 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Plants are the crucial component of floating treatment wetlands (FTWs). However, heavy metal removal capacity varies between plant species, and the relationships between plant traits and differences in removal capacity remain unclear. This study sought to determine: (1) the relationships between plant traits and removal of Cd, Cu, Pb, and Zn from water, and (2) the relationships between the removal patterns of these metals. Plants of 34 wetland plant species were exposed to heavy metal concentrations common in stormwater for five days, and 20 traits were measured on each plant. Results indicate that the most important plant traits for heavy metal removal from water are transpiration and high total biomass, especially large amounts of fine roots and leaves. The same traits were generally related to removal both initially and after longer exposure, with stronger correlations found after longer exposure. Plant removal of one metal was likely correlated with removal of the other metals, and the plant removal capacity after 30 min of exposure was correlated with the removal capacity five days later. The present results can be used in selecting plants for enhanced heavy metal removal by FTWs and in identifying additional useful plant species, allowing adaptation to local conditions.
Introduction
Floating treatment wetlands (FTWs) consisting of vegetated rafts have been proposed for the improved removal of dissolved heavy metals from stormwater, with their potential demonstrated in several mesocosm and field studies (e.g. Tanner and Headley Citation2011; Borne et al. Citation2014; Tara et al. Citation2019). Plants were grown hydroponically play an indispensable role in the function of FTWs (Tanner and Headley Citation2011), removing metals from water through uptake and accumulation in plant tissues and through adsorption on root surfaces. Plants also increase the sedimentation of metals by reducing water velocity and promoting precipitation. Metals can thereafter be removed from the system through plant tissue harvest and sediment dredging. Importantly, the ability of plants to accumulate metals differs between plant species and is suggested to be linked to their morphology and development (Fritioff and Greger Citation2003; Ladislas et al. Citation2013). However, little attention has been paid to what plant species are useful for FTWs removing heavy metals, and even less to understanding what plant traits increase metal removal. As the use of FTWs spreads to new regions, guidelines for identifying plants with high removal capacity would be useful for constructing FTWs for various conditions.
Several studies have analyzed the relationships between plant traits and heavy metal removal capacity for other types of nature-based stormwater treatment systems to provide selection guidelines. In treatments based on free-floating plants, the removal efficacy for heavy metals depends on the plant’s growth rate, heavy metal tolerance, and adaptability to various conditions (Rezania et al. Citation2016). In wetlands and rivers, the root biomass of aquatic plants is an essential trait, and plant species accumulating one metal are likely to accumulate other present metals as well (Li et al. Citation2015). In biofilters, where polluted water is filtered through vegetated coarse soil material, only weak correlations between heavy metal removal and plant traits were found because the heavy metals were mainly bound to the filter material (Read et al. Citation2009). For nitrogen (N) and phosphorus (P) removal in biofilters and constructed wetlands, high growth rate and root traits such as fibrous roots, long roots, and large root surface area have been identified as key traits (Cheng et al. Citation2009; Read et al. Citation2009; Payne et al. Citation2018).
To our knowledge, no investigations of what morphologies or growth traits promote efficient metal removal by FTWs have yet been published. In a recent study, we compared the metal removal capacity of 34 wetland plants, finding that Carex pseudocyperus and C. riparia quickly removed most Cd, Cu, Pb, and Zn from water (Schück and Greger Citationsubmitted). These are both tall sedge species with large root systems and high leaf biomass. In addition, C. pseudocyperus was also among the investigated plants with the highest fine root biomass and C. riparia had the second lowest root: shoot ratio of all examined species. Based on these findings, we examine whether these and other measured traits are correlated with plant removal of metals in general.
In this study, we investigated what plant traits are associated with the high removal of heavy metals from water, providing guidance for plant selection for FTWs. The specific objectives were: (1) to determine whether high root and leaf biomass, as well as high growth and transpiration rates, are properties related to high metal removal capacity; and (2) to determine the relationships between uptake patterns of Cd, Cu, Pb, and Zn.
