Res Int. 21(13):8261–8270. doi:10.1007/s11356-014-2764-4.
- Arbeli Z, Garcia-Bonilla E, Pardo C, Hidalgo K, Velásquez T, Peña L, Ramos EC, Avila-Arias H, Molano-Gonzalez N, Brandão PFB, et al. 2016. Persistence of pentolite (PETN and TNT) in soil microcosms and microbial enrichment cultures. Environ Sci Pollut Res Int. 23(9):9144–9155. doi:10.1007/s11356-016-6133-3.
- Arthur JD, Mark NW, Taylor S, Šimůnek J, Brusseau ML, Dontsova KM. 2018. Dissolution and transport of insensitive munitions formulations IMX-101 and IMX-104 in saturated soil columns. Sci of the Total Envir. 624:758–768. doi:10.1016/j.scitotenv.2017.11.307.
- Badgujar DM, Talawar MB, Asthana SN, Mahulikar PP. 2008. Advances in science and technology of modern energetic materials: an overview. J Hazard Mater. 151(2–3):289–305. doi:10.1016/j.jhazmat.2007.10.039.
- BCC Research LLC. 2018. Global market for explosives to gain $7.4 billion from 2017–2022 [Accessed 2018 June 6]. https://www.bccresearch.com/pressroom/chm/global-market-for-explosives-to-gain-$74-billion-from-2017-2022.
- Becker NM. 1995. Fate of selected high explosives in the environment: a literature review. Los Alamos, NM: Los Alamos National Laboratory.
- Beynon ER, Symons ZC, Jackson RG, Lorenz A, Rylott EL, Bruce NC. 2009. The role of oxophytodienoate reductases in the detoxification of the explosive 2,4,6-trinitrotoluene by Arabidopsis. Plant Physiol. 151(1):253–261. doi:10.1104/pp.109.141598.
- Bhatnagar N, Kamath G, Potoff JJ. 2013. Prediction of 1-octanol-water and air-water partition coefficients for nitro-aromatic compounds from molecular dynamics simulations. Phys Chem Chem Phys. 15(17):6467–6474. doi:10.1039/c3cp44284e.
- Bhattacherjee A, Mandal RS, Das S, Kundu S. 2014. Sequence and 3D structure based analysis of TNT degrading proteins in Arabidopsis thaliana. J Molec Modeling. 20:2174. doi:10.1007/s00894-014-2174-z.
- Bhushan B, Halasz A, Spain J, Thiboutot S, Ampleman G, Hawari J. 2002a. Biotransformation of hexahydro-1,3,5-trinitro-1,3,5-triazine catalyzed by a NAD(P)H: nitrate oxidoreductase from Aspergillus niger. Environ Sci Technol. 36:3104–3108. doi:10.1021/es011460a.
- Bhushan B, Halasz A, Spain JC, Hawari J. 2002b. Diaphorase catalyzed biotransformation of RDX via N-denitration mechanism. Biochem Biophys Res Commun. 296(4):779–784. doi:10.1016/S0006-291X(02)00874-4.
- Boddu VM, Abburi K, Maloney SW, Damavarapu R. 2008. Thermophysical properties of an insensitive munitions compound, 2,4-dinitroanisole. J Chem Eng Data. 53:1120–1125. doi:10.1021/je7006764.
- Callaway E. 2018. CRISPR plants now subject to tough GM laws in European Union. Nature. 560(7716):16. doi:10.1038/d41586-018-05814-6.
- Chaturvedi S, Dave PN. 2015. Solid propellants: AP/HTPB composite propellants. Arabian J of Chem. In Press. doi:10.1016/j.arabjc.2014.12.033.
- Clausen J, Robb J, Curry D, Korte N. 2004. A case study of contaminants on military ranges: Camp Edwards, Massachusetts, USA. Environ Pollut. 129(1):13–21. doi:10.1016/j.envpol.2003.10.002.
- Correa-Torres SN, Pacheco-Londono LC, Espinosa-Fuentes EA, Rodriguez L, Souto-Bachiller FA, Hernandez-Rivera SP. 2012. TNT removal from culture media by three commonly available wild plants growing in the Caribbean. JEM. 14:30–33. doi:10.1039/C1EM10602C.
- Crocker F, Indest K, Fredrickson H. 2006. Biodegradation of the cyclic nitramine explosives RDX, HMX, and CL-20. Appl Microbiol Biotechnol. 73(2):274–290. doi:10.1007/s00253-006-0588-y.