Materials and methods
Plant selection and preparation
A hydroponic greenhouse experiment was performed to study the relationships between plant characteristics and removal of heavy metals from water. Plants from 34 emergent and terrestrial wetland species were used, either collected from the wild near Stockholm, Sweden, grown from seed, or purchased as plug plants propagated from seeds collected in Sweden (Vegtech AB, Vislanda, Sweden) (). The plants came from a wide range of habitats, including lakes, seas, and several types of wetlands, and represented a total of 13 families; they ranged in size from 0.26 to 13.06 g DW, with 0.05–1.96 g DW fine root biomass. After carefully cleaning the roots of soil by hand and rinsing them with tap water, all plants were grown in modified Hoagland solution (Eliasson Citation1978) with aeration for at least two weeks in a greenhouse (13.5 h light, 60% RH, 22 °C) to ensure uniformity.
Table 1. Emergent and terrestrial wetland species included in the experiment.
Experimental design
At the start of the experiment, the plant roots were rinsed with deionized water and any dead plant parts were removed. Each plant was gently shaken to remove water and weighed to determine its fresh weight. The plants were attached to Styrofoam plates and placed in 1-L plastic containers filled with 850 mL of aerated saline heavy metal solution (1.2 µg Cd L−1, 68.5 µg Cu L−1¸ 78.4 µg Pb L−1, 559 µg Zn L−1, and 55.4 mg Cl L−1 added as CdCl2, CuCl2, PbCl2, ZnCl2, and NaCl) to represent springtime stormwater containing chloride from deicing salt (Billberger Citation2011). Three replicates were made for each species; in addition, three more plants of each species were prepared as above but dried (105 °C, 45 h) after their fresh weights were determined, to serve as controls for the dry: fresh biomass ratio. The containers were placed in randomized blocks in the greenhouse described above. Three containers without plants but otherwise identical were used to account for the evaporation and adsorption of metals to non-plant surfaces.
Sampling
The solution was sampled before the addition of plants, after 0.5 h, and at the end of the experiment after 119 h; 12 species were also sampled after 5 h and six species after 24 h. At all samplings, 20 mL samples were taken after vigorous stirring to ensure representative samples. The samples were stored in 24 mL polyethylene bottles at 3 °C until further analysis. At the end of the experiment, the volumes of water remaining in the containers were determined, as were the fresh weights of the plants. The plants were rinsed in deionized water and dried (105 °C, 45 h), after which the fine roots (<1 mm diameter), coarse roots (>1 mm diameter), rhizomes, leaves, and stems were weighed.
Heavy metal analysis
The samples were analyzed for heavy metal concentration using atomic absorption spectrophotometry after filtering through 0.45 µm filters (Sarstedt, Nümbrecht, Germany) to remove particles. Cadmium, Cu, and Pb contents were analyzed using a SpectrAA 240, GTA 120 furnace (Agilent, Santa Clara, CA, USA). The detection limits were 0.006, 0.04, and 0.06 µg L−1 for Cd, Cu, and Pb, respectively. Before the analysis, 5 µL of 65% HNO3 per mL of sample was added, and 2.2% NaCl solution was used as a modifier to prevent interference from the matrix. Zinc was analyzed using a SpectrAA 55B (Varian, Palo Alto, CA, USA) equipped with a flame atomizer (detection limit 1 µg L−1). Standard additions were used in all analyses to eliminate influences from the matrix.
Data analysis
The shares of remaining metal in the solution compared with the start and metal removal capacity were calculated as
(1)
(1)
(2)
(2)
where [Me]tx is the concentration in solution at sampling time tx, Vtx is the volume of solution remaining at sampling time tx, [Me]t0 and Vt0 are the starting concentration and volume, and Rctx is the remaining metal for no-plant control at sampling time tx. The volume changes during the first day due to water loss from evaporation and transpiration were assumed to be negligible.
Net uptake per fine root biomass removal was calculated as
(3)
(3)
where [Me]ctx is the concentration in containers without plants at sampling time tx, Vctx is the volume in containers without plants at sampling time tx, and mfine root (DW) is the dry weight of the fine roots.
Water uptake by plants was calculated as
(4)
(4)
where Vs is the combined volume of samples taken at 0.5, 5, and 24 h.
Transpiration was calculated as:
(5)
(5)
where Wu is the water uptake from (4), FWt0 is the initial fresh weight of the treated plant, DWcorr and FWcorr are the dry and fresh weights of an untreated plant specimen of the same species at t0, and FWt119 and DWt119 are the plant fresh and dry weights at 119 h, respectively.
Statistical analysis
Correlations between parameters and metal removal were tested using the Spearman rank correlation test, p < 0.05, on species averages datasets. Multiple linear regression was used to develop models of metal removal. Parameters with intercorrelations of more than 0.8 (Spearman rank correlation test, p < 0.05) were not used in the same model. All models were checked for the normality of residuals and for heteroscedasticity. Cook’s distance was used to identify influential outliers, which subsequently were removed. All statistical analysis was conducted using the packages stats and psych in R (version 3.6.1).
Results
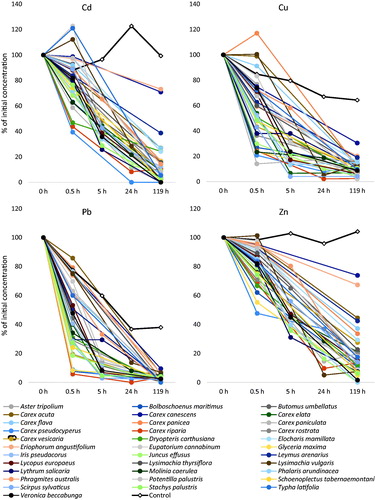
The concentration of all metals in the solution was decreased by the plants according to a similar pattern (). The results indicate that most of the removal took place within the first 24 h of exposure, especially during the first hours, whereas little change was seen afterward. There was large variation between species, with some having removed almost all the metal after 0.5 h of exposure, while others had concurrently increased the metal concentration in the water by releasing previously-stored metals. The plants’ highest removal capacities were seen for Cu and Pb, whereas those for Zn and Cd were slightly lower. In the controls, i.e. beakers without plants, the concentrations of Cu and Pb declined with time, likely caused by adsorption to the beakers; in contrast, Cd and Zn concentrations in the control water did not change with time.
Figure 1. Plant-caused decreases in heavy metal concentrations in water during 119 h of exposure. Note the nonlinear time axis. n = 3 for all plant species, n = 21 for the no-plant treatment (i.e. control).
Relationships between plant traits, sampling occasions, and metal removal
The removal capacities of plants were tested for correlations with plant biomass, growth, and transpiration to understand whether these traits determine the removal of metals by plants (). All biomass traits, except fresh weight for total biomass, are given in dry weights. The results indicate high correlations between metal removal and biomass on the first sampling occasion (0.5 h), especially for the dry and fresh total biomass and the biomass of aboveground parts, leaves, and fine roots for all included metals. After 119 h of exposure, Zn removal was positively influenced by almost all biomass parameters, water uptake, and transpiration. Cadmium and Cu removal were positively influenced by rhizome biomass, aboveground biomass, fresh and dry total biomass, transpiration, and water uptake. Copper removal was also influenced by leaf biomass and belowground biomass. All these correlations between metal removal and plant traits were weaker after 119 h of exposure versus after 0.5 h of exposure, except for rhizome biomass, which was not correlated with removal in the earlier samplings but later displayed a positive correlation with metal removal. Since all plant species removed Pb to a similar, low level, there were only small differences in Pb removal capacity between plant species after 119 h of exposure. Hence, only one correlation was found between Pb removal and the fine root biomass:total biomass ratio.
Table 2. Spearman rank correlations, ρ, between measured parameters and metal removal from the solution.
The differences in metal removal capacity between species were also evaluated as the net uptake per fine root biomass since most uptake occurs via fine roots (). The results indicate clear differences between the metals in net uptake per fine root biomass and other measured parameters after 0.5 h of exposure. Cadmium net uptake per fine root biomass was positively influenced by aboveground biomass, total biomass, and fine root biomass. Zinc net uptake per fine root biomass was negatively influenced by the fine root biomass, leaf biomass, aboveground biomass, and total dry biomass. Copper net uptake per fine root biomass was correlated only with fine root biomass. Lead net uptake per biomass was positively influenced by a high fresh:dry biomass ratio but negatively influenced by a high fine root:total biomass ratio.
Table 3. Spearman rank correlations, ρ, between measured parameters and net uptake of metals per fine root biomass.
The depletion of all metals from the water by many plants towards the end of the study, after 119 h of exposure, resulted in negative correlations between biomass size and distribution traits, on one hand, and between net uptake per fine root biomass and % removal, on the other hand, as the largest plants with most fine roots were restricted in their uptake by the depletion. Besides biomass parameters, only transpiration, which in turn is correlated with leaf biomass (ρ = 0.52, p < 0.05, not shown), is correlated with net uptake after 119 h of exposure.