- Crocker FH, Indest KJ, Jung CM, Hancock DE, Fuller ME, Hatzinger PB, Vainberg S, Istok JD, Wilson E, Michalsen MM. 2015. Evaluation of microbial transport during aerobic bioaugmentation of an RDX-contaminated aquifer. Biodegradation 26(6):443–451. doi:10.1007/s10532-015-9746-1.
- Defense Science Board Task Force. 1998. Task force on unexploded ordnance (UXO) clearance, active range UXO clearance, and explosive ordnance disposal (EOD) programs. Washington, DC, US: Office of the Undersecretary of Defense for Aquisition and Technology.
- Dessaux Y, Grandclement C, Faure D. 2016. Engineering the Rhizosphere. Trends Plant Sci. 21(3):266–278. doi:10.1016/j.tplants.2016.01.002.
- Dodard SG, Sarrazin M, Hawari J, Paquet L, Ampleman G, Thiboutot S, Sunahara GI. 2013. Ecotoxicological assessment of a high energetic and insensitive munitions compound: 2,4-dinitroanisole (DNAN). J Hazard Mater. 262:143–150. doi:10.1016/j.jhazmat.2013.08.043.
- Duringer JM, Morrie Craig A, Smith DJ, Chaney RL. 2010. Uptake and transformation of soil [14C]-trinitrotoluene by cool-season grasses. Environ Sci Technol. 44(16):6325–6330. doi:10.1021/es903671n.
- Eaton HL, De Lorme M, Chaney RL, Craig AM. 2011. Ovine ruminal microbes are capable of biotransforming hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX). Microb Ecol. 62(2):274–286. doi:10.1007/s00248-011-9809-8.
- Eaton HL, Duringer JM, Murty LD, Craig AM. 2013. Anaerobic bioremediation of RDX by ovine whole rumen fluid and pure culture isolates. Appl Microbiol Biotechnol. 97(8):3699–3710. doi:10.1007/s00253-012-4172-3.
- Eberly JO, Indest KJ, Hancock DE, Jung CM, Crocker FH. 2016. Metagenomic analysis of denitrifying wastewater enrichment cultures able to transform the explosive, 3-nitro-1,2,4-triazol-5-one (NTO). J Ind Microbiol Biotechnol. 43(6):795–805. doi:10.1007/s10295-016-1755-5.
- Eisentraeger A, Reifferscheid G, Dardenne F, Blust R, Schofer A. 2007. Hazard characterization and identification of a former ammunition site using microarrays, bioassays, and chemical analysis. Environ Toxicol Chem. 26(4):634–646. doi:10.1897/06-285R.1.
- Ekman DR, Lorenz WW, Przybyla AE, Wolfe NL, Dean JF. 2003. SAGE analysis of transcriptome responses in Arabidopsis roots exposed to 2,4,6-trinitrotoluene. Plant Physiol. 133(3):1397–1406. doi:10.1104/pp.103.028019.
- Esteve-Nunez A, Caballero A, Ramos JL. 2001. Biological degradation of 2,4,6-trinitrotoluene. Microbiol Mol Biol Rev. 65(3):335–352. doi:10.1128/MMBR.65.3.335-352.2001.
- Fida TT, Palamuru S, Pandey G, Spain JC. 2014. Aerobic biodegradation of 2,4-dinitroanisole by Nocardioides sp. strain JS1661. Appl Environ Microbiol. 80(24):7725–7731. doi:10.1128/AEM.02752-14.
- Finkel OM, Castrillo G, Herrera Paredes S, Salas Gonzalez I, Dangl JL. 2017. Understanding and exploiting plant beneficial microbes. Curr Opin Plant Biol. 38:155–163. doi:10.1016/j.pbi.2017.04.018.
- Fuller M, McClay K, Hawari J, Paquet L, Malone T, Fox B, Steffan R. 2009. Transformation of RDX and other energetic compounds by xenobiotic reductases XenA and XenB. Appl Microbiol Biotechnol. 84(3):535–544. doi:10.1007/s00253-009-2024-6.
- Fuller ME, Hatzinger PB, Condee CW, Andaya C, Rezes R, Michalsen MM, Crocker FH, Indest KJ, Jung CM, Alon Blakeney G, et al. 2017. RDX degradation in bioaugmented model aquifer columns under aerobic and low oxygen conditions. Appl Microbiol Biotechnol. 101(13):5557–5567. doi:10.1007/s00253-017-8269-6.
- Gandia-Herrero F, Lorenz A, Larson T, Graham IA, Bowles DJ, Rylott EL, Bruce NC. 2008. Detoxification of the explosive 2,4,6-trinitrotoluene in Arabidopsis: discovery of bifunctional O- and C-glucosyltransferases. Plant J. 56(6):963–974. doi:10.1111/j.1365-313X.2008.03653.x.
- Gong P, Guan X, Pirooznia M, Liang C, Perkins EJ. 2012. Gene expression analysis of CL-20-induced reversible neurotoxicity reveals GABA(A) receptors as potential targets in the earthworm Eisenia fetida. Environ Sci Technol. 46(2):1223–1232. doi:10.1021/es203642e.
- Gorecki S, Nesslany F, Hube D, Mullot JU, Vasseur P, Marchioni E, Camel V, Noel L, Le Bizec B, Guerin T, et al. 2017. Human health risks related to the consumption of foodstuffs of plant and animal origin produced on a site polluted by chemical munitions of the First World War. Sci Tot Env. 599–600:314–323. doi:10.1016/j.scitotenv.2017.04.213.
- Gunning V, Tzafestas K, Sparrow H, Johnston EJ, Brentnall AS, Potts JR, Rylott EL, Bruce NC. 2014. Arabidopsis glutathione transferases U24 and U25 exhibit a range of detoxification activities with the environmental pollutant and explosive, 2,4,6-trinitrotoluene. Plant Physiol. 165(2):854–865. doi:10.1104/pp.114.237180.
- Hagan FL, Koeser AK, Dawson JO. 2016. Growth changes of eighteen herbaceous angiosperms induced by Hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) in soil. Int J Phytoremediation. 18(1):94–102. doi:10.1080/15226514.2015.1073665.
- Halasz A, Hawari J, Perreault NN. 2018. New insights into the photochemical degradation of the insensitive munition formulation IMX-101 in water. Environ Sci Technol. 52(2):589–596. doi:10.1021/acs.est.7b04878.
- Hawari J, Halasz A, Beaudet S, Paquet L, Ampleman G, Thiboutot S. 1999. Biotransformation of 2, 4, 6-trinitrotoluene with Phanerochaete chrysosporium in agitated cultures at pH 4.5. Appl Env Microbiol. 65(7):2977–2986. doi:10.1007/s002530000445.
- Hawari J, Monteil-Rivera F, Perreault NN, Halasz A, Paquet L, Radovic-Hrapovic Z, Deschamps S, Thiboutot S, Ampleman G. 2015. Environmental fate of 2,4-dinitroanisole (DNAN) and its reduced products. Chemosphere 119:16–23. doi:10.1016/j.chemosphere.2014.05.047.
- Hawari J, 2014. Environmental fate and ecological impact of emerging energetic chemicals (ADN, DNAN and its amino-derivatives, PETN, NTO,NQ, FOX-7 and FOX-12) and insensitive formulation. Montreal, Canada: EME NRC-Montreal.
- Hou D, Li F. 2017. Complexities surrounding China’s soil action plan. Land Degrad Develop. 28:2315–2320. doi:10.1002/ldr.2741.
- Jackson R, Rylott E, Fournier D, Hawari J, Bruce N. 2007. Exploring the biochemical properties and remediation applications of the unusual explosive-degrading P450 system XpIA/B. PNAS. 104(43):16822–16827. doi:10.1073/pnas.0705110104.
- Jiamjitrpanich W, Parkpian P, Polprasert C, Laurent F, Kosanlavit R. 2012. The tolerance efficiency of Panicum maximum and Helianthus annuus in TNT-contaminated soil and nZVI-contaminated soil. J Env Sci Health Part A, Toxic/Hazardous Subst Env Eng. 47(11):1506–1513. doi:10.1080/10934529.2012.680320.
- Johnston EJ, Rylott EL, Beynon E, Lorenz A, Chechik V, Bruce NC. 2015. Monodehydroascorbate reductase mediates TNT toxicity in plants. Science. 349(6252):1072–1075. doi:10.1126/science.aab3472.
- Johnson GR, Spain JC. 2003. Evolution of catabolic pathways for synthetic compounds: bacterial pathways for degradation of 2,4-dinitrotoluene and nitrobenzene. Appl Microbiol Biotechnol. 62(2–3):110–123. doi:10.1007/s00253-003-1341-4.