Strong correlations were generally found between the uptakes of the different metals on the same sampling occasions, and between the net uptakes per fine root biomass of the same metal on different sampling occasions (). The uptake of Zn displays the highest correlation of the investigated metals both with the other metals and with sampling occasions. In contrast, the uptake per fine root biomass of Cd after 0.5 h of exposure displays no correlation with the other metals or sampling occasions, except for a weak negative correlation with Cu and Pb uptake after 119 h of exposure.
Table 4. Spearman rank correlations, ρ, between metal uptakes and sampling occasions.
Only minor changes in plant biomass were found due to the short experimental duration, and no correlations with the uptake of metals were found. All correlation results for the 5 h and 24 h sampling occasions are found in Supplementary Tables S1 and S2.
Model of metal removal
Using multiple linear regression to predict the metal removal capacity after 0.5 h of exposure resulted in several possible models. A general trend is that removal capacity after 0.5 h of exposure can be predicted by fine root biomass, leaf biomass, and transpiration measured after 119 h of exposure, either alone or in combination (). Zinc removal can be explained with the best accuracy, followed by Pb, Cu, and Cd. For Cu and Pb, the best model included transpiration data. In these cases, a model with worse fit based only on biomass parameters is also included. The same type of analysis was conducted for the net uptake per fine root biomass after 0.5 h of exposure, but it resulted in much weaker predictions compared with metal removal in % (). The net uptake of Cu per fine root biomass could be predicted by the amount of fine root biomass, Pb by the fine root: total biomass ratio, and Zn by a combination of aboveground biomass and fine root: total biomass ratio. It was impossible to develop a model that predicted net uptake of Cd per fine root biomass.
Table 5. Estimated linear regression models of metal removal capacity (MRC) and net uptake per fine root biomass (NUFRB) after 0.5 h of exposure.
Discussion
This work demonstrates that the ability of plants to remove heavy metals is strongly influenced by their morphology. Overall, the correlations between plant traits and metal removal were stronger at the beginning than the end of the experiment, when most plants had been able to remove almost all available metal, whereas the correlations between metals were strongest at the end of the experiment (). The positive influence of biomass size, in terms of both dry and fresh biomass, on metal removal was clear for all included metals, in agreement with many other studies (Weiss et al. Citation2006; Wang et al. Citation2014; Ladislas et al. Citation2015; Ge et al. Citation2016). Larger plants tend to be more exposed to the pollutants and can store more pollutants while maintaining low tissue concentrations. However, our results indicate that the various parts of the plant are differently correlated with metal removal.
Influence of roots and belowground parts
Plants with large fibrous root systems removed markedly more metals than did plants with coarser roots (). Larger root systems have greater surface areas, which absorb and adsorb more metals (Li et al. Citation2015); this is especially important for Pb, which readily binds to organic matter such as roots (Ignatius et al. Citation2014). The lack of restricting tissue, such as exodermis, in young fine roots enables high absorption to the apoplast. In addition, the influence on the surrounding water, such as pH change, oxygen release, and exudate release, can be expected to increase with increasing root system size. Similar results have previously been reported for Cd, Cu, and Zn and root biomass (R2=0.66–0.90) (Nguyen et al. Citation2016).
As the fine root biomass increased, the net uptake efficacy per unit of fine root biomass decreased for Cu and Zn (). For the first period of the experiment, this can likely be explained by: (1) the dilution effect described for increasing root biomass by Ekvall and Greger (Citation2003); and (2) the decreased mass flow and diffusion of metals from water to roots when most metals had already been removed, thus decreasing the uptake. Similar relationships between small root biomass and high metal concentration in plant tissue were also seen by Sricoth et al. (Citation2018).
The coarse root biomass did not influence the uptake of any metal (), likely due to their small surface area in relation to their volume, giving low exposure to the pollutant, and to the presence of tissues such as exodermis, restricting the exchange of ions, oxygen, and water (White Citation2012). For the 19 of 34 species that had rhizomes, rhizome biomass had a positive influence on the removal of Cd, Cu, and Zn, but only on the last sampling occasion after 119 h. We speculate that this was caused by the translocation of these metals from roots to rhizomes, a slower process than metal adsorption on fine roots that is only important after longer exposure. However, rhizomes generally have lower tissue concentrations of metals than do roots (Zhang et al. Citation2010; Bonanno and Cirelli Citation2017), so larger root systems should be preferred to rhizomes for metal removal.