- Kalderis D, Juhasz A, Boopathy R, Comfort SD. 2011. Soils contaminated with explosives: environmental fate and evaluation of state-of-the-art remediation processes (IUPAC Technical Report). Pure & Applied Chem. 83(7):1407–1484. doi:10.1351/PAC-REP-10-01-05.
- Karthikeyan S, Spain JC. 2016. Biodegradation of 2,4-dinitroanisole (DNAN) by Nocardioides sp. JS1661 in water, soil and bioreactors. J Hazard Mater. 312:37–44. doi:10.1016/j.jhazmat.2016.03.029.
- Katseanes CK, Chappell MA, Hopkins BG, Durham BD, Price CL, Porter BE, Miller LF. 2017. Multivariate soil fertility relationships for predicting the environmental persistence of 2,4,6-trinitrotoluene (TNT) and 1,3,5-trinitro-1,3,5-tricyclohexane (RDX) among taxonomically distinct soils. J Environ Management. 203:383–390. doi:10.1016/j.jenvman.2017.08.005.
- Kennen K, Kirkwood N. 2015. Phyto: principles and resources for site remediation and landscape design. London, UK: Taylor & Francis Group. doi:10.4324/9781315746661.
- Khilyas IV, Lochnit G, Ilinskaya ON. 2017. Proteomic analysis of 2,4,6-trinitrotoluene degrading yeast Yarrowia lipolytica. Front Microbiol. 8:2600.doi:10.3389/fmicb.2017.02600.
- Kloepper JW, Leong J, Teintze M, Schroth MN. 1980. Pseudomonas siderophores: a mechanism explaining disease-suppressive soils. Curr Microbiol. 4(5):317–320. doi:10.1007/BF02602840.
- Labrou NE, Papageorgiou AC, Pavli O, Flemetakis E. 2015. Plant GSTome: structure and functional role in xenome network and plant stress response. Curr Opin Biotechnol. 32:186–194. doi:10.1016/j.copbio.2014.12.024.
- Lamichhane KM, Babcock RW, Jr., Turnbull SJ, Schenck S. 2012. Molasses enhanced phyto and bioremediation treatability study of explosives contaminated Hawaiian soils. J Hazard Mater. 243:334–339. doi:10.1016/j.jhazmat.2012.10.043.
- Le Campion L, Delaforge M, Noel JP, Ouazzani J. 1997. Metabolism of 14C-labelled 5-nitro-1,2,4-triazol-3-one by rat liver microsomes–evidence for the participation of cytochrome P-450. Eur J Biochem. 248(2):401–406. doi:10.1111/j.1432-1033.1997.00401.x.
- Le Campion L, Vandais A, Ouazzani J. 1999. Microbial remediation of NTO in aqueous industrial wastes. FEMS Microbiol Lett. 176(1):197–203. doi:10.1111/j.1574-6968.1999.tb13662.x.
- Liang J, Olivares C, Field JA, Sierra-Alvarez R. 2013. Microbial toxicity of the insensitive munitions compound, 2,4-dinitroanisole (DNAN), and its aromatic amine metabolites. J Hazard Mater. 262:281–287. doi:10.1016/j.jhazmat.2013.08.046.
- Lorenz A, Rylott EL, Strand SE, Bruce NC. 2013. Towards engineering degradation of the explosive pollutant hexahydro-1,3,5-trinitro-1,3,5-triazine in the rhizosphere. FEMS Microbiol Lett. 340(1):49–54. doi:10.1111/1574-6968.12072.
- Lotufo GR, Biedenbach JM, Sims JG, Chappell P, Stanley JK, Gust KA. 2015. Bioaccumulation kinetics of the conventional energetics TNT and RDX relative to insensitive munitions constituents DNAN and NTO in Rana pipiens tadpoles. Environ Toxicol Chem. 34(4):880–886. doi:10.1002/etc.2863.
- Madeira CL, Speet SA, Nieto CA, Abrell L, Chorover J, Sierra-Alvarez R, Field JA. 2017. Sequential anaerobic-aerobic biodegradation of emerging insensitive munitions compound 3-nitro-1,2,4-triazol-5-one (NTO). Chemosphere 167:478–484. doi:10.1016/j.chemosphere.2016.10.032.
- Maestri E, Marmiroli N. 2011. Transgenic plants for phytoremediation. Int J Phytoremed. 13(1):264–279. doi:10.1080/15226514.2011.568549.
- Margot A. 2009. Environmentalists adopt new weapon: seed balls. National Public Radio. 18 June 2018.
- Mezzari MP, Walters K, Jelinkova M, Shih MC, Just CL, Schnoor JL. 2005. Gene expression and microscopic analysis of arabidopsis exposed to chloroacetanilide herbicides and explosive compounds. A phytoremediation approach. Plant Physiol. 138(2):858–869. doi:10.1104/pp.104.056168.
- Michalsen MM, King AS, Rule RA, Fuller ME, Hatzinger PB, Condee CW, Crocker FH, Indest KJ, Jung CM, Istok JD. 2016. Evaluation of biostimulation and bioaugmentation to stimulate hexahydro-1,3,5-trinitro-1,3,5,-triazine degradation in an aerobic groundwater aquifer. Environ Sci Technol. 50(14):7625–7632. doi:10.1021/acs.est.6b00630.
- Musdal Y, Mannervik B. 2015. Substrate specificities of two tau class glutathione transferases inducible by 2,4,6-trinitrotoluene in poplar. Biochim Biophys Acta. 1850(9):1877–1883. doi:10.1016/j.bbagen.2015.05.015.
- Naumann JC, Anderson JE, Young DR. 2010. Remote detection of plant physiological responses to TNT soil contamination. Plant Soil. 329(1–2):239–248. doi:10.1007/s11104-009-0148-1.
- Nishino SF, Paoli GC, Spain JC. 2000. Aerobic degradation of dinitrotoluenes and pathway for bacterial degradation of 2,6-dinitrotoluene. Appl Environ Microbiol. 66(5):2139–2147. doi:10.1128/AEM.66.5.2139-2147.2000.
- Olivares CI, Abrell L, Khatiwada R, Chorover J, Sierra-Alvarez R, Field JA. 2016. (Bio)transformation of 2,4-dinitroanisole (DNAN) in soils. J Hazard Mater. 304:214–221. doi:10.1016/j.jhazmat.2015.10.059.
- Olivares CI, Liang J, Abrell L, Sierra-Alvarez R, Field JA. 2013. Pathways of reductive 2,4-dinitroanisole (DNAN) biotransformation in sludge. Biotechnol Bioeng. 110(6):1595–1604. doi:10.1002/bit.24820.
- Olivares CI, Madeira CL, Sierra-Alvarez R, Kadoya W, Abrell L, Chorover J, Field JA. 2017. Environmental fate of 14C radiolabeled 2,4-dinitroanisole in soil microcosms. Environ Sci Technol. 51(22):13327–13334. doi:10.1021/acs.est.7b03699.
- Panja S, Sarkar D, Datta R. 2018. Vetiver grass (Chrysopogon zizanioides) is capable of removing insensitive high explosives from munition industry wastewater. Chemosphere 209:920–927. doi:10.1016/j.chemosphere.2018.06.155.
- Panz K, Miksch K. 2012. Phytoremediation of explosives (TNT, RDX, HMX) by wild-type and transgenic plants. J Environ Manage. 113:85–92. doi:10.1016/j.jenvman.2012.08.016.
- Paquet L, Monteil-Rivera F, Hatzinger PB, Fuller ME, Hawari J. 2011. Analysis of the key intermediates of RDX (hexahydro-1,3,5-trinitro-1,3,5-triazine) in groundwater: occurrence, stability and preservation. J Environ Monit. 13(8):2304–2311. doi:10.1039/c1em10329f.
- Perreault NN, Manno D, Halasz A, Thiboutot S, Ampleman G, Hawari J. 2012. Aerobic biotransformation of 2,4-dinitroanisole in soil and soil Bacillus sp. Biodegradation. 23(2):287–295. doi:10.1007/s10532-011-9508-7.
- Pichtel J. 2012. Distribution and fate of military explosives and propellants in soil: a review. Appl Env Soil Sci. 2012:1–33. doi:10.1155/2012/617236.
- Platten WE, 3rd, Bailey D, Suidan MT, Maloney SW. 2010. Biological transformation pathways of 2,4-dinitro anisole and N-methyl paranitro aniline in anaerobic fluidized-bed bioreactors. Chemosphere 81(9):1131–1136. doi:10.1016/j.chemosphere.2010.08.044.
- Qasim M, Gorb L, Magers D, Honea P, Leszczynski J, Moore B, Taylor L, Middleton M. 2009. Structure and reactivity of TNT and related species: application of spectroscopic approaches and quantum-chemical approximations toward understanding transformation mechanisms. J Hazard Mater. 167(1–3):154–163. doi:10.1016/j.jhazmat.2008.12.105.