Influence of leaves, aboveground parts, and transpiration
There was a clear correlation between leaf biomass and the uptake of all metals on the first sampling occasion. However, as the leaves were not directly exposed to the metals, their influence was indirect. A likely explanation is that large leaf biomass is related to high transpiration (ρ = 0.52, p < 0.05, not shown), which was also correlated with metal removal (), resulting in higher water uptake that increases the metal uptake (Chien et al. Citation2006; Salah and Barrington Citation2006). A less likely explanation is that the leaves act as storage space for the metals, increasing the total storage capacity. It was not measured whether the leaves accumulated any metals, but most of the accumulated metal was likely stored in belowground parts (Deng et al. Citation2004). Another possible explanation for the correlation between metal uptake and leaf biomass is that the larger leaf biomass promotes root growth due to higher photosynthetic yield (Li et al. Citation2013). The correlation we found between leaf and fine root dry biomass was 0.72 (p < 0.05, not shown), similar to the correlations between leaf dry biomass and metal removal ().
Relationship between uptake patterns of metals
The correlations between the uptakes of the various metals were mostly strong, indicating that plants that successfully removed one studied metal likely also removed the others (), corroborating Li et al. (Citation2015), who found correlations of r = 0.74–0.90 between the uptakes of Cd, Cu, and Zn in a large range of species. In fact, the correlations between the uptakes of different metals in our study were often stronger than the correlations between metal uptake and the other measured traits, indicating that there are traits not included in this study that could further explain the variation between species.
Strong correlations were also observed between different sampling occasions for Cu, Pb, and Zn (). The strongest correlations between the uptakes of the various metals and other sampling occasions were found after 119 h of exposure, indicating that investigating the removal capacity for one metal on this occasion could be enough to predict the removal of the other metals on other occasions. Similar uptake patterns have been found for several metals, including all those studied here (Rai et al. Citation1995; Tanner and Headley Citation2011). In addition, although the duration of our test was only 5 days, we found similar uptake capacities when comparing individual species exposed to similar concentrations of metals for longer periods in other studies (Weiss et al. Citation2006; Ladislas et al. Citation2013; Schück and Greger, Citationsubmitted).
Agreement with other trait studies
Extensive fine root systems and large leaf biomass, identified as important traits for metal removal in this study, are also found in Carex riparia, Carex virgata, Cyperus ustulatus, and Juncus effusus, demonstrated in other studies to perform well for metal removal in FTWs (Tanner and Headley Citation2011; Borne et al. Citation2014; Ladislas et al. Citation2015), confirming our findings. Plants in FTWs that efficiently remove metals will also likely remove other pollutants, such as excess nutrients, phytoplankton, and particles, for which plants with large, dense root systems with long roots are preferred (Borne et al. Citation2013; Castro-Castellon et al. Citation2016; Ge et al. Citation2016). In addition, extensive fine root systems, high aboveground biomass, and high total biomass were identified as important traits both for heavy metal removal in this study and for the removal of N and P by plants growing in biofilters and constructed wetlands (Cheng et al. Citation2009; Read et al. Citation2009; Payne et al. Citation2018), indicating that large exposed surfaces and high storage capacity are useful plant traits for removing several types of pollutants under various growth conditions.
Choosing plants for FTWs
Additional species with potential for FTW metal removal due to their large fibrous root systems and shoots dominated by leaves can be found in the Cyperacae and Poaceae families, among others. The metal removal capacity of a plant can generally be predicted using the models developed here (), but testing species before the full-scale application is recommended, as the models do not explain all observed variation in metal uptake capacity between species. In addition, field conditions, including seasonal changes and interaction with other substances in the water, could also change the dynamics of the uptake behavior. However, due to the high correlation between uptakes of all studied metals, a species proven to efficiently remove one metal can be assumed to efficiently remove other metals as well.
We conclude that specific plant traits can be used to predict what plants have a high probability of efficient heavy metal removal. The removal of heavy metals by FTWs might be increased by selecting plants with high transpiration and high total biomass, especially if they have high fine root biomass and high leaf biomass. The present results can guide the identification of additional plant species with potentially high heavy metal removal capacities, allowing adaptation to local conditions and further increasing the versatility of FTWs.