- Richard T, Weidhaas J. 2014a. Biodegradation of IMX-101 explosive formulation constituents: 2,4-dinitroanisole (DNAN), 3-nitro-1,2,4-triazol-5-one (NTO), and nitroguanidine. J Hazard Mater. 280:372–379. doi:10.1016/j.jhazmat.2014.08.019.
- Richard T, Weidhaas J. 2014b. Dissolution, sorption, and phytoremediation of IMX-101 explosive formulation constituents: 2,4-dinitroanisole (DNAN), 3-nitro-1,2,4-triazol-5-one (NTO), and nitroguanidine. J Hazard Mater. 280:561–569. doi:10.1016/j.jhazmat.2014.08.042.
- Robertson BK, Jjemba PK. 2005. Enhanced bioavailability of sorbed 2,4,6-trinitrotoluene (TNT) by a bacterial consortium. Chemosphere 58(3):263–270. doi:10.1016/j.chemosphere.2004.08.080.
- Rylott E, Bruce N. 2009. Plants disarm soil: engineering plants for the phytoremediation of explosives. Trends in biotechnol. 27(2):73–81. doi:10.1016/j.tibtech.2008.11.001.
- Rylott E, Jackson R, Sabbadin F, Seth-Smith H, Edwards J, Chong C, Strand S, Grogan G, Bruce N. 2011. The explosive-degrading cytochrome P450 XplA: Biochemistry, structural features and prospects for bioremediation. Biochimica Et Biophysica Acta-Proteins and Proteomics 1814(1):230–236. doi:10.1016/j.bbapap.2010.07.004.
- Rylott EL, Budarina MV, Barker A, Lorenz A, Strand SE, Bruce NC. 2011. Engineering plants for the phytoremediation of RDX in the presence of the co-contaminating explosive TNT. New Phytol. 192(2):405–413. doi:10.1111/j.1469-8137.2011.03807.x.
- Rylott EL, Gunning V, Tzafestas K, Sparrow H, Johnston EJ, Brentnall AS, Potts JR, Bruce NC. 2015. Phytodetoxification of the environmental pollutant and explosive 2,4,6-trinitrotoluene. Plant Signal Behav. 10(1):e977714. doi:10.4161/15592324.2014.977714.
- Rylott EL, Jackson RG, Edwards J, Womack GL, Seth-Smith HM, Rathbone DA, Strand SE, Bruce NC. 2006. An explosive-degrading cytochrome P450 activity and its targeted application for the phytoremediation of RDX. Nat Biotechnol. 24(2):216–219. doi:10.1038/nbt1184.
- Rylott EL, Johnston EJ, Bruce NC. 2015. Harnessing microbial gene pools to remediate persistent organic pollutants using genetically modified plants–a viable technology? EXBOTJ. 66(21):6519–6533. doi:10.1093/jxb/erv384.
- Rylott EL, Lorenz A, Bruce NC. 2011. Biodegradation and biotransformation of explosives. Curr Opin Biotechnol. 22(3):434–440. doi:10.1016/j.copbio.2010.10.014.
- Schoenmuth B, Mueller JO, Scharnhorst T, Schenke D, Buttner C, Pestemer W. 2014. Elevated root retention of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) in coniferous trees. Environ Sci Pollut Res Int. 21(5):3733–3743. doi:10.1007/s11356-013-2306-5.
- Schoenmuth BW, Pestemer W. 2004. Dendroremediation of trinitrotoluene (TNT). Part 2: fate of radio-labelled TNT in trees. Environ Sci & Pollut Res. 11(5):331–339. doi:10.1007/BF02979648.
- Schroer HW, Langenfeld KL, Li X, Lehmler H-J, Just CL. 2015. Stable isotope-enabled pathway elucidation of 2,4-dinitroanisole metabolized by Rhizobium lichtii. Env Sci Technol Lett. 2(12):362–366. doi:10.1021/acs.estlett.5b00278.
- Schroer HW, Langenfeld KL, Li X, Lehmler HJ, Just CL. 2017. Biotransformation of 2,4-dinitroanisole by a fungal Penicillium sp. Biodegradation 28(1):95–109. doi:10.1007/s10532-016-9780-7.
- Schroer HW, Li X, Lehmler HJ, Just CL. 2017. Metabolism and photolysis of 2,4-dinitroanisole in Arabidopsis. Environ Sci Technol. 51(23):13714–13722. doi:10.1021/acs.est.7b04220.
- Sens C, Scheidemann P, Klunk A, Werner D. 1998. Distribution of 14C TNT and derivatives in different biochemical compartments of Phaseolus vulgaris. Environ Sci & Pollut Res. 5(4):202–208. doi:10.1007/BF02986402.
- Sens C, Scheidemann P, Werner D. 1999. The distribution of 14C-TNT in different biochemical compartments of the monocotyledonous Triticum aestivum. Environ Pollut. 104(1):113–119. doi:10.1016/S0269-7491(98)00142-0.
- Smith DJ, Craig AM, Duringer JM, Chaney RL. 2008. Absorption, tissue distribution, and elimination of residues after 2,4,6-trinitro[14C]toluene administration to sheep. Environ Sci Technol. 42(7):2563–2569. doi:10.1021/es702601n.
- Spain JC, Hughes JB, Knackmuss HJ. 2000. Biodegradation of nitroaromatic compounds and explosives. Boca Raton, Florida: Lewis.
- Tanaka S, Brentner LB, Merchie KM, Schnoor JL, Yoon JM, Van Aken B. 2007. Analysis of gene expression in poplar trees (Populus deltoides x nigra, DN34) exposed to the toxic explosive hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX). Int J Phytoremed. 9(1):15–30. doi:10.1080/15226510601139375.
- Thijs S, Sillen W, Truyens S, Beckers B, van Hamme J, van Dillewijn P, Samyn P, Carleer R, Weyens N, Vangronsveld J. 2018. The Sycamore Maple bacterial culture collection from a TNT polluted site shows novel plant-growth promoting and explosives degrading bacteria. Frontiers Plant Sci. 9(1134) doi:10.3389/fpls.2018.01134.
- Thijs S, Van Dillewijn P, Sillen W, Truyens S, Holtappels M, D´Haen J, Carleer R, Weyens N, Ameloot M, Ramos J-L, Vangronsveld J. 2014. Exploring the rhizospheric and endophytic bacterial communities of Acer pseudoplatanus growing on a TNT-contaminated soil: towards the development of a rhizocompetent TNT-detoxifying plant growth promoting consortium. Plant Soil. 385(1–2):15–36. doi:10.1007/s11104-014-2260-0.
- Thijs S, Weyens N, Sillen W, Gkorezis P, Carleer R, Vangronsveld J. 2014. Potential for plant growth promotion by a consortium of stress-tolerant 2,4-dinitrotoluene-degrading bacteria: isolation and characterization of a military soil. Microbial Biotechnol. 7(4):294–306. doi:10.1111/1751-7915.12111.
- Toghiani RK, Toghiani H, Maloney SW, Boddu VM. 2008. Prediction of physicochemical properties of energetic materials. Fluid Phase Equilibria. 264(1–2):86–92. doi:10.1016/j.fluid.2007.10.018.
- Tzafestas K, Razalan MM, Gyulev I, Mazari AM, Mannervik B, Rylott EL, Bruce NC. 2017. Expression of a Drosophila glutathione transferase in Arabidopsis confers the ability to detoxify the environmental pollutant, and explosive, 2,4,6-trinitrotoluene. New Phytol. 214(1):294–303. doi:10.1111/nph.14326.
- Tzafestas K, Ahmad LM, Dani MP, Grogan G, Rylott EL, Bruce NC. 2018. Structure-guided mechanisms behind the metabolism of 2,4,6-trinitrotoluene by glutathione transferases U25 and U24 that lead to alternate product distribution Frontiers Plant Sci. In Press.
- United States General Accounting Office, Department of Defense Operational Ranges: More Reliable Cleanup Cost Estimates and a Proactive Approach to Identifying Contamination Are Needed, GAO-04-601 (Washington D.C.: May 2004). https://www.gao.gov/products/GAO-04-601
- Valli K, Brock BJ, Joshi DK, Gold MH. 1992. Degradation of 2,4-dinitrotoluene by the lignin-degrading fungus Phanerochaete chrysosporium. Appl Environ Microbiol. 58(1):221–228.
- Van Aken B. 2008. Transgenic plants for phytoremediation: helping nature to clean up environmental pollution. Trends in Biotechnol. 26(5):225–227. doi:10.1016/j.tibtech.2008.02.001.
- Van Aken B. 2009. Transgenic plants for enhanced phytoremediation of toxic explosives. Curr Op Biotechnol. 20(2):231–236. doi:10.1016/j.copbio.2009.01.011.