Supplemental Material
Download MS Word (16.8 KB)Acknowledgements
We acknowledge Tommy Landberg for advice on metal analysis, Lark Davis and Léa Laguillaumie for help during the plant collection, and Dr. Jan-Olov Persson for suggestions regarding statistical analysis.
Additional information
Funding
References
- Billberger M. 2011. Road runoff - advice and recommendations for choosing environmental measures (in Swedish). Vol. 2011: 112. Borlänge, (SE): Swedish transport administration.
- Bonanno G, Cirelli GL. 2017. Comparative analysis of element concentrations and translocation in three wetland congener plants: Typha domingensis, Typha latifolia and Typha angustifolia. Ecotoxicol Environ Saf. 143:92–101. doi:10.1016/j.ecoenv.2017.05.021.
- Borne KE, Fassman-Beck EA, Tanner CC. 2014. Floating treatment wetland influences on the fate of metals in road runoff retention ponds. Water Res. 48:430–442. doi:10.1016/j.watres.2013.09.056.
- Borne KE, Fassman EA, Tanner CC. 2013. Floating treatment wetland retrofit to improve stormwater pond performance for suspended solids, copper and zinc. Ecol Eng. 54:173–182. doi:10.1016/j.ecoleng.2013.01.031.
- Castro-Castellon AT, Chipps MJ, Hankins NP, Hughes J. 2016. Lessons from the “Living-filter”: An in-reservoir floating treatment wetland for phytoplankton reduction prior to a water treatment works intake. Ecol Eng. 95:839–851. doi:10.1016/j.ecoleng.2016.07.023.
- Cheng XY, Chen WY, Gu BH, Liu XC, Chen F, Chen ZH, Zhou XY, Li YX, Huang H, Chen YJ. 2009. Morphology, ecology, and contaminant removal efficiency of eight wetland plants with differing root systems. Hydrobiologia. 623(1):77–85. doi:10.1007/s10750-008-9649-9.
- Chien SWC, Das B, Liao YC, Hung PL, Shen Y, Wang MC. 2006. Effect of transpiration on Pb uptake by lettuce and on water soluble low molecular weight organic acids in rhizosphere. Chemosphere. 65:343–351. doi:10.1016/j.chemosphere.2006.02.010.
- Deng H, Ye Z, Wong M. 2004. Accumulation of lead, zinc, copper and cadmium by 12 wetland plant species thriving in metal-contaminated sites in China. Environ Pollut. 132:29–40. doi:10.1016/j.envpol.2004.03.030.
- Ekvall L, Greger M. 2003. Effects of environmental biomass-producing factors on Cd uptake in two Swedish ecotypes of Pinus sylvestris. Environ Pollut. 121:401–411. doi:10.1016/s0269-7491(02)00232-4.
- Eliasson L. 1978. Effects of nutrients and light on growth and root formation in Pisum sativum cuttings. Physiol Plant. 43(1):13–18. doi:10.1111/j.1399-3054.1978.tb01560.x.
- Fritioff Å, Greger M. 2003. Aquatic and terrestrial plant species with potential to remove heavy metals from stormwater. Int J Phytoremediation. 5(3):211–224. doi:10.1080/713779221.
- Ge Z, Feng C, Wang X, Zhang J. 2016. Seasonal applicability of three vegetation constructed floating treatment wetlands for nutrient removal and harvesting strategy in urban stormwater retention ponds. Int Biodeterior Biodegrad. 112:80–87. doi:10.1016/j.ibiod.2016.05.007.
- Ignatius A, Arunbabu V, Neethu J, Ramasamy EV. 2014. Rhizofiltration of lead using an aromatic medicinal plant Plectranthus amboinicus cultured in a hydroponic nutrient film technique (NFT) system. Environ Sci Pollut Res. 21(22):13007–13016. doi:10.1007/s11356-014-3204-1.
- Ladislas S, Gérente C, Chazarenc F, Brisson J, Andrès Y. 2013. Performances of two macrophytes species in floating treatment wetlands for cadmium, nickel, and zinc removal from urban stormwater runoff. Water Air Soil Pollut. 224:1408. doi:10.1007/s11270-012-1408-x
- Ladislas S, Gérente C, Chazarenc F, Brisson J, Andrès Y. 2015. Floating treatment wetlands for heavy metal removal in highway stormwater ponds. Ecol Eng. 80:85–91. doi:10.1016/j.ecoleng.2014.09.115.