- Van Aken B, Yoon JM, Just CL, Schnoor JL. 2004. Metabolism and mineralization of hexahydro-1,3,5-trinitro-1,3,5-triazine inside poplar tissues (Populus deltoides x nigra DN-34). Environ Sci Technol. 38(17):4572–4579. doi:10.1021/es049837a.
- van Dillewijn P, Couselo J, Corredoira E, Delgado A, Wittich R, Ballester A, Ramos J. 2008. Bioremediation of 2,4,6-trinitrotoluene by bacterial nitroreductase expressing transgenic aspen. Environ Sci Technol. 42(19):7405–7410. doi:10.1021/es801231w.
- Via SM, Zinnert JC. 2016. Impacts of explosive compounds on vegetation: a need for community scale investigations. Environ Pollut. 208(Pt B):495–505. doi:10.1016/j.envpol.2015.10.020.
- Via SM, Zinnert JC, Young DR. 2015. Differential effects of two explosive compounds on seed germination and seedling morphology of a woody shrub, Morella cerifera. Ecotoxicology 24(1):194–201. doi:10.1007/s10646-014-1372-x.
- Via SM, Zinnert JC, Young DR. 2017. Multiple metrics quantify and differentiate responses of vegetation to composition B. Int J Phytoremediation. 19(1):56–64. doi:10.1080/15226514.2016.1216080.
- Vila M, Pascal-Lorber S, Rathahao E, Debrauwer L, Canlet C, Laurent F. 2005. Metabolism of [14C]-2,4,6-trinitrotoluene in tobacco cell suspension cultures. Environ Sci Technol. 39:663–672.
- Weidhaas J, Panaccione A, Bhattacharjee AS, Goel R, Anderson A, Acharya SP. 2018. Whole community transcriptome of a sequencing batch reactor transforming 2,4-dinitroanisole (DNAN) and 3-nitro-1,2,4-triazol-5-one (NTO). Biodegradation. 29(1):71–88. doi:10.1007/s10532-017-9814-9.
- Wijker RS, Bolotin J, Nishino SF, Spain JC, Hofstetter TB. 2013. Using compound-specific isotope analysis to assess biodegradation of nitroaromatic explosives in the subsurface. Environ Sci Technol. 47(13):6872–6883. doi:10.1021/es3051845.
- Williams RE, Rathbone DA, Scrutton NS, Bruce NC. 2004. Biotransformation of explosives by the old yellow enzyme family of flavoproteins. Appl Environ Microbiol. 70(6):3566–3574. doi:10.1128/AEM.70.6.3566-3574.2004.
- Wittich R, Ramos J, van Dillewijn P. 2009. Microorganisms and explosives: mechanisms of nitrogen release from TNT for use as an N-Source for growth. Environ Sci Technol. 43(8):2773–2776. doi:10.1021/es803372n.
- Wittich RM, Haidour A, Van Dillewijn P, Ramos JL. 2008. OYE flavoprotein reductases initiate the condensation of TNT-derived intermediates to secondary diarylamines and nitrite. Environ Sci Technol. 42(3):734–739. doi:10.1021/es071449w.
- Wu H, Lai C, Zeng G, Liang J, Chen J, Xu J, Dai J, Li X, Liu J, Chen M, et al. 2017. The interactions of composting and biochar and their implications for soil amendment and pollution remediation: a review. Crit Rev Biotechnol. 37(6):754–764. doi:10.1080/07388551.2016.1232696.
- Yamamoto H, Morley MC, Speitel GE, Clausen JAY. 2004. Fate and transport of high explosives in a sandy soil: adsorption and desorption. Soil Sedi Contam: An Int J. 13(5):361–379. doi:10.1080/10588330490500419.
- Zhang L, Routsong R, Nguyen Q, Rylott EL, Bruce NC, Strand SE. 2017. Expression in grasses of multiple transgenes for degradation of munitions compounds on live-fire training ranges. Plant Biotechnol J. 15(5):624–633. doi:10.1111/pbi.12661.
- Zhang L, Rylott EL, Bruce NC, Strand SE. 2017. Phytodetoxification of TNT by transplastomic tobacco (Nicotiana tabacum) expressing a bacterial nitroreductase. Plant Mol Biol. 95(1–2):99–109. doi:10.1007/s11103-017-0639-z.
- Zhang L, Rylott EL, Bruce NC, Strand SE. 2018. Genetic modification of western wheatgrass (Pascopyrum smithii) for the phytoremediation of RDX and TNT. Planta. 249:1007–1015. doi:10.1007/s00425-018-3057-9.