- Li J, Yu H, Luan Y. 2015. Meta-analysis of the copper, zinc, and cadmium absorption capacities of aquatic plants in heavy metal-polluted water. IJERPH. 12(12):14958–14973. doi:10.3390/ijerph121214959.
- Li L, Yang Y, Tam NFY, Yang L, Mei X-Q, Yang F-J. 2013. Growth characteristics of six wetland plants and their influences on domestic wastewater treatment efficiency. Ecol Eng. 60:382–392. doi:10.1016/j.ecoleng.2013.09.044.
- Nguyen C, Soulier AJ, Masson P, Bussière S, Cornu JY. 2016. Accumulation of Cd, Cu and Zn in shoots of maize (Zea mays L.) exposed to 0.8 or 20 nM Cd during vegetative growth and the relation with xylem sap composition. Environ Sci Pollut Res Int. 23(4):3152–3164. doi:10.1007/s11356-015-5782-y.
- Payne EGI, Pham T, Deletic A, Hatt BE, Cook PLM, Fletcher TD. 2018. Which species? A decision-support tool to guide plant selection in stormwater biofilters. Adv Water Resour. 113:86–99. doi:10.1016/j.advwatres.2017.12.022.
- Rai UN, Sinha S, Tripathi RD, Chandra P. 1995. Wastewater treatability potential of some aquatic macrophytes: removal of heavy metals. Ecol Eng. 5(1):5–12. doi:10.1016/0925-8574(95)00011-7.
- Read J, Fletcher TD, Wevill T, Deletic A. 2009. Plant traits that enhance pollutant removal from stormwater in biofiltration systems. Int J Phytoremediation. 12(1):34–53. doi:10.1080/15226510902767114.
- Rezania S, Taib SM, Md Din MF, Dahalan FA, Kamyab H. 2016. Comprehensive review on phytotechnology: heavy metals removal by diverse aquatic plants species from wastewater. J Hazard Mater. 318:587–599. doi:10.1016/j.jhazmat.2016.07.053.
- Salah SA, Barrington SF. 2006. Effect of soil fertility and transpiration rate on young wheat plants (Triticum aestivum) Cd/Zn uptake and yield. Agric Water Manag. 82(1–2):177–192. doi:10.1016/j.agwat.2005.06.002.
- Schück M, Greger M. Capacity of 34 wetland plant species to remove heavy metals from water. Environ Sci Pollut Res.
- Sricoth T, Meeinkuirt W, Saengwilai P, Pichtel J, Taeprayoon P. 2018. Aquatic plants for phytostabilization of cadmium and zinc in hydroponic experiments. Environ Sci Pollut Res Int. 25(15):14964–14976. doi:10.1007/s11356-018-1714-y.
- Tanner CC, Headley TR. 2011. Components of floating emergent macrophyte treatment wetlands influencing removal of stormwater pollutants. Ecol Eng. 37(3):474–486. doi:10.1016/j.ecoleng.2010.12.012.
- Tara N, Arslan M, Hussain Z, Iqbal M, Khan QM, Afzal M. 2019. On-site performance of floating treatment wetland macrocosms augmented with dye-degrading bacteria for the remediation of textile industry wastewater. J Clean Prod. 217:541–548. doi:10.1016/j.jclepro.2019.01.258.
- Wang C, Zheng S, Wang P, Qian J. 2014. Effects of vegetations on the removal of contaminants in aquatic environments: a review. J Hydrodyn. 26(4):497–511. doi:10.1016/S1001-6058(14)60057-3.
- Weiss J, Hondzo M, Biesboer D, Semmens M. 2006. Laboratory study of heavy metal phytoremediation by three wetland macrophytes. Int J Phytoremediation. 8(3):245–259. doi:10.1080/15226510600846798.
- White P. 2012. Ion uptake mechanisms of individual cells and roots: short-distance transport. In: Marschner P, editor. Marschner’s mineral nutrition of higher plants. London (UK): Elsevier.
- Zhang Z, Rengel Z, Meney K. 2010. Cadmium accumulation and translocation in four emergent wetland species. Water Air Soil Pollut. 212(1–4):239–249. doi:10.1007/s11270-010-0339-7.
